Abstract
CD4 and CD8 lymphocyte numbers in the gut lamina propria are grossly altered in HIV-1 infection, out of proportion to alterations in the circulation. Such alterations in lymphocyte counts in the tissues may be due to altered leucocyte migration from the blood. One factor affecting leucocyte migration is adhesion molecule expression. Levels of adhesion molecule expression on peripheral CD4 and CD8 lymphocytes, monocytes and neutrophils from HIV-1-infected (AIDS and non-AIDS) and low-risk control individuals were compared. CD11a, CD62L, CD44, CD49d and β7 integrin expression were examined by FACS analysis of fresh whole blood. Significant alterations in adhesion molecule expression were detected in HIV infection. The most striking alterations were observed in the CD8 lymphocyte population. CD11a expression was increased and CD62L and CD44 decreased. The CD4 lymphocyte population followed a similar, though less striking, pattern of alteration in adhesion molecule expression. Neutrophils displayed significantly reduced expression of both CD11a and CD62L, but only after onset of AIDS. Monocytes from infected individuals without AIDS displayed a different pattern of altered adhesion molecule expression compared with individuals with AIDS. These findings suggest that in HIV infection, leucocyte functions, such as migration, which require adhesion molecules are abnormal.
Keywords: HIV-1, adhesion molecules, CD44, blood leucocytes
INTRODUCTION
The increasing immunological deterioration associated with HIV infection is obviously a more complex process than a simple reduction in CD4 lymphocyte numbers, although this change remains the most easily available marker to assess disease progression [1]. The reduction in CD4 lymphocyte count is thought to be primarily related to destruction by HIV, but might also result from altered lymphocyte distribution and migration within the body [2]. One major factor affecting leucocyte distribution, migration and function is the interaction between cells facilitated by adhesion molecules [3].
Alterations in expression of a variety of adhesion molecules by leucocytes might result in alterations in leucocyte migration, thereby affecting leucocyte numbers in the peripheral blood and tissues. Whereas HIV infection appears to result in greater retention of lymphocytes within the lymph node [4], other tissues, such as the lamina propria of the intestine, harbour greatly reduced populations of CD4 lymphocytes, with reductions out of proportion to the reductions observed in the peripheral blood [5,6].
The present study aimed to assess the level of expression of a variety of adhesion molecules on the predominant peripheral leucocyte populations, the CD4 and CD8 lymphocytes, monocytes and neutrophils, by FACS analysis. Fresh whole blood was analysed, thereby minimizing in vitro alterations in adhesion molecule expression [7]. HIV-infected individuals with and without AIDS were compared with individuals at low risk of HIV infection. Adhesion molecules associated with leucocyte trans-endothelial migration were studied: CD11a, CD62L (l-selectin), CD44, CD49d and integrin β7 [8].
SUBJECTS AND METHODS
Subjects and sampling
Thirty-two HIV-1-infected and 15 laboratory control individuals were studied. Infected individuals were all anti-retroviral treatment-naive and were subdivided into two groups according to blood CD4 count and AIDS-defining illness: Group 1, n = 15, CD4 ≥ 200/μl (mean = 447, s.d. = 144), and Group 2, n = 17, CD4 < 200/μl (mean = 80, s.d. = 65), with an AIDS-defining illness according to the CDC classification. Blood was collected into potassium EDTA tubes, placed on ice immediately and processed for flow cytometric analysis within 1 h.
Monoclonal antibodies
The following directly conjugated mouse anti-human MoAbs (all IgG1) were used along with mouse IgG1 isotype controls for three-colour FACS analysis: CD4–PE (Dako, Cambridge, UK), CD8–PE–Cy5 (Dako), CD11a–FITC (Dako), CD62L–FITC (R&D Systems, Abingdon, UK), CD44–FITC (Sigma, St Louis, MO) and CD49d–FITC (Serotec, Oxford, UK). Unconjugated rat anti-human integrin β7 and FITC-conjugated goat anti-rat immunoglobulin (multiple adsorption) were purchased from Pharmingen (San Diego, CA). Unconjugated rat IgG was used as a control. CD14–PE (Sigma), mouse IgG2a–PE (Sigma), CD15–FITC (Dako) and mouse IgM–FITC (Dako) were used in single-colour FACS analysis.
Flow cytometry
All procedures were performed at 4°C or on ice to minimize in vitro alterations in adhesion molecule expression [7]. Blood (50 μl) was incubated with 5 μl of anti-CD4, anti-CD8 and one of the anti-adhesion molecule antibodies for 45 min in the dark. Erythrocytes were lysed with freshly prepared Tris (0.017 m) ammonium chloride (0.14 m) and the remaining leucocytes washed with 0.1% w/v bovine serum albumin (BSA)–PBS. Leucocytes incubated with directly conjugated antibodies were fixed in 2% w/v paraformaldehyde in PBS. Leucocytes incubated with unconjugated rat antibodies were incubated with FITC-conjugated goat anti-rat immunoglobulin (10 μl added to residual BSA–PBS after washing) for 30 min followed by washing and fixation.
Leucocytes were analysed with a Becton Dickinson (San Jose, CA) Facscalibur between 18 h and 72 h post-fixation. Lymphocyte, monocyte and neutrophil populations were gated on the basis of forward and side scatter. Lymphocytes were further gated on the basis of CD4 and CD8 expression. Mean fluorescent intensities (MFI) were determined for all adhesion molecules studied by comparison with isotype controls. In separate tubes the validity of the monocyte and neutrophil gates was confirmed by CD14 and CD15 staining, respectively. Levels of significance were determined with a two-tailed two-sample t-test assuming unequal variance using Microsoft Excel software.
RESULTS
Significant differences in MFI of expression of CD11a, CD62L and CD44 were detected in the two HIV-infected groups compared with the control group. However, no significant differences in the levels of CD49d or integrin β7 were detected on any of the blood leucocyte populations studied from HIV-infected individuals, compared with control individuals (data not shown).
In the CD4 lymphocyte population (Fig. 1a) mean CD11a expression was significantly higher in both HIV-infected groups. CD62L expression was lower on CD4 lymphocytes from HIV-infected individuals, though not significantly. CD44 expression was significantly reduced only in individuals with AIDS.
Fig. 1.
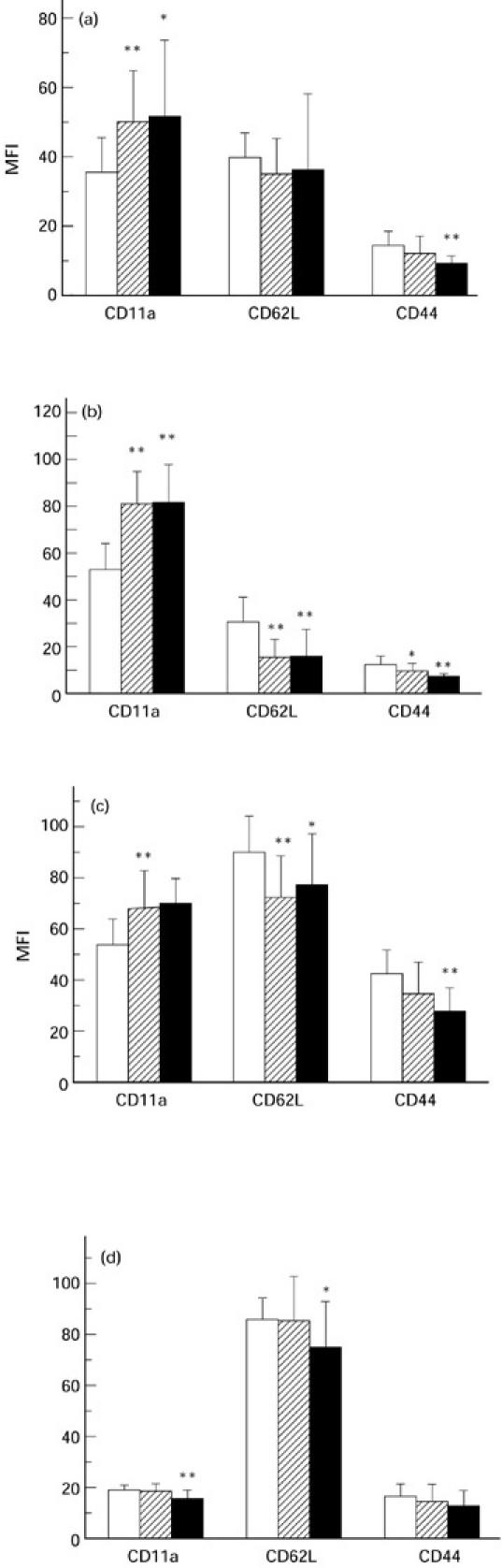
Mean fluorescent intensities (MFI) of CD11a, CD62L and CD44 expression on peripheral blood CD4 lymphocytes (a), CD8 lymphocytes (b), monocytes (c), and neutrophils (d). Three groups of individuals were assessed: laboratory controls (□), HIV-1-infected with CD4 counts ≥ 200/μl (hatched), and HIV-1-infected with CD4 counts < 200/μl and an AIDS-defining illness (▪). Error bars indicate + 1 s.d. The two HIV-infected groups were compared with the control group for statistically significant differences: *P < 0.05; **P < 0.01.
Compared with CD4 lymphocytes, CD8 lymphocytes (Fig. 1b) displayed a more abnormal adhesion molecule profile in both HIV-infected groups. CD11a expression was significantly increased and CD62L significantly decreased in both HIV-infected groups. Decreased CD44 expression on CD8 lymphocytes was significant in both HIV-infected groups but, as in the CD4 lymphocyte population, was more pronounced in individuals with AIDS.
Lymphocyte expression of CD62L by both CD4 and CD8 lymphocyte populations was variable in both HIV-infected and control populations (from absent to high). However, similar results, in terms of statistically significant differences, to those seen with MFI expression were obtained when the proportion of lymphocytes displaying CD62L from HIV-infected individuals were compared with control individuals (data not shown).
Monocytes (Fig. 1c) displayed abnormal adhesion molecule profiles in both HIV-infected groups. Highly significant increased CD11a and decreased CD62L expression were detected only in HIV-infected individuals without AIDS. These abnormalities were not as pronounced on monocytes from AIDS patients. However, highly significant reductions in CD44 expression were detected on monocytes from AIDS patients.
Neutrophils (Fig. 1d) displayed both significantly decreased CD11a and CD62L expression, but only in AIDS.
DISCUSSION
This study confirms the previous observation [9] of altered adhesion molecule expression in neutrophils in HIV infection, and extends these observations to the CD4 lymphocyte, CD8 lymphocyte and monocyte populations. The most consistent finding was a considerable diminution in CD44 expression that was observed in the CD4 lymphocyte, CD8 lymphocyte and monocyte populations from individuals with AIDS, but not the neutrophil population.
In general the abnormalities were most pronounced in the CD8 lymphocyte population in both HIV-infected groups. This sub-population displayed elevated levels of CD11a and reduced CD62L expression.
The causes of the altered adhesion molecule profiles are unknown. The pattern of CD11a and CD62L expression would suggest an activated phenotype [10,11]. In HIV infection, chronic immune activation is known to affect particularly the CD8 lymphocyte population [1] where the changes in this study were more pronounced. Similarly the highly altered CD11a and CD62L profiles in the monocyte population would suggest activation of this cell type during the asymptomatic phase, but to a lesser extent during AIDS.
Memory CD8 lymphocytes, in contrast to naive lymphocytes, express elevated levels of CD11a and reduced CD62L [12]. The CD8 CD45RO+ memory lymphocyte population is expanded in HIV infection [13], which could contribute to the altered profiles observed in this study. However, human CD4 and CD8 CD45RO+ memory lymphocytes are CD44high [14] and the reduced levels of expression of this adhesion molecule on CD4 lymphocytes, CD8 lymphocytes and monocytes from individuals with AIDS remain unexplained. Among the possibilities are direct infection of CD4 lymphocytes and monocytes with HIV and/or alterations in the cytokine milieu with advancing infection-causing changes in CD44 expression in CD4 lymphocytes, CD8 lymphocytes and monocytes.
The observed changes in cell adhesion molecule expression may have important implications for the pathogenesis of HIV infection. Abnormal levels of adhesion molecule expression on the leucocyte cell surface would affect leucocyte interactions and communication with other cells, thereby altering the ability of a leucocyte to respond effectively to infection or migrate to the appropriate tissue. Some of the adhesion molecules studied were expressed at normal levels on leucocytes from HIV-infected individuals. However, immune and particularly migratory cellular interactions are multistep processes requiring orchestrated sequences of adhesive events. Therefore, an adhesion molecule defect at any one stage in the adhesive process could inhibit the entire process, even if other adhesive interactions are intact.
Alterations in levels of integrin β7 (α4β7) or CD49d (α4β1) integrins were not detected in this study. These two integrins appear to have related though opposing functions, as expression of one or the other of these adhesion molecules appears to define a subdivision of lymphocytes as either MAdCAM-1 binding mucosal homing (α4β7) or vascular cell adhesion molecule-1 (VCAM-1) binding non-mucosal homing (α4β1) [15,16]. Integrin activity is dependent upon qualitative changes in function as well as quantitative levels of cell surface expression [3]. The former were not studied in this work.
Lymphocytes with migratory capabilities are predominantly memory cells with a CD45RO+ CD11ahigh, CD62Llow and CD44high phenotype [17]. Whilst this study demonstrates CD8 lymphocytes form AIDS patients display a predominantly CD11ahigh and CD62Llow profile, the CD44low nature of these cells would suggest their migratory capabilities would be reduced. It is notable that with the onset of AIDS, CD44 is down-regulated on the surface of the three mononuclear leucocyte populations studied, the CD4 lymphocytes, the CD8 lymphocytes and the monocytes. CD44 has been associated with lymphocyte migration to mucosal tissues and inflamed tissues [18]. The CD4 lymphocyte population of the gut lamina propria is greatly reduced in AIDS patients [5,6]. Therefore, in addition to the possible direct effects of viral replication, reduced migration of lymphocytes from the blood to the gut due to deficiencies in CD44 expression could contribute to the reduced CD4 lymphocyte numbers observed in the gut of AIDS patients.
Changes in both the CD8 intraepithelial lymphocyte population and the CD4 lymphocyte population within the lamina propria may have important implications for the vulnerability of the gut mucosa of such individuals to persistent infection with protozoal organisms, such as Cryptosporidium and Microsporidium, and also villous height and crypt replication rates may be affected by such changes.
Acknowledgments
This research was funded by the St Stephen's AIDS trust.
REFERENCES
- 1.Pantaleo G, Fauci AS. New concepts in the pathogenesis of HIV infection. Ann Rev Immunol. 1995;13:487–512. doi: 10.1146/annurev.iy.13.040195.002415. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg YJ, Anderson AO, Pabst R. HIV-induced decline in blood CD4/CD8 ratios: viral killing or altered lymphocyte trafficking. Immunol Today. 1998;19:10–16. doi: 10.1016/s0167-5699(97)01183-3. [DOI] [PubMed] [Google Scholar]
- 3.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–34. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 4.Pakker NG, Notermans DW, de Boer RJ, et al. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nature Med. 1998;4:208–14. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 5.Lim SG, Condez A, Lee CA, Johnson MA, Elia C, Poulter LW. Loss of mucosal CD4 lymphocytes is an early feature of HIV infection. Clin Exp Immunol. 1993;92:448–54. doi: 10.1111/j.1365-2249.1993.tb03419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider T, Jahn HU, Schmidt W, Riecken EO, Zeitz M, Ullrich R. Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Gut. 1995;37:524–9. doi: 10.1136/gut.37.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forsyth KD, Levinsky RJ. Preparative procedures of cooling and re-warming increase leukocyte integrin expression and function on neutrophils. J Immunol Methods. 1990;128:159–63. doi: 10.1016/0022-1759(90)90206-b. [DOI] [PubMed] [Google Scholar]
- 8.Bevilacqua MP. Endothelial leukocyte adhesion molecules. Ann Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 9.Moore DAJ, Henderson D, Gazzard BG. Neutrophil adhesion molecules in HIV disease. Clin Exp Immunol. 1998;114:73–77. doi: 10.1046/j.1365-2249.1998.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimuzu Y, van Seventer GA, Horgan KJ, Shaw S. Roles of adhesion molecules in T cell recognition: fundamental similarities between four integrins on resting human T cells (LFA-1, VLA-4, VLA-5, VLA-6) in expression, binding, and costimulation. Immunol Rev. 1990;114:109–43. doi: 10.1111/j.1600-065x.1990.tb00563.x. [DOI] [PubMed] [Google Scholar]
- 11.Jung TM, Gallatin WM, Weissman IL, Dailey MO. Down regulation of homing receptors after T cell activation. J Immunol. 1988;141:4110–7. [PubMed] [Google Scholar]
- 12.Hamann D, Baars PA, Rep MHG, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RAW. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–18. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sachsenberg N, Perelson AS, Yerly S, Schockmel GA, Leduc D, Hirschel B, Perrin L. Turnover of CD4+ and CD8+ T lymphocytes in HIV-1 infection as measured by Ki-67 antigen. J Exp Med. 1998;187:1295–303. doi: 10.1084/jem.187.8.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanders ME, Makgoba MW, Sharrow SO, Stephany D, Springer TA, Young HA, Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-γ production. J Immunol. 1988;140:1401–7. [PubMed] [Google Scholar]
- 15.Rott LS, Briskin MJ, Andrew DP, Berg EL, Butcher EC. A fundamental subdivision of circulating lymphocytes defined by adhesion to mucosal addressin cell adhesion molecule-1. J Immunol. 1996;156:3727–36. [PubMed] [Google Scholar]
- 16.Schweighoffer T, Tanaka Y, Tidswell M, et al. Selective expression of integrin α4β7 on a subset of human CD4+ memory T cells with hallmarks of gut-tropism. J Immunol. 1993;151:717–29. [PubMed] [Google Scholar]
- 17.Brezinschek RI, Lipsky PE, Galea P, Vita R, Oppenheimer-Marks N. Phenotypic characterization of CD4+ T cells that exhibit a transendothelial migratory capacity. J Immunol. 1995;154:3062–77. [PubMed] [Google Scholar]
- 18.Borland G, Ross JA, Guy K. Forms and functions of CD44. Immunology. 1998;93:139–48. doi: 10.1046/j.1365-2567.1998.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


