Abstract
The immunoglobulin receptor FcγRIIIa (CD16) is distributed on natural killer (NK) cells, macrophages, and γδ T cells, and is polymorphic. FcγRIIIa-158V has a higher affinity for both monomeric and immune complexed IgG1, IgG3, and IgG4 than IIIa-158F. We determined FcγRIIIa-158V/F genotypes of Japanese patients with adult periodontitis. A significant over-representation of FcγRIIIa-158F was found in patients with recurrence, compared with patients without recurrence, making FcγRIIIA a candidate gene for recurrence risk of adult periodontitis.
Keywords: FcγRIIIa-158V-F, FcγR polymorphism, adult periodontitis
INTRODUCTION
Leucocyte receptors specific for the constant, or Fc-part of antibodies (FcR) create an important link between humoral and cellular defence mechanisms. Human IgG receptors constitute a highly heterogeneous family and are divided into three main classes, FcγRI (CD64), FcγRII (CD32) and FcγRIII (CD16). Cross-linking of FcγRs potently triggers functions such as phagocytosis, respiratory burst, degranulation, antibody-dependent cellular cytotoxicity and antigen presentation [1,2]. Three receptor subclasses (FcγRIIa, FcγRIIIa, FcγRIIIb) have been shown functionally polymorphic [1,2]. On FcγRIIa, polymorphism at amino acid position 131 in the membrane-proximal, IgG-binding domain affects interaction with human IgG2 [3]. On neutrophils, the FcγRIIIb-NA1 and IIIb-NA2 allotypes interact differently with IgG1- and IgG3-opsonized particles [4,5].
FcγRIIIa, a medium affinity receptor, capable of interaction with complexed as well as monomeric IgG, is expressed on natural killer (NK) cells, macrophages, and subsets of monocytes and γδ T cells [6,7]. FcγRIIIa mediates antibody-dependent cell-mediated cytotoxicity (ADCC) by NK cells and T cells [8], phagocytosis by macrophages [9], cytokine production by NK cells and lymphocytes [10,11], and regulation of immunoglobulin production [12]. A G to T point mutation at nucleotide 559 within FcγRIIIa results in an amino acid substitution at position 158 (valine to phenylalanine) in the second immunoglobulin-like domain [13]. The FcγRIIIa-158V allotype exhibits higher affinity for IgG1 and IgG3 than does FcγRIIIa-158F, and is capable of binding IgG4 [14,15]. An over-representation of FcγRIIIa-158F allele has been reported in patients with systemic lupus erythematosus (SLE) [15,16]. Though the FcγRIIIa-158 V-F polymorphism may have impact beyond autoimmune diseases [15], the clinical significance of FcγRIIIa in bacterial infections is unclear.
Adult periodontitis (AP), a major cause of tooth loss, is an infectious disease resulting from the direct effects of periodontopathic bacteria along with the specific host inflammatory response. Both efficient humoral and cellular responses are considered essential for host defence against periodontopathic bacteria. Some patients respond poorly to conventional treatment and suffer from recurrence, which results in progressive attachment loss [17,18]. Bacteria are essential for the initiation and progression of periodontitis, but the basis for inter-individual differences, especially in the prognosis of the disease, may be associated with genetically determined variance in immune responses [19,20].
Approximately 90% of cells in gingival crevicular fluid are neutrophils [21]. Kobayashi et al. found a significant over-representation of the FcγRIIIb-NA2 allotype in patients with AP recurrence [22]. In contrast, inflamed human gingival tissue contains fewer neutrophils but significant numbers of activated macrophages [23–25], NK cells [26–29] and γδ T cells [30], which may express FcγRIIIa. In human inflammatory gingival tissues, the major IgG subclass expressed in cells is IgG1 [31].
The FcγRIIIa-158V-F polymorphism may thus play a role in the pathogenesis of AP. In this study, we assessed the relevance of the FcγRIIIa-158V-F polymorphism in Japanese patients with and without recurrence of AP.
PATIENTS AND METHODS
Subjects
Peripheral blood (PB) samples were obtained from 100 Japanese patients with AP (48 males and 52 females; age range 34–67 years; mean age 47.8 years) referred to the periodontal clinic of the Niigata University Dental Hospital and 104 Japanese healthy volunteers (73 males and 31 females; age range 23–63 years; mean age 27.0 years). Informed consent was obtained from all participants. None of the participants had a history or current signs of systemic disease.
Clinical assessments
Clinical assessment of the patients was performed by the same periodontist at the first visit (baseline), the completion of treatment (follow-up start), and the latest recall appointment. Probing depth (from free gingival margin to bottom of pocket) and attachment level (from cementoenamel junction to bottom of pocket) of all teeth were assessed using a Williams probe at six sites: mesiobuccal, midbuccal, distobuccal, mesiolingual, midlingual, and distolingual. Measurements were recorded to the nearest millimetre. All Japanese patients with AP were treated by conventional periodontal therapy, consisting of oral hygiene instruction, scaling, root planing, and periodontal surgery. When the patients were confirmed to be free from all periodontal lesions by the re-examinations, they were monitored clinically at 3-month intervals for more than 1 year (mean 38.9 months) post-therapy. The mean number of teeth in the patients was 25.7 (s.d. = 3.4) at the follow-up start. Recurrence of periodontitis was defined as having more than one diseased site with a loss of attachment level ≥ 2 mm in the entire dentition during follow up.
Determination of FcγRIIIa-158V-F polymorphism
Ninety-eight patients and 55 healthy controls were genotyped by DNA sequencing. Genomic DNA was isolated from PB (Easy-DNA kit; Invitrogen, San Diego, CA). Portion of exon 4 of FcγRIIIA gene which corresponds to extracellular domain-2 was amplified by polymerase chain reaction (PCR) using primers as described by Wu et al. [15]. The 162-bp PCR product containing the nt559 polymorphic site was purified, followed by fluorescence-based automated cycle sequencing on an ABI 377 (Applied Biosystems, Foster City, CA). Genotypes of 55 healthy controls were determined by allele-specific PCR with primers described by Wu et al. [15]. The PCR was performed with 100 ng DNA, 200 nm of each primer, 500 μm dNTP, 2.8 mm MgCl2 and 1 U Ampli Taq Gold DNA Polymerase in a 50-μl reaction volume starting with 95°C for 9 min, 37 cycles at 95°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 20 s with a final extension at 72°C for 10 min. Three DNA samples established as FcγRIIIa-158V/V, V/F and F/F genotypes were provided by Dr H. R. Koene (Central Laboratory of Netherlands Red Cross Blood Transfusion Service, Department of Experimental Immunohematology, Amsterdam, The Netherlands), and included in each allele-specific amplification as internal controls. The end product of 138 bp was assayed on a 2% agarose gel with ethidium bromide.
Statistical analysis
The χ2 test was used to compare the FcγRIIIa genotype distributions between Japanese patients with AP and race-matched healthy controls, and between patients with and without recurrence (3 × 2 contingency table). The same test was used to assess the role of FcγRIIIA 559T gene as a risk factor for AP, or recurrence (2 × 2 contingency table; patients with versus without recurrence, FcγRIIIa-158V/V versus FcγRIIIa-158V/F and FcγRIIIa-158F/F, 2 × 2 contingency table; patients with versus without recurrence, FcγRIIIa-158V-F allelic frequency in the absolute numbers of each alleles). For each analysis, Fisher's exact probability test was also performed.
RESULTS
All genomic DNA sequences obtained in this study were confirmed to encode the FcγRIIIA gene, and not FcγRIIIB gene, by the documented presence of homozygous C at nt 531. End products of allele-specific PCR revealed either a single band of approximately 138 bp or no amplified fragment. Allele-specific PCR determinations of FcγRIIIa genotypes agreed with results of genomic DNA sequencing in eight of eight cases (six healthy controls and two verified controls). We did not find differences in the distribution of disease recurrence between subjects with mild and those with severe AP at baseline (data not shown). The percentages of patients who were reported to be cigarette smokers did not differ significantly among the FcγRIIIa-158V-F genotypes (data not shown).
Distribution of FcγRIIIa-158V-F genotypes in periodontitis patients and healthy controls
We first assessed the distribution of FcγRIIIa genotypes in Japanese patients with AP and race-matched healthy controls (Table 1). Genotype frequencies of FcγRIIIa did not deviate from Hardy–Weinberg equilibrium. We only included Japanese subjects and found that in healthy individuals the FcγRIIIa-158F allele was more frequently present (72%) compared with healthy Caucasian controls (57%) [14,16], and ethnically diverse normal subjects (56%) [15]. No skewing in the distribution of genotypes and the allelic frequency was found between patients and controls (Table 1).
Table 1.
Distribution of FcγRIIIa genotypes and alleles in Japanese patients with adult periodontitis and race-matched healthy controls*
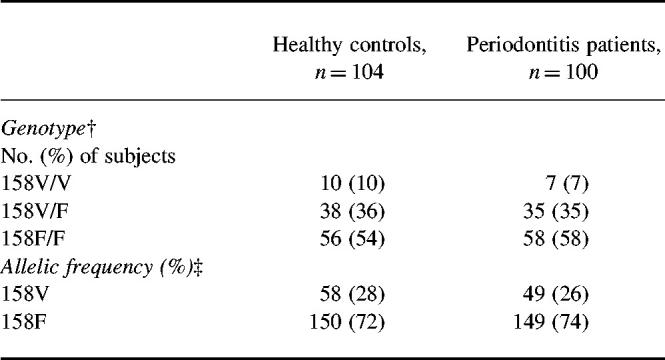
*FcγRIIIa genotype was determined by genomic DNA sequencing and allele-specific polymerase chain reaction (PCR).†Frequency of genotype (3 × 2 contingency table): χ2 = 0.61, P = 0.74.‡Allelic frequency: odds ratio 1.18 (95% confidence interval (CI) 0.72–1.84); χ2 = 0.51. P = 0.47.
Distribution of FcγRIIIa-158V-F genotypes in periodontitis patients with and without disease recurrence
We next studied whether there was a relationship between FcγRIIIa genotype and recurrence of periodontitis. A significant skewing was observed in the distribution of the FcγRIIIa genotypes between patients with and without recurrence (χ2 = 6.80, P = 0.03 in 3 × 2 contingency table).
Notably, there was a significant skewing in the allelic frequency between patients with and without recurrence. A significant over-representation of FcγRIIIa-158F allele was found in patients with disease recurrence compared with patients without recurrence (Table 2; odds ratio 2.85, 95% confidence interval (CI) 0.19–18.3, χ2 = 6.76, P = 0.009).
Table 2.
Distribution of FcγRIIIa genotypes and alleles in Japanese adult periodontitis patients with and without disease recurrence*
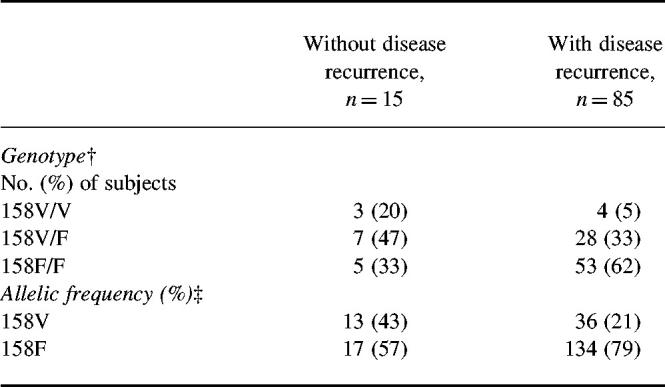
*Disease recurrence was defined as the presence of more than one diseased site with a loss of ≥ 2 mm in attachment level during follow up. FcγRIIIa genotype was determined by genomic DNA sequencing and allele-specific polymerase chain reaction (PCR).†Frequency of genotype (3 × 2 contingency table): χ2 = 6.80, P = 0.03. Odds ratio for risk of periodontitis recurrence in FcγRIIIa 158V/F and 158F/F compared with 158V/V: 5.06 (95% confidence interval (CI) 1.01–25.4); χ2 = 4.58, P = 0.03; Fisher's exact probability = 0.067.‡Allelic frequency: odds ratio 2.85 (95% CI 0.19–18.3); χ2 = 6.76. P = 0.009; Fisher's exact probability = 0.01.
Relationship between FcγRIIIa-158V-F genotypes and annual rate of disease recurrence
Noteworthy was that all four patients of the highest annual rate of recurrence (> 12% diseased sites with loss of ≥ 2 mm in attachment level in the entire dentition per year (dLA ≥ 2 mm/year)) belonged to FcγRIIIa-158F/F genotype group (Fig. 1, Kruskal–Wallis test, P > 0.05).
Fig. 1.
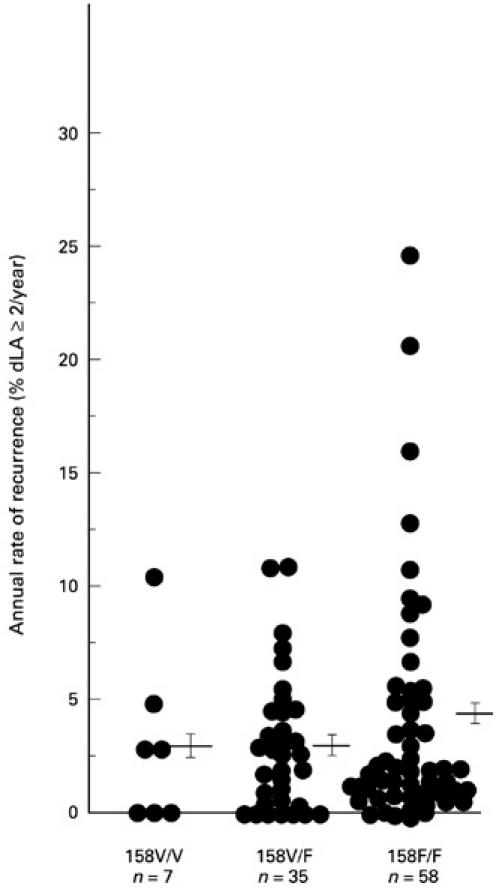
Relationship between FcγRIIIa genotypes and annual rates of disease recurrence. The annual rate of recurrence was expressed as the percentage of diseased sites with a loss of ≥ 2 mm in attachment level in the entire dentition per year. Each point represents the annual recurrence rate for one subject. The mean and s.e.m. are indicated for each genotype (FcγRIIIa-158V/V, -IIIa-158V/F, and -IIIa-158F/F, 2.98 ± 1.42%, 2.99 ± 0.51%, and 3.69 ± 0.65%). % dLA ≥ 2/year, Percentage of diseased sites with a loss of ≥ 2 mm in attachment level in the entire dentition per year.
Associations between FcγRIIIa-158V/F and FcγRIIIb-NA1/NA2 alleles
We analysed associations between FcγRIIIa-158V/F and FcγRIIIb-NA1/NA2 alleles because of the proximity of these two genes, both on chromosome 1q 23–24 [1]. No significant association was observed between FcγRIIIa-158V/F genotypes and FcγRIIIb-NA1/NA2 genotypes in patients [22] (3 × 3 contingency table: χ2 = 5.26, P = 0.26, 2 × 2 contingency table for homozygous: Fisher's exact probability = 0.65). The association between allelic frequency of FcγRIIIb-NA1/NA2 and FcγRIIIa-158V/F was not significant in patients (2 × 2 contingency table: χ2 = 0.40, P = 0.53). The D values for the frequency of haplotypes were 0.0067 for patients, 0.0536 for patients without recurrence, and −0.0100 for patients with recurrence.
DISCUSSION
In this study, we found a significant over-representation of FcγRIIIa-158F allele in patients with recurrence of AP compared with those without recurrence (Table 2).
The number of patients without recurrence was lower (n = 15) than that of patients with recurrence (n = 85), probably due to the strict definition of recurrence, compared with other studies [32]. Thus, we used Fisher's exact probability test in the case of low expectation values in 2 × 2 contingency tables, and observed a significant difference in the allelic frequency between patients with and without recurrence (Fisher's exact probability = 0.01).
FcγRIIIa-158V-F polymorphism is located in the extracellular membrane-proximal domain which is considered crucial for IgG binding [13,33,34]. Compared with 158F/F homozygotes, FcγRIIIa expressed on NK cells and monocytes in V/V homozygotes bound IgG1, IgG3 and IgG4 more effectively [14,15]. In response to aggregated human IgG, FcγRIIIa engagement on NK cells from V/V homozygotes led to a larger rise in intracellular calcium and more prominent cell activation [15]. These differences of IgG binding levels between FcγRIIIa-158V-F genotypes may affect functions mediated by FcγRIIIa expressed on not only NK cells, but also monocytes, macrophages and γδ T cells [1,6], though monocytes express a different glycoform of FcγRIIIa, and monocyte FcγRIIIa exhibit a lower ligand binding affinity than FcγRIIIa on NK cells [35].
FcγRIIIa has been reported to mediate different functions: FcγRIIIa on NK cells and γδ T cells are capable of mediating ADCC [8]. In the absence of other FcRs, macrophage FcγRIIIa can mediate phagocytosis [9]. Binding of human monomeric IgG to FcγRIIIa induced stimulatory signals in human NK cells, leading to up-regulated IL-2Rα expression, cell proliferation and cytokine release (IL-1β, interferon-gamma (IFN-γ), and tumour necrosis factor-alpha (TNF-α)) [10]. Cross-linking of human FcγRIIIa, but not FcγRI or FcγRII, stimulates FcγRIIIa-bearing lymphocytes to produce IL-1β [11].
Actually, inflamed human gingival tissue contains activated macrophages [23–25], NK cells [26–29] and γδ T cells [30]. Recently, two-colour flow cytometric analysis demonstrated that CD16+ NK cells were increased in PB from periodontitis patients compared with healthy controls [36]. NK cell-mediated cytolysis was predominantly observed in gingival mononuclear cell populations [27]. However, the relevance of NK cells to the pathogenesis of periodontitis is still equivocal. Although T and B lymphocyte populations increased approximately 20-fold progressing from healthy to gingivitis to periodontitis specimens, the NK cell population showed only a three-fold increase, which represented 19%, 6.6%, and 7% of the total of all positively stained lymphocytes across biopsy groups [28].
In addition to FcγRIIIa, macrophages also express FcγRIa and FcγRIIa [37]. FcγRIa is probably not involved in mediating the first contact between immune complexes and macrophages in the presence of high concentrations of soluble IgG in tissue fluids. This receptor has a high affinity for monomeric IgG, and is probably occupied with plasma IgG in vivo [16]. FcγRIIa (CD32) only binds immune complexes due to its low affinity (Ka: < 106 M−1). This supports a role of importance for FcγRIIIa on macrophages.
Our results show the FcγRIIIa-158V-F polymorphism to be associated with recurrence of AP. The lower levels of IgG1 and IgG3 binding to NK cells, macrophages, and lymphocytes in individual subjects carrying FcγRIIIA-158F gene might result in a relatively diminished function of these cells, though the exact cell types with FcγRIIIa affecting recurrence of AP remain to be determined. Our results identify FcγRIIIA-158F as a candidate gene for recurrence risk of AP.
Acknowledgments
The authors are indebted to Dr Harry R. Koene for kindly providing genomic DNA samples of FcγRIIIa-V/V, V/F, and F/F genotypes used as controls in our study. We also appreciate the excellent technical guidance for allele-specific PCR by Nomdo A. C. Westerdaal and Ronald Scheepers (Department of Immunology, University Medical Center Utrecht). We express special thanks to Dr Ryozo Kuwano (Research Laboratory for Molecular Genetics, Niigata University) for his scientific guidance in PCR, and to Dr Masatoshi Yano and Dr Akihiro Yoshihara (Department of Preventive Dentistry, Niigata University School of Dentistry) for technical help with the statistical analysis. This study was supported by a grant for Development of Advanced Medical Technology from the Ministry of Education, Science, Sports and Culture of Japan; a Fund for Scientific Promotion of Tanaka Industries Co. Ltd (Niigata, Japan).
REFERENCES
- 1.van de Winkel JGJ, Capel PJA. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol Today. 1993;14:215–21. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]
- 2.van der Pol W-L, van de Winkel JGJ. IgG receptor polymorphisms: risk factors for disease. Immunogenetics. 1998;48:222–32. doi: 10.1007/s002510050426. [DOI] [PubMed] [Google Scholar]
- 3.Parren PWHI, Warmerdam PAM, Boeije LCM, et al. On the interaction of IgG subclasses with the low affinity FcγRIIa (CD32) on human monocytes, neutrophils, and platelets. Analysis of functional polymorphism to human IgG2. J Clin Invest. 1992;90:1537–46. doi: 10.1172/JCI116022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salmon JE, Edberg JC, Kimberly RP. Fcγ receptor III on human neutrophils. Allelic variants have functionally distinct capacities. J Clin Invest. 1990;85:1287–95. doi: 10.1172/JCI114566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salmon JE, Edberg JC, Brogle NL, Kimberly RP. Allelic polymorphisms of human Fcγ receptor IIA and Fcγ receptor IIIB. Independent mechanisms for differences in human phagocyte function. J Clin Invest. 1992;89:1274–81. doi: 10.1172/JCI115712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vance BA, Huizinga TWJ, Wardwell K, Guyre PM. Binding of monomeric human IgG defines an expression polymorphism of FcγRIII on large granular lymphocyte/natural killer cells. J Immunol. 1993;151:6429–39. [PubMed] [Google Scholar]
- 7.Braakman E, van de Winkel JGJ, van Krimpen BA, Jansze M, Bolhuis RLH. CD16 on human γδ T lymphocytes: expression, function, and specificity for mouse IgG isotypes. Cell Immunol. 1992;143:97–107. doi: 10.1016/0008-8749(92)90008-d. [DOI] [PubMed] [Google Scholar]
- 8.Perussia B, Trinchieri G, Jackson A, Warner NL, Faust J, Rumpold H, Kraft D, Lanier LL. The Fc receptor for IgG on human natural killer cells: phenotypic, functional, and comparative studies with monoclonal antibodies. J Immunol. 1984;133:180–8. [PubMed] [Google Scholar]
- 9.Park J-G, Isaacs RE, Chien P, Schreiber AD. In the absence of other Fc receptors, FcγRIIIA transmits a phagocytic signal that requires the cytoplasmic domain of its γ subunit. J Clin Invest. 1993;92:1967–73. doi: 10.1172/JCI116790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sulica A, Metes D, Gherman M, Whiteside TL, Herberman RB. Divergent effects of FcγRIIIA ligands on the functional activities of human natural killer cells in vitro. Eur J Immunol. 1996;26:1199–203. doi: 10.1002/eji.1830260602. [DOI] [PubMed] [Google Scholar]
- 11.Marsh CB, Lowe MP, Rovin BH, Parker JM, Liao Z, Knoell DL, Wewers MD. Lymphocytes produce IL-1β in response to Fcγ receptor cross-linking: effects on parenchymal cell IL-8 release. J Immunol. 1998;160:3942–8. [PubMed] [Google Scholar]
- 12.Lenz P, Gessner JE, Sautes C, Schmidt RE. Fc gamma-receptor III (CD16) is involved in NK–B cell interaction. Immunobiol. 1996;196:387–98. doi: 10.1016/s0171-2985(96)80061-1. [DOI] [PubMed] [Google Scholar]
- 13.Ravetch JV, Perussia B. Alternative membrane forms of FcγRIII (CD16) on human natural killer cells and neutrophils. Cell type-specific expression of two genes that differ in single nucleotide substitutions. J Exp Med. 1989;170:481–97. doi: 10.1084/jem.170.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koene HR, Kleijer M, Algra J, von Roos D, dem Borne AEG, Kr, de Haas M. FcγRIIIa-158V/F polymorphism influences the binding of IgG by NK cell FcγRIIIa, independently of the FcγRIIIa-48L/R/H phenotype. Blood. 1997;90:1109–14. [PubMed] [Google Scholar]
- 15.Wu J, Edberg JC, Redecha PB, Bansal V, Guyre PM, Coleman K, Salmon JE, Kimberly RP. A novel polymorphism of FcγRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J Clin Invest. 1997;100:1059–70. doi: 10.1172/JCI119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koene HR, Kleijer M, Swaak AJG, et al. The FcγRIIIA-158F allele is a risk factor for systemic lupus erythematosus. Arthritis Rheum. 1998;41:1813–8. doi: 10.1002/1529-0131(199810)41:10<1813::AID-ART13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Hirschfeld L, Wasserman B. A long-term survey of tooth loss in 600 treated periodontal patients. J Periodontol. 1978;49:225–37. doi: 10.1902/jop.1978.49.5.225. [DOI] [PubMed] [Google Scholar]
- 18.McFall WT. Tooth loss in 100 treated patients with periodontal disease. A long-term study. J Periodontol. 1982;53:539–49. doi: 10.1902/jop.1982.53.9.539. [DOI] [PubMed] [Google Scholar]
- 19.Kornman KS, Crane A, Wang H-Y, et al. The interleukin-1 genotype as a severity factor in adult periodontal disease. J Clin Periodontol. 1997;24:72–77. doi: 10.1111/j.1600-051x.1997.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 20.Hart TC, Kornman KS. Genetic factors in the pathogenesis of periodontitis. Periodontology 2000. 1997;14:202–15. doi: 10.1111/j.1600-0757.1997.tb00198.x. [DOI] [PubMed] [Google Scholar]
- 21.Sugita N, Suzuki T, Yoshie H, Yoshida N, Adachi M, Hara K. Differential expression of CR3, FcɛRII and FcγRIII on polymorphonuclear leukocytes in gingival crevicular fluid. J Periodont Res. 1993;28:363–72. doi: 10.1111/j.1600-0765.1993.tb01080.x. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi T, Westerdaal NAC, Miyazaki A, van der Pol W-L, Suzuki T, Yoshie H, van de Winkel JGJ, Hara K. Relevance of immunoglobulin G Fc receptor polymorphism to recurrence of adult periodontitis in Japanese patients. Infect Immun. 1997;65:3556–60. doi: 10.1128/iai.65.9.3556-3560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Topoll HH, Zwadlo G, Lange DE, Sorg C. Phenotypic dynamics of macrophage subpopulations during human experimental gingivitis. J Periodont Res. 1989;24:106–12. doi: 10.1111/j.1600-0765.1989.tb00864.x. [DOI] [PubMed] [Google Scholar]
- 24.Matsuki Y, Yamamoto T, Hara K. Interleukin-1 mRNA-expressing macrophages in human chronically inflamed gingival tissues. Am J Pathol. 1991;138:1299–305. [PMC free article] [PubMed] [Google Scholar]
- 25.Schlegel Gómez R, Langer P, Pelka M, von den Driesch P, Johannessen AC, Simon M. Variational expression of functionally different macrophage markers (27E10, 25F9, RM3/1) in normal gingiva and inflammatory periodontal disease. J Clin Periodontol. 1995;22:341–6. doi: 10.1111/j.1600-051x.1995.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 26.Wynne SE, Walsh LJ, Seymour GJ, Powell RN. In situ demonstration of natural killer (NK) cells in human gingival tissue. J Periodontol. 1986;57:699–702. doi: 10.1902/jop.1986.57.11.699. [DOI] [PubMed] [Google Scholar]
- 27.Komiyama K, Hirsch HZ, Moro I, Umemura S, Mestecky J. HNK-1+ (Leu-7) cells and natural killer cell activity in inflamed human gingival tissue. J Oral Pathol. 1988;17:118–23. doi: 10.1111/j.1600-0714.1988.tb01897.x. [DOI] [PubMed] [Google Scholar]
- 28.Cobb CM, Singla O, Feil PH, Theisen FC, Schultz RE. Comparison of NK-cell (Leu-7+ and Leu-11b+) populations in clinically healthy gingiva, chronic gingivitis and chronic adult periodontitis. J Periodont Res. 1989;24:1–7. doi: 10.1111/j.1600-0765.1989.tb00851.x. [DOI] [PubMed] [Google Scholar]
- 29.Cobb CM, Singla O, Theisen FC, Shultz RE. Ultrastructural evidence of large granular lymphocyte (LGL) activity in lesions of chronic adult periodontitis. J Clin Periodontol. 1990;17:371–8. doi: 10.1111/j.1600-051x.1990.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 30.Gemmel E, Seymour GJ. γδ T Lymphocytes in human periodontal disease tissue. J Periodontol. 1995;66:780–5. doi: 10.1902/jop.1995.66.9.780. [DOI] [PubMed] [Google Scholar]
- 31.Kinane DF, Lappin DF, Koulouri O, Buckley A. Humoral immune responses in periodontal disease may have mucosal and systemic immune features. Clin Exp Immunol. 1999;115:534–41. doi: 10.1046/j.1365-2249.1999.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colombo AP, Eftimiadi C, Haffajee AD, Cugini MA, Socransky SS. Serum IgG2 level, Gm (23) allotype and FcγRIIa and FcγRIIIb receptors in refractory periodontal disease. J Clin Periodontol. 1998;25:465–74. doi: 10.1111/j.1600-051x.1998.tb02475.x. [DOI] [PubMed] [Google Scholar]
- 33.Hibbs ML, Tolvanen M, Carpén O. Membrane-proximal Ig-like domain of FcγRIII (CD16) contains residues critical for ligand binding. J Immunol. 1994;152:4466–74. [PubMed] [Google Scholar]
- 34.Tamm A, Schmidt RE. The binding epitopes of human CD16 (FcγRIII) monoclonal antibodies. Implications for ligand binding. J Immunol. 1996;157:1576–81. [PubMed] [Google Scholar]
- 35.Edberg JC, Kimberly RP. Cell type-specific glycoforms of FcγRIIIa (CD16). Differential ligand binding. J Immunol. 1997;159:3849–57. [PubMed] [Google Scholar]
- 36.Afar B, Engel D, Clark EA. Activated lymphocyte subsets in adult periodontitis. J Periodont Res. 1992;27:126–33. doi: 10.1111/j.1600-0765.1992.tb01814.x. [DOI] [PubMed] [Google Scholar]
- 37.Anderson CL, Looney RJ, Culp DJ, et al. Alveolar and peritoneal macrophages bear three distinct classes of Fc receptors for IgG. J Immunol. 1990;145:196–201. [PubMed] [Google Scholar]


