Abstract
Increased production of proinflammatory cytokines, including tumour necrosis factor-alpha (TNF-α), IL-1β, IL-6 and IL-8, has been demonstrated in Helicobacter pylori-associated gastric mucosal inflammation. IL-12, a newly characterized cytokine, is thought to be a key mediator in host responses to bacterial infections. The aim of this study was to investigate differences in cytokine patterns between H. pylori-positive and -negative gastritis and normal mucosa. Secretion of IL-12, TNF-α, IL-1β, IL-6, IL-8 and IL-10 was measured in 176 patients with chronic gastritis in whole biopsy cultures. Gastritis was graded for chronic inflammation or acute inflammatory activity, respectively, according to the Sydney system. Biopsies with similar scores were matched for analysis from H. pylori-infected and non-infected patients. Secretion of IL-12 was significantly increased in H. pylori-associated gastritis in comparison with H. pylori-negative gastritis (P < 0.0001). In contrast, secretion of TNF-α, IL-1β, IL-6, and IL-8 correlated with the degree of inflammation but was not different between H. pylori-positive and -negative patients. Moreover, IL-10 secretion was found to be higher in H. pylori-positive than in H. pylori-negative patients. IL-12 may play a specific role in H. pylori-associated gastric disease, whereas production of the proinflammatory cytokines TNF-α, IL-1β, IL-6 and IL-8 does not seem to be restricted to H. pylori-induced inflammation. The contra-inflammatory cytokine IL-10 may be a contributor to the chronicity of H. pylori-associated gastritis by impairing clearance of the pathogen.
Keywords: Helicobacter pylori, gastritis, cytokines, gastric immunity, inflammation
INTRODUCTION
Proinflammatory cytokines appear to play an important role in gastrointestinal immunoregulation and physiology [1–9]. Helicobacter pylori is regarded as the causative agent in the great majority of patients with chronic gastritis or ulcer disease. Increased production of proinflammatory cytokines such as tumour necrosis factor-alpha (TNF-α), IL-1β, IL-6 and in particular IL-8 in the gastric mucosa in H. pylori-positive gastritis has been well documented [1–3,5,6]. Recent studies of the pathophysiology of the inflammatory process in the gastric mucosa have mainly focused on H. pylori-associated gastritis in comparison with healthy gastric mucosa without signs of inflammation [1–3,5,6].
Yamaoka et al. investigated cytokine production in different grades of H. pylori-associated gastritis. Detectability of cytokine mRNAs by qualitative polymerase chain reaction (PCR) correlated to the severity of gastritis, but comparisons were only performed within H. pylori-positive patient groups or with normal, H. pylori-negative controls [7]. Karttunen et al. [10] investigated the number of interferon-gamma (IFN-γ)- and IL-4-secreting cells in the gastric mucosa. The investigators compared H. pylori-negative patients who showed only minor polymorphonuclear (PMN) infiltration with H. pylori-positive patients with PMN infiltrates. They found an increase in both groups versus normal mucosa, and surprisingly, even more IFN-γ-secreting cells in H. pylori-negative ‘inactive’ gastritis, while no differences were observed for IL-4 [10].
In our study, for the first time production of the proinflammatory cytokines TNF-α, IL-1β, IL-6 and IL-8 was investigated by comparison of samples of H. pylori-positive and -negative patients which were matched for the histological degree of inflammation. Cytokine production was investigated at the protein level, as the detection of specific mRNA may not always reflect the availability of the corresponding proteins [11].
IL-12, a recently characterized cytokine, is rapidly produced after stimulation with bacterial antigens [12–15]. IL-12 is thought to play a key role in host responses to bacterial infections [15–17]. It has been shown in several bacterial infection models that the host response is initiated by IL-12, with subsequent induction of IFN-γ and therefore promotion of a Th1 immune response [13,15]. The increased secretion of proinflammatory cytokines including TNF-α, IL-1β, IL-6, IL-8 may be a secondary event to IL-12 production in the immune cascade [15–17].
Because IL-12 is induced by bacterial antigens, an increased production of IL-12 in H. pylori-positive gastritis may be expected. However, in gastritis this has not yet been investigated adequately. Karttunen et al. [18] in a small study recently reported IL-12 mRNA to be more frequently expressed in H. pylori-positive patients with PMN infiltrates in comparison with H. pylori- negative patients with only minor cellular infiltrates. In another pilot study, no difference in IL-12 production between H. pylori-positive and -negative patients was found [19]. However, for a detailed analysis of the inflammatory effects induced by H. pylori, it is useful to compare patient groups with similar histopathological changes.
To characterize patterns of IL-12 production in gastritis, the relationship was therefore evaluated between production of IL-12 and different degrees of gastritis in a comparative analysis of H. pylori-positive and -negative patients, who were matched for their degree of histopathological changes.
Helicobacter pylori-induced inflammation is a chronic disease, which does not result in successful clearance of the microorganism. IL-10, a contra-inflammatory cytokine and a strong inhibitor of proinflammatory cytokine secretion [20–22], has recently been proposed as being responsible for the chronicity of the inflammatory process in H. pylori-associated gastritis [23]. In a comparative analysis of H. pylori-positive and -negative patients, who were matched for the histopathological degree of inflammatory changes, we specifically evaluated the role of IL-10 in H. pylori-induced inflammation.
PATIENTS AND METHODS
Patients
Endoscopic biopsies from antrum and corpus (six each from the same location) for the parallel assessment of cytokine production and histology were taken in 176 patients who underwent routine gastroscopy. All antral biopsies were taken at least 3 cm distant from the pyloric region, and in cases of gastric ulcers, biopsies were taken > 2 cm distant from the ulcer. In addition, a set of biopsies for rapid urease testing were taken from different sites of the antrum or corpus (two each). All patients who underwent routine gastroscopy at one of the endoscopy suites of Charité Hospital between July 1995 and February 1996 were screened. Out of 340 consecutive patients, 176 patients were included in the study. Indications for gastroscopy in the included patients were upper abdominal pain (n = 95), dyspepsia (n = 39) and unspecific upper abdominal symptoms (n = 42). Patient characteristics are given in Table 1. All patients gave informed consent and the study received prior approval by the local ethics committee. The vast majority (> 95%) of the patients recruited lived for the previous 5 years in the eastern part of Berlin. Ninety-seven percent of patients were of Caucasian origin.
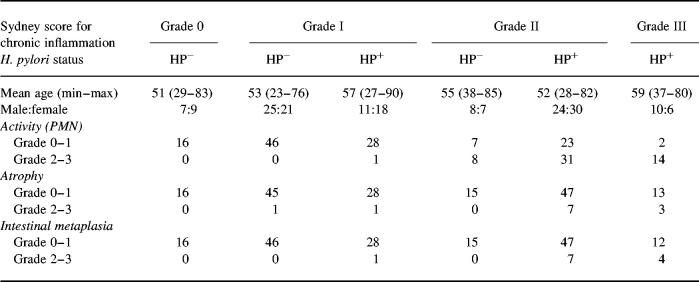
Table 1.
Patient characteristics
Helicobacter pylori-infected and non-infected patients (n = 176) were investigated. Patients were grouped by gastritis grade (Sydney score for chronic inflammatory cells). Mean age, male:female ratio and corresponding scores for inflammatory activity (PMN cell infiltrates), atrophy and intestinal metaplasia are shown.
One hundred and sixty-four patients were excluded from the study. Exclusion criteria were gastric carcinoma (n = 14), prior eradication therapy for H. pylori (n = 37), medication with proton pump inhibitors for more than 5 days in the previous month (n = 28), any treatment with steroids within the preceding 4 weeks (n = 12), immunosuppressants within the previous 4 months (n = 7), treatment with antibiotics (for more than 3 days for other reasons than eradication of H. pylori) within the previous 6 months (n = 41), or non-steroidal anti-phlogistic medication (more than two doses in the preceding 2 weeks and more than six doses in the preceding 4 weeks; n = 47).
Whole biopsy culture
A set of four antrum and four corpus biopsies was cultured in an adaptation of the procedure described by Crabtree et al. [1]. Following mucolysis, biopsies were washed and directly cultured in 1 ml culture medium RPMI 1640 (supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin, 100 mg/ml streptomycin and 50 mg/ml gentamycin). After 24 h (37°C/5% CO2), supernatants were harvested, snap frozen and stored at −70°C until determination of cytokine levels. Then, specimens were homogenized in 2 ml 3.3 mmol/l CaCl2. Total protein in homogenized biopsies was determined [24].
Isolation of gastric lamina propria mononuclear cells
In preliminary experiments (n = 20 patients) lamina propria mononuclear cells (LMNC) were isolated from antrum biopsies to evaluate their contribution to the overall secretion of proinflammatory cytokines. By isolating LMNC by collagenase digestion, density gradient centrifugation and 48-h cultures as previously described [9], we found that most of the TNF-α, IL-1β and IL-12 was secreted by LMNC, whereas other cell types apparently contributed to most of the IL-6 and IL-8 secreted. No IL-12 could be detected in epithelial cell supernatants.
Cytokine measurement and determination of cag A status
Supernatant concentrations of IL-12, TNF-α, IL-1β, IL-6, IL-8, and IL-10 were assessed by specific sandwich ELISA (IL-6, IL-8: R&D Systems/DPC Biermann (Minneapolis, MN/Bad Nauheim, Germany); TNF-α: T-cell Diagnostics (Cambridge, MA); IL-1β and IL-12: Genzyme (Rüsselsheim, Germany); IL-10: Titerzyme Perceptive Diagnostics (Framingham, MA)). The sensitivity of the ELISAs was: 10 pg/ml (IL-12), 1.5 pg/l (TNF-α), 3 pg/ml (IL-1β), 0.7 pg/ml (IL-6), 3 pg/ml (IL-8) and 1 pg/ml (IL-10). All samples were analysed in duplicate. Cag A status was determined in serum samples taken at the time of endoscopy by immunoblotting (DPC Biermann, Bad Nauheim, Germany).
Histological grading/urease test
The degree of inflammation present in histological specimens of antrum and corpus was classified according to the Sydney system. A grade from 0 (absent) to III (severe) was assigned for four histological variables: chronic inflammation (mononuclear cell infiltrate), activity (polymorphonuclear cell infiltration), glandular atrophy, and intestinal metaplasia [25]. Grading of all biopsies was performed by a single pathologist in a blinded fashion (G.N.). For rapid urease testing in tissue, the HUT-Test (Astra Chemicals, Wedel/Holstein, Germany) was used according to the manufacturer's instructions. The presence of H. pylori was determined by either a positive detection by histology (including Giemsa staining) and/or a positive rapid urease test in at least one of the samples. All patients were tested for the presence of H. pylori by both methods.
Statistical analysis
Data were found to be non-normally distributed using the Shapiro-Wilk's W-test [26]. The non-parametric Kruskaal–Wallis test was used to investigate cytokine secretion in relationship to different degrees of gastric inflammation. Differences in levels of cytokine secretion were tested with the Mann–Whitney U-test [27].
RESULTS
Proinflammatory cytokines and degree of inflammation
H pylori-positive gastritis
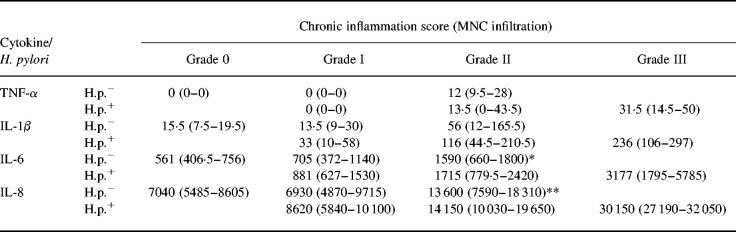
If Helicobacter infection was detected, all patients showed at least histological signs of grade I gastritis (Sydney score for chronic inflammatory cells). No H. pylori-infected patient had a completely normal mucosa. The degree of gastritis correlated with the level of secretion of TNF-α (P < 0.01), IL-1β, IL-8 (P < 0.001 each) and IL-6 (P < 0.002; non-parametric Kruskaal–Wallis test) in antrum biopsies (Table 2, Fig. 1).
Table 2.
Proinflammatory cytokines and chronic inflammation
Proinflammatory cytokine production (median cytokine values in pg/mg (interquartile range)) in antrum biopsy cultures in Helicobacter pylori-infected and non-infected patients with different degrees of chronic inflammation (mononuclear cell (MNC) infiltrate). Tumour necrosis factor-alpha (TNF-α) secretion could not be detected in patients with normal mucosa. No patient with H. pylori infection had a completely normal mucosa, whereas no severe gastritis (grade III) was found in H. pylori-negative patients. Proinflammatory cytokine secretion was not different between matched samples of H. pylori-positive gastritis and H. pylori-negative gastritis. Significance for comparison with specimens without inflammatory changes: *P = 0.009; **P = 0.01.
Fig. 1.
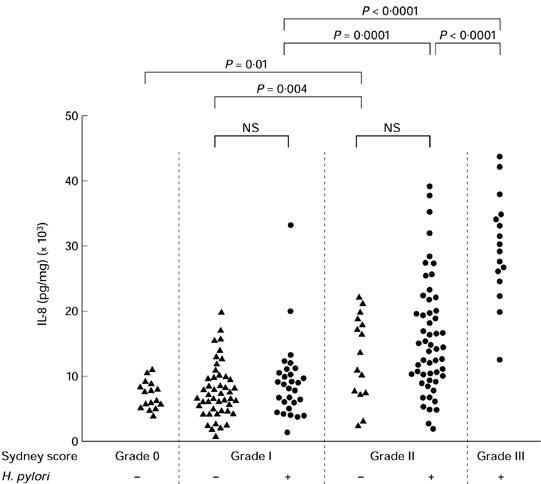
Secretion of IL-8. Relationship between secretion of IL-8 and the histological degree of chronic inflammation (Sydney gastritis score for mononuclear cells (MNC)) and Helicobacter pylori infection. Secretion of IL-8 in antrum biopsy cultures correlated to the severity of chronic gastritis in both H. pylori-positive and H. pylori-negative gastritis. No differences were found between cytokine secretion in histologically matched samples of H. pylori-positive and H. pylori-negative gastritis. Square brackets indicate significance levels.
The histological activity of inflammation is defined by the presence of a PMN infiltrate (Sydney system). We therefore evaluated the degree of PMN infiltration separately from the chronic inflammation score [25]. Higher degrees of PMN infiltration correlated with an increase in the secretion of TNF-α, IL-1β, IL-6 and IL-8 (P < 0.0001 for each; Table 3). Studies on cytokine secretion in corpus biopsies (data not shown) of H. pylori-positive gastritis revealed results similar to the antrum samples with regard to both chronic inflammation and activity scores.
Table 3.
Proinflammatory cytokines and activity of inflammation
Proinflammatory cytokine production (median cytokine values in pg/mg protein (and interquartile range)) in antrum biopsy cultures in Helicobacter pylori-infected and non-infected patients with different activity of gastritis (polymorphonuclear (PMN) cell infiltration). No severe PMN infiltration was found in H. pylori-negative patients. Proinflammatory cytokine secretion was not different in H. pylori-positive gastritis in comparison with H. pylori-negative gastritis (samples matched for histopathological inflammatory activity). Significance for comparison against specimens without inflammatory changes: *P < 0.0001; **P < 0.001; †P < 0.005; ††P < 0.03.
H. pylori-negative gastritis
All patients with a completely normal gastric antral mucosa by histology (n = 16) were H. pylori-negative and did not show any secretion of TNF-α. Higher degrees of chronic inflammation correlated significantly with an increase in the secretion of TNF-α (P = 0.0004), IL-1β (P = 0.02), IL-6 (P = 0.028) and IL-8 (P < 0.001). No severe gastritis (grade III) was observed in the H. pylori-negative patient group (Table 2).
Activity of inflammation was assessed by the degree of PMN infiltration. In 36 H. pylori-negative patients there were no histological signs of PMN infiltration. In 33 patients a mild and in eight patients a moderate degree of PMN infiltration were observed. High inflammatory activity was not seen in any H. pylori-negative patient. Secretion of TNF-α, IL-1β, IL-6 and IL-8 correlated with the degree of PMN infiltration (TNF-α, IL-6 and IL-8: P < 0.0001; IL-1β: P = 0.0003, non-parametric Kruskaal–Wallis test; Table 3). As in H. pylori-positive gastritis, results from corpus biopsies paralleled antrum samples (data not shown).
Influence of H pylori infection on the production of proinflammatory cytokines
The comparative analysis of patients with a matched degree of gastritis demonstrated that secretion of TNF-α, IL-1β, IL-6 and IL-8 was not statistically different between H. pylori-positive and -negative patients (Tables 2 and 3, Fig. 1). Therefore, the degree of chronic inflammation or the activity of inflammation, respectively, were the sole determinants of proinflammatory cytokine secretion, irrespective of disease aetiology.
IL-12 secretion and H. pylori status
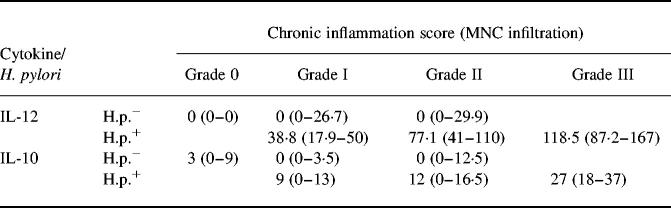
In H. pylori-positive gastritis an increase in the degree of chronic inflammation was related to higher secretion levels of IL-12 (P = 0.0058; Fig. 2,Table 4). A high activity of inflammation in H. pylori-positive gastritis was also related to an increased secretion of IL-12 (P < 0.0002; Table 5). In contrast, in H. pylori-negative gastritis, higher degrees of chronic inflammation or inflammatory activity were not associated with an increase in IL-12 secretion (Fig. 2,Tables 4 and 5).
Fig. 2.
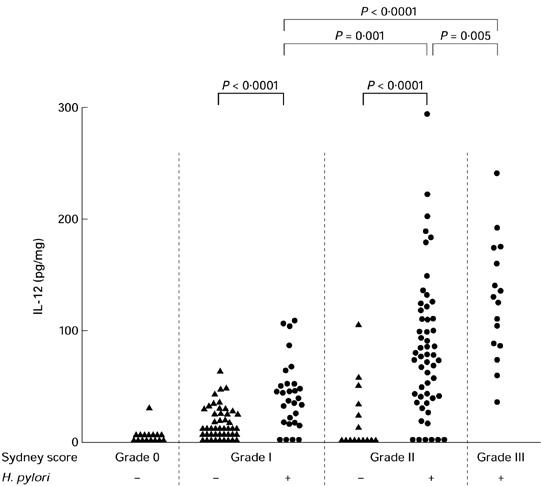
Secretion of IL-12. Relationship between secretion of IL-12 and grade of chronic inflammation (mononuclear cell infiltrate, Sydney system) in Helicobacter pylori-positive and H. pylori-negative gastritis. Secretion of IL-12 in antrum biopsy cultures increased with the severity of chronic gastritis only in H. pylori-positive gastritis. An increased IL-12 secretion was seen in H. pylori infection in comparison with H. pylori-negative patients. Square brackets indicate significance of statistical differences.
Table 4.
IL-12/IL-10 and chronic inflammation
Secretion of IL-12 and IL-10 (median cytokine values in pg/mg (interquartile range)) in antrum biopsy cultures in Helicobacter pylori-infected and non-infected patients with different grades of chronic inflammation (mononuclear cell infiltrate). Secretion of IL-12 was significantly increased in H. pylori-associated gastritis in comparison with H. pylori-negative gastritis (comparison of matched samples grade I or II: P < 0.0001). Similar differences were also observed for IL-10 (comparative analysis: grade I, P = 0.0026; grade II, P = 0.04). For further significances see Figs 2 and 3.
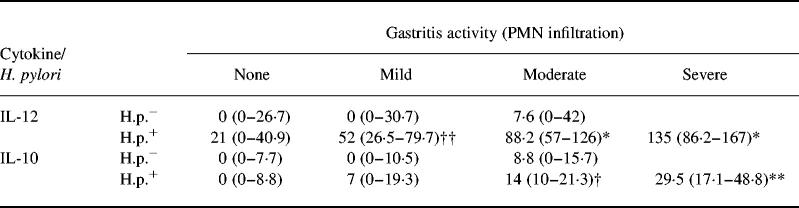
Table 5.
IL-12/IL-10 and activity of inflammation
Secretion of IL-12 and IL-10 (median cytokine values in pg/mg protein (and interquartile range)) in antrum biopsy cultures in Helicobacter pylori-infected and non-infected patients with different activity of gastritis (polymorphonuclear (PMN) cell infiltration). Secretion of IL-12 was significantly associated with inflammatory activity in H. pylori-associated gastritis in contrast to H. pylori-negative gastritis (matched samples for mild activity, P < 0.0001; matched samples for moderate activity, P = 0.0004). Differences were also observed for IL-10 (mild activity, P = 0.04; moderate activity, P = 0.03). Significance for comparison against specimens without inflammatory changes: *P < 0.0001; **P = 0.0001; †P = 0.0002; ††P = 0.01.
Helicobacter pylori-positive and H. pylori-negative individuals were matched for the histological degree of gastritis. Secretion of IL-12 was significantly increased in H. pylori-positive gastritis in comparison with H. pylori-negative gastritis if chronic inflammation was present (P < 0.0001; Fig. 2,Table 4). Similar findings were made if biopsies were matched for the degree of inflammatory activity (mild PMN infiltration: P < 0.0001; moderate PMN infiltration: P = 0.0004). Results of IL-12 secretion in corpus biopsies paralleled results in antrum biopsies (data not shown).
IL-10 secretion and H. pylori status
In H. pylori-positive patients an increase in the Sydney score for chronic inflammation (P = 0.04) as well as an increase in inflammatory activity (P < 0.0001) was related to an increase in the secretion of IL-10 (Fig. 3,Tables 4 and 5). In contrast to these findings, production of IL-10 was not increased in H. pylori-negative patients with chronic inflammation or inflammatory activity in comparison with normal controls (Fig. 3,Tables 4 and 5). Secretion of IL-10 was higher in H. pylori-positive patients than in H. pylori-negative patients with mild (P = 0.04) or moderate inflammatory activity (P = 0.03), respectively. Results of IL-10 secretion in corpus biopsies paralleled results in antrum biopsies (data not shown).
Fig. 3.
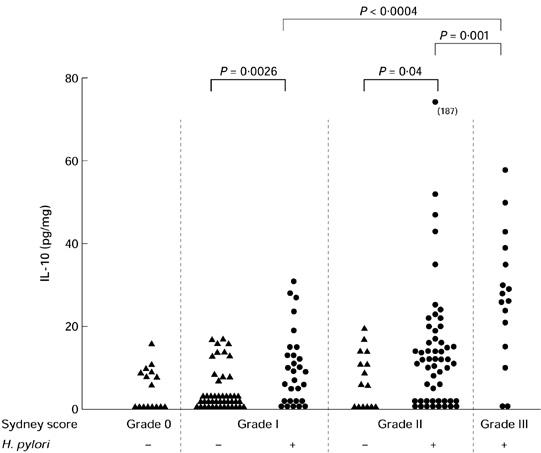
Secretion of IL-10. Relationship between secretion of IL-10 and grade of inflammation (MNC) in Helicobacter pylori-positive and H. pylori-negative gastritis. Secretion of IL-10 in antrum biopsy cultures increased with the severity of chronic gastritis only in H. pylori-positive gastritis. Secretion of IL-10 was increased in H. pylori-positive gastritis grade I and grade II in comparison with H. pylori-negative patients (matched samples, P = 0.0026 for chronic inflammation score I, P = 0.04 for chronic inflammation score II). Square brackets indicate significance of statistical differences.
Cytokine production and cag A status
In 50 of 57 (87.7%) Helicobacter-positive patients, seroreactivity against the cag A antigen was found. Patients with grade I gastritis were anti-cag A+ in 84% (21/25), with grade II gastritis were anti-cag A+ in 90% (18/20) and patients with grade III gastritis in 91.6% (11/12) (NS). Cytokine secretion was not different between anti-cag A+ and anti-cag A− patients with H. pylori-associated gastritis.
DISCUSSION
Gastric infection with H. pylori is now recognized as the leading cause of chronic gastritis and ulcer disease. Increased production of proinflammatory cytokines in the gastric mucosa appears to contribute to the inflammatory response in H. pylori-infected patients. Crabtree et al. found increased levels of TNF-α and IL-6 in patients with Helicobacter-associated gastritis compared with normal healthy Helicobacter-negative controls [1]. Similar findings were reported for IL-1β and IL-8 [2,3,5,6]. From these studies it was suggested that secretion of the proinflammatory cytokines TNF-α, IL-1β, IL-6 and IL-8 is important in the inflammatory process in H. pylori-positive gastritis [1–3,5,6]. Yamaoka et al. [7] qualitatively evaluated the presence of proinflammatory cytokine mRNA in patients with different grades of H. pylori-positive gastritis. The authors found that an increasing severity of H. pylori-positive gastritis is correlated with an increasing percentage of patients being positive for proinflammatory cytokine mRNA expression. IL-8 mRNA was found to be more frequently expressed in patients infected with cag A+H. pylori strains than in those who were cag A− [7].
An increased secretion of proinflammatory cytokines in H. pylori-positive gastritis has been therefore well documented. However, in our opinion only a limited conclusion can be deduced from the data as to whether this immunoregulatory disturbance is specifically caused by H. pylori as a pathogenic agent. This information can be only obtained by a comparison with H. pylori-negative gastritis of a similar degree as a disease specificity control.
We therefore investigated differences in cytokine production between H. pylori-positive and H. pylori-negative patients matched for the grade of gastritis. Surprisingly, we found that the small patient population with H. pylori-negative gastritis showed high levels of proinflammatory cytokine secretion which was similar to those seen in H. pylori-positive gastritis. Use of non-steroidal anti-rheumatics (NSAR), which was an exclusion criterion, cannot account for our findings. The exact cause of gastritis in some of the H. pylori-negative patients remains unclear. No explanation was found by an analysis of the demographics or the ethnicity of the patients.
Our study showed no difference in proinflammatory cytokine secretion levels between infected and non-infected patients if matched for the same histopathological inflammation score. In an extension of the study of Yamaoka et al. [7], our data suggest that proinflammatory cytokine production in the gastric mucosa is not a specific element of the pathophysiology of H. pylori-induced disease, but rather reflects inflammation irrespective of its aetiology. This observation may give rise to the experimental use of new anti-inflammatory strategies in refractory gastric inflammation.
IL-12, which is produced mainly by macrophages and monocytes in host defence against bacteria, bacterial products or intracellular parasites [12–14], is thought to play an initiating role in the inflammatory cascade [15–17]. In a recent study of Karttunen et al. [18], IL-12 mRNA was detected in 21/27 (78%) patients with H. pylori-associated gastritis, in comparison with 6/11 (55%) H. pylori-negative patients. However, gastritis in H. pylori-negative patients was less active (only minor cellular infiltrates, P = 0.15) than in H. pylori-positive patients. In H. pylori-negative non-inflamed mucosa, IL-12 mRNA was detected in only three of seven (43%) patients [18]. Another pilot study could not show differences in IL-12 mRNA expression by semiquantitative PCR between H. pylori-positive gastritis and non-infected patients [19].
In our study, IL-12 was highly significantly increased in H. pylori-associated gastritis in comparison with H. pylori- negative gastritis patients matched for a similar degree of histological inflammation. Also, increases in the secretion of IL-12 parallel gastritis grades and inflammatory activity scores only in H. pylori-positive gastritis but not in H. pylori-negative gastritis. Increased levels of IL-12 secretion in H. pylori-positive gastritis may therefore indicate a pivotal role for this cytokine in H. pylori-associated gastric disease, whereas TNF-α, IL-1β, IL-6 and IL-8 may be rather unspecific mediators in the pathophysiology of gastric inflammation.
It has been suggested that the inflammatory reaction in the gastric mucosa may be suppressed by counter-regulatory mechanisms, thereby contributing to an ineffective clearance of H. pylori [23]. In a pilot study, Bodger et al. reported increased levels of IL-10 in patients with chronic active H. pylori-positive gastritis in comparison with normal or H. pylori-negative patients with only a minor degree of inflammation [22]. In contrast, Yamaoka et al. [7] could detect IL-10 mRNA only in a small number of H. pylori-positive gastritis patients. Karttunen et al. [18] reported the detection of IL-10 mRNA in 23/27 (85%) patients with H. pylori-associated gastritis, in comparison with 7/12 (58%) H. pylori-negative patients (P = 0.08) and 7/7 H. pylori-negative patients with normal mucosa.
Our results indicate that IL-10 up-regulation relates to the intensity of gastric inflammation only in H. pylori-positive patients. In the matched comparative analysis, secretion of IL-10 was increased in H. pylori-positive over H. pylori-negative patients with gastritis. The possibility therefore exists that immunosuppression by IL-10 may be indeed a contributor to the chronicity of H. pylori-associated gastritis. An adequate explanation may be also that IL-10 would be a preventive factor for mucosal injury but cannot overcome the pathogenic Th1 cytokine-driven response mounted against the bacteria.
Cag A is thought to be a marker of H. pylori strains with increased pathogenicity [28,29] also associated with an increased secretion of IL-8 [7,30,31]. No differences were observed between cytokine secretion in cag A+ and cag A− patients. However, this finding may be related to the small number of cag A− patients in our population (7/57).
Secretion of proinflammatory cytokines by mucosal biopsies correlates with the degree of gastric inflammation irrespective of H. pylori infection. In contrast, increased secretion of IL-12 and IL-10 is important in H. pylori-associated gastritis, but is not seen in H. pylori-negative gastritis. We conclude that increased secretion of IL-12 and IL-10 may indicate a specific pathomechanism in H. pylori-induced gastric disease. IL-12 could be a candidate for evaluation as a mediator with possible importance for the development of gastric lymphoma [32] and carcinoma [2,33,34] in future studies. The possible contribution of IL-10 to chronicity of H. pylori infection should be further characterized.
REFERENCES
- 1.Crabtree JE, Shallcross TM, Heatley RV, Wyatt JI. Mucosal tumor necrosis factor α and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991;32:1473–7. doi: 10.1136/gut.32.12.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crabtree JE, Wyatt JI, Trejdosiewicz LK, et al. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J Clin Pathol. 1994;47:61–66. doi: 10.1136/jcp.47.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan XG, Chua A, Fan XJ, Keeling PW. Increased gastric production of interleukin-8 and tumour necrosis factor in patients with Helicobacter pylori infection. J Clin Pathol. 1995;48:133–6. doi: 10.1136/jcp.48.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowe SE, Alvarez L, Dytoc M, et al. Expression of interleukin 8 and CD54 by human gastric epithelium after Helicobacter pylori infection in vitro. Gastroenterol. 1995;108:65–74. doi: 10.1016/0016-5085(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 5.Noach LA, Bosma NB, Jansen J, Hoek FJ, van Deventer SJ, Tytgat GN. Mucosal tumor necrosis factor α, interleukin 1 beta, and interleukin 8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994;29:425–9. doi: 10.3109/00365529409096833. [DOI] [PubMed] [Google Scholar]
- 6.Moss SF, Legon S, Davies J, Calam J. Cytokine gene expression in Helicobacter pylori associated antral gastritis. Gut. 1994;35:1567–70. doi: 10.1136/gut.35.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamaoka Y, Kita M, Kodama T, Sawai N, Imanishi J. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterol. 1996;110:1744–52. doi: 10.1053/gast.1996.v110.pm8964399. [DOI] [PubMed] [Google Scholar]
- 8.Reinecker HC, Steffen M, Witthöft T, Pflueger I, Schreiber S, MacDermott RP, Raedler A. Enhanced secretion of tumor necrosis factor-alpha, IL-6 and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993;94:174–81. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schreiber S, Heinig T, Panzer U, Reinking R, Bouchard A, Stahl PD, Raedler A. Impaired response of activated mononuclear phagocytes to interleukin 4 in inflammatory bowel disease. Gastroenterol. 1995;108:21–33. doi: 10.1016/0016-5085(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 10.Karttunen R, Karttunen T, Ekre H-PT, Macdonald TT. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995;36:341–5. doi: 10.1136/gut.36.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amakawa R, Fukuhara S, Ohno H, et al. Genomic organization of IgH gene compared with the expression of Bcl-2 gene in T (14;18)-positive lymphoma. Blood. 1991;77:1970–6. [PubMed] [Google Scholar]
- 12.Stern AS, Podlaski FJ, Hulmes JD, et al. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc Natl Acad Sci USA. 1990;87:6808–12. doi: 10.1073/pnas.87.17.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh C, Macatonia SE, Tripp CS, Wolf SF, O'garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–9. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 14.Song F, Matsuzaki G, Mitsuyama M, Nomoto K. Differential effects of viable and killed bacteria on IL-12 expression of macrophages. J Immunol. 1996;156:2979–84. [PubMed] [Google Scholar]
- 15.Trinchieri G. Interleukin 12 and its role in the generation of TH1 cells. Immunol Today. 1993;14:335–7. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- 16.Wolf SF, Sieburth D, Sypek J. Interleukin 12: a key modulator of immune function. Stem Cells Dayt. 1994;12:154–68. doi: 10.1002/stem.5530120203. [DOI] [PubMed] [Google Scholar]
- 17.Biron CA, Gazzinelli RT. Effects of IL-12 on immune responses to microbial infections: a key mediator in regulating disease outcome. Curr Opin Immunol. 1995;7:485–96. doi: 10.1016/0952-7915(95)80093-x. [DOI] [PubMed] [Google Scholar]
- 18.Karttunen RA, Karttunen TJ, Yousfi MM, El-Zimaity HMT, Graham DY, El-Zaatari FAK. Expression of mRNA for interferon gamma, interleukin 10 and interleukin 12 (p40) in normal gastric mucosa and in mucosa infected with Helicobacter pylori. Scand J Gastroenterol. 1997;32:22–27. doi: 10.3109/00365529709025058. [DOI] [PubMed] [Google Scholar]
- 19.Haeberle HA, Kubin M, Bamford KB, et al. Differential stimulation of interleukin 12 (IL-12) and IL-10 by live and killed Helicobacter pylori in vitro and association of IL-12 production with gamma interferon-producing T cells in the human gastric mucosa. Infect Immun. 1997;65:4229–35. doi: 10.1128/iai.65.10.4229-4235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'garra A. Interleukin-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 21.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin-10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of interleukin-10 produced by human monocytes. J Exp Med. 1991;174:2109–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreiber S, Heinig T, Thiele HG, Raedler A. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterol. 1995;108:1434–44. doi: 10.1016/0016-5085(95)90692-4. [DOI] [PubMed] [Google Scholar]
- 23.Bodger K, Wyatt JI, Heatley RV. Gastric mucosal secretion of interleukin 10: relations to histopathology, Helicobacter pylori status, and tumor necrosis factor-α secretion. Gut. 1997;40:739–44. doi: 10.1136/gut.40.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83:346–56. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 25.Misiewicz JJ, Tytgat G, Goodwin CS, et al. Working Party Reports of the World Congress of Gastroenterology. Oxford: Blackwell Scientific Publications; 1990. The Sydney system: a new classification of gastritis; pp. 1–10. [Google Scholar]
- 26.Shapiro SS, Wilk MB, Chen HJ. Comparative study of various tests of normality. J Am Stat Assoc. 1968;63:1343–72. [Google Scholar]
- 27.Sachs L. Heidelberg. 7. Germany: Springer-Verlag; 1992. Angewandte Statistik. [Google Scholar]
- 28.Covacci A, Censini S, Bugnoli M, et al. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791–5. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crabtree JE, Taylor JD, Wyatt JI, Heatley RV, Shallcross TM, Tompkins DS, Rathbone BJ. Mucosal recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991;338:332–5. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- 30.Crabtree JE, Covacci A, Farmery SM, et al. Helicobacter pylori induced interleukin 8 expression in gastric epithelial cells is associated with CagA positive phenotype. J Clin Pathol. 1995;48:41–45. doi: 10.1136/jcp.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crabtree JE, Xiang Z, Lindley IJ, Tompkins DS, Rappuoli R, Covacci A. Induction of interleukin 8 secretion from gastric epithelial cells by a cagA negative isogenic mutant of Helicobacter pylori. J Clin Pathol. 1995;48:967–9. doi: 10.1136/jcp.48.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang C, Mayo MW, Baldwin AS. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–7. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 33.Bayerdorffer E, Neubauer A, Rudolph B, Thiede C, Lehn N, Eidt S, Stolte M. Regression of primary gastric lymphoma of mucosa- associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet. 1995;345:1591–4. doi: 10.1016/s0140-6736(95)90113-2. [DOI] [PubMed] [Google Scholar]
- 34.Kuipers EJ, Uyterlinde AM, Pena AS, et al. Long-term sequelae of Helicobacter pylori gastritis. Lancet. 1995;345:1525–8. doi: 10.1016/s0140-6736(95)91084-0. [DOI] [PubMed] [Google Scholar]
- 35.Blaser MJ, Pérez-Pérez GI, Kleanthous H, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–5. [PubMed] [Google Scholar]