Abstract
Lymphocyte emigration from the intestinal wall via lymphatics is necessary to maintain gastrointestinal immunity and also to connect the different parts of the mucosal immune system. In the present study the numbers and time kinetics of proliferating lymphocyte subsets leaving the gut wall via intestinal lymphatics were analysed in mesenteric lymph node adenectomized minipigs (n = 8). After cannulation of the major intestinal lymph duct, afferent lymph was collected under non-restraining conditions. In four pigs lymphocytes taken from the intestinal lymph and blood were incubated in vitro with the thymidine analogue bromodesoxyuridine (BrdU) to label all lymphocytes in the S-phase of the cell cycle. The other four pigs received a single i.v. injection of BrdU 1 week after cannulation. The initial percentage of BrdU+ lymphocyte subsets in the intestinal lymph 15 min after BrdU injection was comparable to that after the in vitro labelling (1.5 ± 0.7% in T cells, 10.6 ± 1.6% in IgM+ cells and 30.0 ± 11.9% in IgA+ cells). From this level onwards, the percentage of in vivo labelled BrdU+ lymphocyte subsets reached a maximum at 12 h after BrdU application. A different pattern of BrdU+ subsets was seen in the blood. After an early peak at around 3–4 h, the frequency of BrdU in vivo labelled cells decreased. Each subset had a maximum between 12 h and 48 h after BrdU application (maximum of BrdU+ CD2+ T cells at 12 h, 4.6 ± 1.5%; IgM+ BrdU+ at 48 h, 8.8 ± 3.3%). The present results provide a basis to determine the time necessary for induction of specific intestinal immunity during oral vaccination studies.
Keywords: pig, time kinetics, lymphocyte subsets, emigration, proliferation
INTRODUCTION
In the intestine humoral as well as cellular immunity plays an important role in the defence against harmful antigens and in inducing oral tolerance. The details of the different processes that regulate immunity, oral tolerance, or inflammation of the host are still unknown. Many aspects of intestinal immune reactions have been studied and reviewed recently [1–3], but the local proliferation of B and T lymphocytes in the compartments of the gut wall (Peyer's patches, lamina propria and epithelium) and especially their emigration behaviour have only rarely been studied.
So far it is known that an intestinal immune reaction starts within the organized lymphoid tissues. After controlled antigen uptake by M cells, which are specialized cells of the dome epithelium [4], antigen presentation, stimulation and induction of antigen-specific B and T cells initiate the immune response, mainly in the Peyer's patches. Many of the induced lymphocytes emigrate and recirculate preferentially into the lamina propria of the intestinal mucosa [5,6], where T cells and cytokines regulate plasma cell differentiation (for review see [3]). This continuous cell traffic is important, especially for those antigen-specific lymphocytes mediating immunity after oral vaccination [7,8]. The present study focuses on the detailed time kinetics of proliferating lymphocytes leaving the intestinal wall via lymphatics as an essential aspect for understanding immune responses to enteric pathogens as well as for the development of oral vaccination protocols. In previous vaccination studies in humans, antibody-secreting cells were detected in the blood about 7 days after oral immunization with cholera toxin [9], but it is not clear at what point of time antigen-specific cells proliferate and emigrate from the place of antigen contact.
In this study all lymphoid cells in the cell cycle of the whole animal were labelled using one single injection of the thymidine analogue bromodesoxyuridine (BrdU). The labelled cells—lymphoblasts and their progeny, which are newly formed cells—were followed in the intestinal lymph and in the blood. The incorporated BrdU and the subsets were detected by a double-labelling procedure. Thus, the appearance of the gut-emigrating proliferating lymphocytes was analysed and quantified in the intestinal lymph using an established pig model [10,11]. The omnivorous pig was kept non-restrained under nearly physiological conditions. Using this model the data on lymphocyte recirculation from the intestine back to the lamina propria have not only been confirmed, but the pool sizes of these cells accumulated in the lamina propria have also been quantified in vivo [12]. To study lymphocyte proliferation without the effects of the operation, it was necessary to sustain the lymph collection for a period of up to 2 weeks. This essential step was made possible by a new cannula.
The present results are a basis for experimental strategies in the development of effective vaccination protocols, e.g. using the pig model for human rotaviral disease [13] or vaccination protocols against typhoid fever in humans [14].
MATERIALS AND METHODS
Surgical techniques
The experiments were performed on eight female minipigs of the Göttingen breed. At age 3 months the animals were laparotomized under i.v. thiobarbiturate anaesthesia (Trapanal; Byk Gulden, Konstanz, Germany) and all mesenteric lymph nodes draining the small intestine were removed (animal weight 10.3 ± 1.6 kg). After the operation the afferent and the efferent lymph vessels reanastomose by physiological regeneration, so that it is possible after a few weeks to collect gut-derived lymph directly without influence of the mesenteric lymph nodes. At around 8 months old (animal weight 34.7 ± 5.1 kg) a venous cannula was established in the external jugular vein of all animals, before the major intestinal lymph duct was cannulated. Using a midline laparotomy the peritoneum was opened. The intestinal lymph duct was found below the pancreas near the confluence of the left renal vein and the posterior vena cava. The cannulation was performed with a special cannula (Teflon TPE Micro Tube; Novodirekt GmbH, Kehl/Rhein, Germany) in a silastic leading tube (Silastic; Dow Corning GmbH, Meerbusch, Germany). The end of the cannula was exteriorized through an incision in the right abdominal wall. The intestinal lymph was collected in a 250-ml flask fixed in a bag tied on the animal. Afterwards the minipigs were kept under non-restraining conditions with free access to food and water. They recovered quickly from the operation and were not affected by the surgery.
Collecting and handling the samples
During the experiment the intestinal lymph was collected continuously, the flasks being changed twice a day, on average about every 12 h. The lymph flasks contained 5 ml sterile RPMI 1640 (Seromed Biochrom KG, Berlin, Germany) supplemented with antibiotics (6000 U penicillin, 6 mg streptomycin, 75 μg amphotericin, 3 mg gentamycin; Seromed) and 1500 IE heparin to prevent clotting (Liquemin N 250000; Roche, Grenzach-Wyhlen, Germany). For each lymph sample the volume and the time of the collecting period were determined, before the cells were centrifuged at 400 g for 10 min and resuspended in RPMI 1640. This step was repeated with a defined volume of RPMI 1640. Using a phase contrast microscope at × 500 magnification the nucleated cells, lymphocytes and erythrocytes were counted in a Neubauer's counting chamber. Based on the data obtained, the lymph flow/h and the hourly output of lymphocytes were calculated. In addition, blood samples were taken from the external jugular vein. To remove the erythrocytes by osmotic shock, 1 ml EDTA blood was incubated with 10 ml lysis solution (8.3 g NH4Cl + 0.1 g EDTA + 1.0 g KHCO3 and 1 l distilled water) for 10 min at room temperature. The remaining nucleated cells were centrifuged at 200 g and resuspended in 1 ml RPMI 1640. Using a haemocytometer these cells were counted. Giemsa-stained cytospots were used to determine the percentage of lymphoid cells among the nucleated cells.
Determination of lymphocyte subpopulations
In brief, an indirect immunofluorescence staining was used to determine the lymphocyte subpopulations in the lymph and blood samples. In a microtitre plate 1.5 × 106 lymph or blood cells were incubated with seven porcine-specific MoAbs against CD2 (preferentially α/β T cells in the pig), CD4 (T helper cells), CD8 (cytotoxic/suppressor T cells), IgA, IgM, macrophages and Null cells, which are mainly CD2−γ/δ T cells in the pig [15,16]. As a secondary antibody a PE-labelled goat anti-mouse antibody (Dianova, Hamburg, Germany) was applied to identify the lymphocyte subpopulation using a flow cytometric method. Additionally, a double immunofluorescence staining (CD4/CD8) with isotype-specific secondary antibodies (PE- or FITC-labelled goat anti-mouse antibody) was used to determine the CD4+CD8+ lymphocytes in the intestinal lymph and in the blood. Flow cytometric analysis of the cells was performed with a FACScan HP 9000 (Hewlett Packard, Ford Collins, CO). The FACScan program was used for acquisition and the MDI-Software (WinMDI 2.7, facs.scripps.edu) for evaluation of the data.
Proliferation study
In vitro labelling
In four animals the lymph and blood samples were taken immediately after the cannulation and on the following days and the cells were used for BrdU in vitro labelling to characterize all cells in the S-phase of the cell cycle. With regard to the intestinal lymph the flasks contained BrdU (10 μmol/l) for a collecting period of 1 h per day. The blood samples were incubated for 30 min with BrdU in a water bath at 37°C. Each lymph and blood sample was treated as described.
In vivo labelling
One week after successful cannulation of the intestinal lymph duct four of the minipigs received a single i.v. dose of BrdU (20 mg/kg body weight; Sigma, München, Germany). The lymph and also the blood samples were collected at defined intervals after the injection. The first samples were obtained at 5, 10, 15 and 30 min, hourly from 1 h to 6 h after injection, then at 9 h and 12 h, and during the next 3 days at 12-h intervals.
Markers for porcine lymphocytes (CD2, CD4, CD8, IgM, IgA and Null cells [15]) were used in an immunocytochemical stain (bridging antibody, alkaline phosphatase–anti-alkaline phosphatase (APAAP) complex (Dako, Hamburg, Germany) and fast blue salt (Sigma)). After fixing and denaturation of the DNA the incorporated BrdU was detected by an immunoperoxidase method [10,11].
Statistical analysis
The percentage of BrdU+ lymphoid cells was determined by analysing at least 500 subset positive cells for incorporated BrdU. Means and s.d. were calculated. Differences were taken as significant when they reached at least P < 0.05 in the Wilcoxon Mann–Whitney test.
RESULTS
After the cannulation of the intestinal lymph duct the minipigs showed no clinical problems, and they had normal food intake and bowel movements during the whole observation period (12.1 ± 3.5 days (mean ± s.d.)). The lymph flow was relatively constant (17.9 ± 7.1 ml/h) and the hourly lymphocyte yield ranged between 8.1 ± 2.7 × 106 (animal 1) and 43.4 ± 12.2 × 106 (animal 8), so that on average 27.5 ± 18.0 × 106 lymphocytes/h left the intestinal wall via lymphatics. The lymph flow remained stable, but the lymphocyte output decreased within the first 24 h after the operation. From the second post-operative day onwards the lymphocyte yield increased again and remained at a comparable high level until the end of the experiments.
Lymphocyte subset distribution
Using flow cytometry the lymphocyte composition was analysed in the intestinal lymph and in the circulating blood in parallel. The percentages of immunoglobulin-positive, CD2+ and Null cell subsets were comparable over the whole experimental period with no influence of the i.v. BrdU application. In the intestinal lymph 83.2 ± 6.9% and in the blood 54.6 ± 8.7% CD2+ T cells were detected. The CD4+ T helper cells (39.5 ± 3.4%) outnumbered the CD8+ T cytotoxic/suppressor cells (30.2 ± 5.5%) in the lymph, whereas in the blood more CD8+ cells (31.1 ± 6.6%) than CD4+ cells (25.8 ± 3.8%) were found. The double immunofluorescence staining showed a simultaneous expression of CD4 and CD8 antigens on lymphocytes in the intestinal lymph (4.6 ± 0.7%) and in the blood (8.8 ± 2.0%). About 10% of all lymphocytes in the lymph were Null cells (10.3 ± 4.3%). In contrast, the blood contained approximately three times more Null cells (27.9 ± 7.9%). The frequency of immunoglobulin-positive lymphocytes in the intestinal lymph (IgM, 9.2 ± 3.4%; IgA, 1.2 ± 0.5%) and in the blood (IgM, 8.6 ± 2.9%; IgA, 1.9 ± 2.1%) was similar. The sum of CD2+ cells, immunoglobulin-positive cells and Null cells, including cells identified as macrophages (lymph, 1.4 ± 0.9%; blood, 12.8 ± 8.9%), was approx. 100%. These calculations provide evidence of identification of all lymphocyte subsets in the intestinal lymph and in the blood. Among all nucleated cells of intestinal lymph there was a proportion of up to approx. 4% dendritic cells, as revealed by microscopic analysis of Giemsa-stained cytospin preparations.
Proportion of BrdU+ cells in the intestinal lymph
Using a double-labelling technique the proliferative behaviour of lymphocyte subsets was determined after in vitro and in vivo labelling with the thymidine analogue bromodesoxyuridine. The indices, which were obtained after in vitro labelling (Fig. 1) of lymphocytes (n = 4), showed no variation at the different time points after the cannulation (CD2+, 2.1 ± 0.4%; IgM+, 9.1 ± 2.4%; IgA+, 29.6 ± 7.3%). Furthermore, the number of BrdU+ subsets was lower in blood lymphocytes, but remained stable over the whole observation period.
Fig. 1.
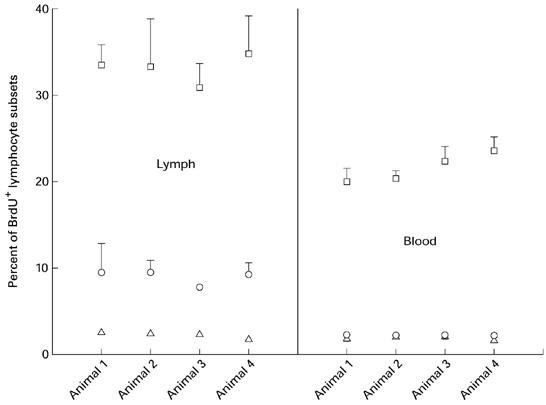
In vitro bromodesoxyuridine (BrdU) labelling of lymphocytes taken from the intestinal lymph and the blood. The relative proportions of cells, which were in the S-phase of the cell cycle, are given as mean ± s.d. □, IgA+ BrdU+; ○, IgM+ BrdU+; ▵, CD2+ BrdU+.
The BrdU in vivo application at a defined time point (pulse label) was necessary to examine the emigration of proliferating or already newly formed lymphocytes. In the intestinal lymph 15 min after BrdU injection 1.5 ± 0.7% CD2+ T cells had incorporated the label (CD8+, 0.7 ± 0.1%; CD4+, 2.2 ± 0.6%). However, a higher BrdU labelling index was found for the immunoglobulin-positive lymphocyte subsets (IgM+, 10.6 ± 1.6%; IgA+, 30.0 ± 11.9%). These indices obtained after 15 min in vivo were comparable to those after in vitro labelling. During the following collection periods the amount of BrdU+ lymphocytes increased and reached a maximum at 12 h after BrdU application. In Fig. 2a the emigration behaviour of BrdU-labelled IgA+ cells is shown for each of the four animals. At the maximum a mean of 57.4 ± 5.7% IgA+ lymphocytes had incorporated the label. The frequency of BrdU+ cells leaving the gut wall 12 h after the application was 3.6 ± 1.0% in the CD2 subset and 17.1 ± 3.0% in the IgM subset (Fig. 2b). In the later sampling periods the frequency of BrdU+ cells decreased in all subsets. About 12 h after BrdU application, lymphocytes which showed only a dull labelling of BrdU were observed in each subset. The time kinetics of these populations was determined separately (Fig. 2b).
Fig. 2.
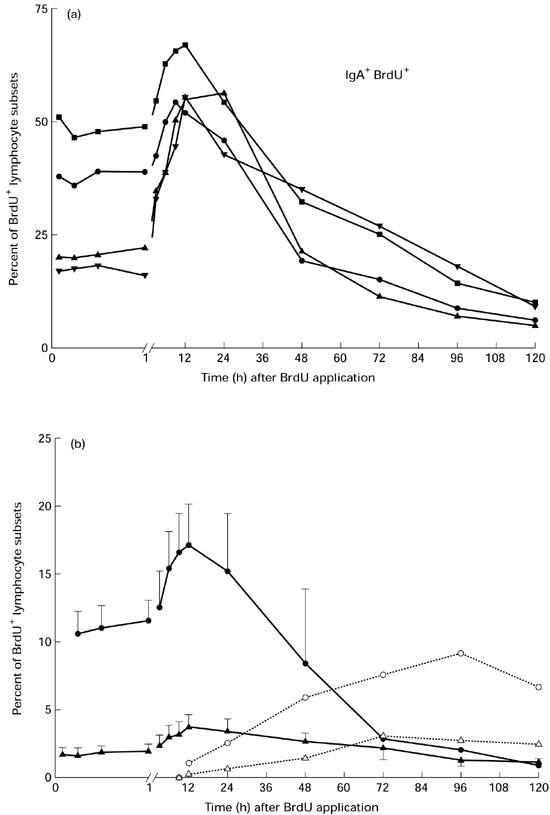
(a) Frequency of proliferating or already newly formed IgA+ lymphocytes in the intestinal lymph for each of the four animals after bromodesoxyuridine (BrdU) injection. For clarity, only results of selected time points during the first 12 h after BrdU application were included in this and the following figures. •, Animal 5; ▪, animal 6; ▴, animal 7; ▾, animal 8. (b) Detailed time kinetics of in vivo BrdU-labelled IgM+ and CD2+ lymphocytes in the intestinal lymph (pooled results of four minipigs, mean ± s.d.). Additionally the appearance of only dull-labelled BrdU+ lymphocytes, which were detected from 12 h after BrdU injection onwards, are shown. Note that the scale of the ordinate is different from that in (a). •, IgM+ BrdU+; ○, IgM+ BrdU+ dull; ▴, CD2+ BrdU+; ▵, CD2+ BrdU+ dull.
The total numbers of BrdU+ lymphocyte subsets leaving the intestinal wall were calculated based on the lymphocyte yield/h, the lymphocyte subset evaluation by flow cytometry and the counts performed using the double-stained subset/BrdU cytospot. The maximum output per hour was 0.5 ± 0.2 × 106 CD2+ BrdU+ cells, 0.3 ± 0.1 × 106 IgM+ BrdU+ and 0.1 ± 0.1 × 106 IgA+ BrdU+ B lymphocytes 12 h after BrdU in vivo labelling.
Time kinetics of BrdU+ cells in the circulating blood
The kinetics of BrdU+ lymphocytes in the circulating blood was different from that observed in the intestinal lymph. Moreover, differences could also be seen between the B and T cells. The percentage of BrdU-labelled CD2+ cells in the blood increased from 0.8 ± 0.2% at 15 min, which was comparable to the results of in vitro labelling (Fig. 1), to an early peak at 3 h (2.0 ± 0.9%) after BrdU application (Fig. 3). During the following hour the BrdU+ CD2+ subset decreased (1.5 ± 0.8%). Then the maximum percentage of BrdU+ CD2+ lymphocytes was achieved 12 h after the injection (4.6 ± 1.5%). The proportions of BrdU-labelled CD4 and CD8 subsets were similar to the CD2 subset (peak at 3 h and maximum at 12 h after BrdU injection). The frequency of BrdU+ immunoglobulin-positive cells rose from the initial value (IgM+, 1.3 ± 0.6%; IgA+, 21.7 ± 4.1%) to a peak at 4 h and then decreased over the next 2 h (Fig. 3). From 6 h onwards the immunoglobulin-positive subset increased again and reached a maximum between 24 h and 48 h after the injection (IgM+, 8.8 ± 3.3% at 48 h; IgA+, 54.5 ± 16.5% at 24 h). Based on the small numbers in the circulating blood it was not possible to determine dull-labelled BrdU+ cells separately.
Fig. 3.
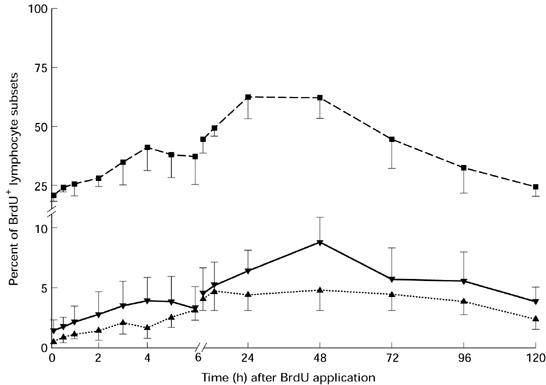
Comparison of the bromodesoxyuridine (BrdU+) lymphocytes within the CD2, IgM and IgA subsets in the circulating blood after i.v. BrdU application (mean ± s.d.). ▪, IgA+ BrdU+; ▾, IgM+ BrdU+; ▴, CD2+ BrdU+.
DISCUSSION
In this study the appearance, numbers and subset composition of stimulated B and T cells in the intestinal lymph and in the blood were studied under physiological conditions in vivo. Analysing the kinetics of lymphocyte emigration from the gut in detail will help to understand the time schedule of an intestinal immune reaction. The omnivorous, monogastric pig was used as model because its gastrointestinal tract is comparable to that of humans, e.g. the post-natal, antigen-dependent development of the Peyer's patches [17]. The mesenteric lymph nodes of the pig are unique in their direct vascular exit of lymphocytes [18,19], with only a few lymphocytes leaving the mesenteric lymph nodes via lymphatics to reach the thoracic duct. After mesenteric lymph node adenectomy it was possible to obtain afferent intestinal lymph almost exclusively. The use of a new Teflon cannula allowed the cannulation of the major intestinal lymph duct in the pig model for up to approx. 2 weeks.
In our investigations the lymph flow (17.9 ± 7.1 ml/h) and the lymphocyte yield (27.5 ± 18.0 × 106/h) were comparable to those reported in earlier studies [10,15]. In total these pigs lose only 0.2% of their total lymphocyte pool per day [20] through the cannulation of intestinal lymph. There is no evidence that this amount of lymphocyte loss has any relevance for the results of lymphocyte proliferation.
The addition of the percentages of CD2+, immunoglobulin-positive and Null cells, which are preferentially CD2−γ/δ T cells in the pig [16], resulted in approx. 100%. Under the supposition that the three populations were different and effectively non-overlapping [21], all lymphocytes in the intestinal lymph were characterized by flow cytometric analysis. A significant population of CD4+CD8+ T cells was present among the gut-emigrant lymphocytes. The frequency of these CD4+CD8+ lymphocytes in the circulating blood was approximately double that in the intestinal lymph. Other investigators showed that in the pig CD4+CD8+ T cells are memory T helper cells [22], which can be induced by vaccination [23]. A double expression of CD4/CD8 by rat T lymphocytes was also linked to activation [24]. These results are in accordance with results in humans, where some CD4+CD8− T lymphocytes express CD8 after in vitro activation [25].
The main objective of the present study was to determine the emigration behaviour of gut-derived lymphocyte subsets. The time kinetics was analysed in detail using the thymidine analogue BrdU, which is incorporated in proliferating cells during the S-phase of the cell cycle and can be revealed by immunocytology. An interesting aspect of the in vivo study was the high initial value of BrdU+ cells observed within the first samples. The indices obtained after BrdU in vitro labelling, which were comparable to those after 15 min in vivo, suggest that these lymphocytes proliferate while leaving the intestinal wall, which has also been shown in other species [26,27]. Moreover, our in vivo results showed a maximum emigration of newly formed B as well as T cell subsets 12 h after injection of the DNA precursor. Due to the high absolute numbers of T cells, more BrdU-labelled T than B lymphocytes emigrate from the intestinal wall in total numbers. This is in accordance with the results of earlier studies [10,11]. The appearance of only dull-labelled lymphocytes in the intestinal lymph from 12 h after BrdU injection onwards provides evidence that these lymphocytes were daughter cells from compartments of the gut wall. The concept of semiconservative replication accounts for the low colour reaction of these cells. However, the present experimental arrangement did not allow the origin of the bright and dull BrdU+ gut-emigrating lymphocytes or the contribution of fast recirculating lymphocytes to the pool of BrdU+ emigrants to be clarified.
Regarding the recirculating lymphocyte pool, earlier studies were based on the use of only thoracic duct lymphocytes from rats [28] and mice [29], which represented a balance of lymphocytes from different sources including the gut. Recent studies, especially in sheep, in which lymph-derived lymphocytes were labelled and retransfused intravenously, showed that these actively recirculating cells reached peak levels in various lymph samples 20–30 h after retransfusion [30,31]. In those experiments only a few labelled cells were detected in the gut lymph within the first few hours after reinjection. Most of these cells were large lymphocytes or lymphoblasts, which showed that they were in the S-phase of the cell cycle, a process that was presumably initiated by antigenic stimulation [32]. In the pig the majority of fluorescein-labelled lymphocytes taken from the intestinal lymph need at least 12 h to migrate from the blood through the gut wall back to the intestinal lymph [10]. Therefore, most BrdU-labelled lymphocytes that emigrate from the intestine within the period up to 12 h after BrdU application will have their origin in the compartments of the gut wall. Moreover, earlier studies pointed out that these lymphocytes with the described time kinetics have their origin mainly in the Peyer's patches. The metaphase-arrest technique using vincristine demonstrated a large quantity of proliferating cells not only in the follicles but also in the corona and interfollicular area [33]. These results suggest that the BrdU-labelled cells have their origin mainly in the interfollicular area (T cell area) and the follicles (B cell area) of the Peyer's patches. In mice it was demonstrated that Peyer's patch cells possess an inherent capacity to continue dividing in vitro, whereas most lymphoid cell populations do not exhibit strong proliferative reactions in culture under normal circumstances [34]. Proliferating cells were observed in the lamina propria [35], and in the epithelium cells were detected 1 h after BrdU injection, which had incorporated the label in the S-phase of the cell cycle [36]. The frequency of these proliferating lymphocytes in both compartments is not high enough to contribute in marked numbers to the newly formed cell pool observed in the gut lymph.
A different migratory pattern of BrdU-labelled B and T lymphocytes was observed in the blood. In the present study the early peak at 3 h for T and at 4 h for B cell subsets after BrdU application may be explained by an early emigration of stimulated lymphocyte subsets, not from the gut but from different lymphoid or non-lymphoid tissues, e.g. the thymus, spleen, bone marrow, and other sources [33]. Because the blood lymphocyte pool is kept in equilibrium with its large reservoirs of migrating cells, lymphocyte recirculation experiments are now necessary to clarify the early peak of BrdU+ cells in the blood.
In conclusion, our results provide evidence that in the intestinal lymph most newly formed lymphocytes 12 h after BrdU injection predominantly comprise lymphocytes, which have their origin in the Peyer's patches and are developed during intestinal immune responses. Moreover, it was possible to propose a hypothesis about the appearance of BrdU+ lymphocytes, including the only dull-labelled cells in the intestinal lymph (Fig. 4). Based on these results, it has to be investigated whether this pattern is also typical of the appearance of specific cells after antigen exposition. So far the parameters of a successful oral vaccination, e.g. dose, single or repetitive application, and in particular the time intervals, have been arrived at by trial and error and not because the different steps of the pathomechanisms are really understood. Thus, the present data provide the basis to determine the time necessary for the development of intestinal immune responses.
Fig. 4.
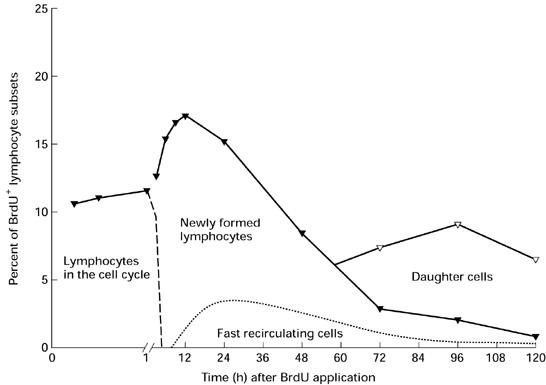
Schematic summary of the appearance of proliferating cells in the intestinal lymph after labelling all cells in the cell cycle by bromodesoxyuridine (BrdU) at a defined time point (pulse label). The continuous line (—–) shows the typical appearance of bright and only dull BrdU-labelled lymphocytes for the BrdU+ IgM+ cells in the lymph (see Fig. 2b). Based on the patterns described the distribution of the BrdU+ lymphocytes was estimated in relation to the cell cycle. Left of the dashed line (–—––) the cells have entered the cell cycle but not yet completed mitosis. With regard to the results of the BrdU in vitro labelling, the lymphocytes emigrating within the first hour after BrdU in vivo labelling proliferate while leaving the intestinal wall. On the right side preferentially post-mitotic cells are present (see Discussion). The proportion of fast recirculating lymphocytes (- - - - -) is based on data from the literature [37]. The daughter cells represent the only dull-labelled BrdU+ cell population (see Discussion).
Acknowledgments
The study was supported by the Deutsche Forschungsgemeinschaft, SFB 280 ‘Gastrointestinal Barrier’, Project C1. The authors thank R. M. Binns (Babraham, Cambridge, UK), A. T. C. Bianchi (Lelystad, The Netherlands) and A. Saalmüller (Tübingen, Germany), for providing monoclonal antibodies. Also the technical assistance of A. Herden, I. Trotz and S. Schlecht as well as the correction of the English by S. Fryk are gratefully acknowledged.
REFERENCES
- 1.Mowat AM, Viney JL. The anatomical basis of intestinal immunity. Immunol Rev. 1997;156:145–66. doi: 10.1111/j.1600-065x.1997.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 2.Dunkley M, Pabst R, Cripps A. An important role for intestinally derived T cells in respiratory defence. Immunol Today. 1995;16:231–6. doi: 10.1016/0167-5699(95)80165-0. [DOI] [PubMed] [Google Scholar]
- 3.McGhee JR, Mestecky J, Dertzbaugh MT, Eldridge JH, Hirasawa M, Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10:75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- 4.Gebert A, Rothkötter HJ, Pabst R. M cells in Peyer's patches of the intestine. Int Rev Cytol. 1996;167:91–159. doi: 10.1016/s0074-7696(08)61346-7. [DOI] [PubMed] [Google Scholar]
- 5.Pabst R. The anatomical basis for the immune function of the gut. Anat Embryol. 1987;176:135–44. doi: 10.1007/BF00310046. [DOI] [PubMed] [Google Scholar]
- 6.Bienenstock J, Befus D, McDermott M, Mirski S, Rosenthal K. Regulation of lymphoblast traffic and localization in mucosal tissues, with emphasis on IgA. Fed Proc. 1983;42:3213–7. [PubMed] [Google Scholar]
- 7.Quiding M, Nordström I, Kilander A, et al. Intestinal immune responses in humans. J Clin Invest. 1991;88:143–8. doi: 10.1172/JCI115270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmgren J, Svennerholm AM. Bacterial enteric infections and vaccine development. Gastroenterol Clin North Am. 1992;21:283–302. [PubMed] [Google Scholar]
- 9.Quiding-Järbrink M, Nordstrom I, Granstrom G, et al. Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric, and nasal immunizations. A molecular basis for the compartmentalization of effector B cell responses. J Clin Invest. 1997;99:1281–6. doi: 10.1172/JCI119286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothkötter HJ, Huber T, Barman NN, Pabst R. Lymphoid cells in afferent and efferent intestinal lymph: lymphocyte subpopulations and cell migration. Clin Exp Immunol. 1993;92:317–22. doi: 10.1111/j.1365-2249.1993.tb03398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothkötter HJ, Hriesik C, Pabst R. More newly formed T than B lymphocytes leave the intestinal mucosa via lymphatics. Eur J Immunol. 1995;25:866–9. doi: 10.1002/eji.1830250336. [DOI] [PubMed] [Google Scholar]
- 12.Rothkötter HJ, Hriesik C, Barman NN, Pabst R. B and also T lymphocytes migrate via gut lymph to all lymphoid organs and the intestinal wall, but only IgA+ cells accumulate in the lamina propria of the intestinal mucosa. Eur J Immunol. 1999;29:327–33. doi: 10.1002/(SICI)1521-4141(199901)29:01<327::AID-IMMU327>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 13.Yuan L, Kang SY, Ward LA, To TL, Saif LJ. Antibody-secreting cell responses and protective immunity assessed in gnotobiotic pigs inoculated orally or intramuscularly with inactivated human rotavirus. J Virol. 1998;72:330–8. doi: 10.1128/jvi.72.1.330-338.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanoff B, Levine MM, Lambert PH. Vaccination against typhoid fever: present status. Bull World Health Organ. 1994;72:957–71. [PMC free article] [PubMed] [Google Scholar]
- 15.Davis WC, Zuckermann FA, Hamilton MJ, et al. Analysis of monoclonal antibodies that recognize γδ T/null cells. Vet Immunol Immunopathol. 1998;60:305–16. doi: 10.1016/s0165-2427(97)00107-4. [DOI] [PubMed] [Google Scholar]
- 16.Saalmüller A, Pauly T, Lunney JK, et al. Overview of the Second International Workshop to define swine cluster of differentiation (CD) antigens. Vet Immunol Immunopathol. 1998;60:207–28. doi: 10.1016/s0165-2427(97)00098-6. [DOI] [PubMed] [Google Scholar]
- 17.Barman NN, Bianchi ATJ, Zwart RJ, Pabst R, Rothkötter HJ. Jejunal and ileal Peyer's patches in pigs differ in their postnatal development. Anat Embryol. 1997;195:41–50. doi: 10.1007/s004290050023. [DOI] [PubMed] [Google Scholar]
- 18.Binns RM, Hall JG. The paucity of lymphocytes in the lymph of unanaesthetised pigs. Br J Exp Pathol. 1966;47:275–80. [Google Scholar]
- 19.Bennell MA, Husband AJ. Route of lymphocyte migration in pigs. I. Lymphocyte circulation in gut-associated lymphoid tissue. Immunology. 1981;42:469–74. [PMC free article] [PubMed] [Google Scholar]
- 20.Pabst R, Trepel F. Quantitative evaluation of the total number and distribution of lymphocytes in young pigs. Blut. 1975;31:77–86. doi: 10.1007/BF01633723. [DOI] [PubMed] [Google Scholar]
- 21.Binns RM, Duncan IA, Powis SJ, Hutchings A, Butcher GW. Subsets of null and γδ T-cell receptor+ T lymphocytes in the blood of young pigs identified by specific monoclonal antibodies. Immunology. 1992;77:219–27. [PMC free article] [PubMed] [Google Scholar]
- 22.Pescovitz MD, Sakopoulos AG, Gaddy JA, Husmann RJ, Zuckermann FA. Porcine peripheral blood CD4+/CD8+ dual expressing T-cells. Vet Immunol Immunopathol. 1994;43:53–62. doi: 10.1016/0165-2427(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 23.Ober BT, Summerfield A, Mattlinger C, et al. Vaccine-induced, pseudorabies virus-specific, extrathymic CD4+CD8+ memory T-helper cells in swine. J Virol. 1998;72:4866–73. doi: 10.1128/jvi.72.6.4866-4873.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bevan DJ, Chisholm PM. Co-expression of CD4 and CD8 molecules and de novo expression of MHC class II antigens on activated rat T cells. Immunology. 1986;59:621–5. [PMC free article] [PubMed] [Google Scholar]
- 25.Blue ML, Daley JF, Levine H, Craig KA, Schlossman SF. Biosynthesis and surface expression of T8 by peripheral blood T4+ cells in vitro. J Immunol. 1986;137:1202–7. [PubMed] [Google Scholar]
- 26.Gowans JL, Knight EJ. The route of re-circulation of lymphocytes in the rat. Proc R Soc Lond B Biol Sci. 1964;159:257–82. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- 27.Guy-Grand D, Griscelli C, Vassalli P. The gut-associated lymphoid system: nature and properties of the large dividing cells. Eur J Immunol. 1974;4:435–43. doi: 10.1002/eji.1830040610. [DOI] [PubMed] [Google Scholar]
- 28.Howard JC. The life-span and recirculation of marrow-derived small lymphocytes from the rat thoracic duct. J Exp Med. 1972;135:185–99. doi: 10.1084/jem.135.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sprent J. Fate of H2-activated lymphocytes in syngeneic hosts. Cell Immunol. 1975;21:278–302. doi: 10.1016/0008-8749(76)90057-5. [DOI] [PubMed] [Google Scholar]
- 30.Witherden DA, Kimpton WG, Washington EA, Cahill RN. Non-random migration of CD4+, CD8+ and γδ+ T19+ lymphocytes through peripheral lymph nodes. Immunology. 1990;70:235–40. [PMC free article] [PubMed] [Google Scholar]
- 31.Andrade WN, Johnston MG, Hay JB. The relationship of blood lymphocytes to the recirculating lymphocyte pool. Blood. 1998;91:1653–61. [PubMed] [Google Scholar]
- 32.Reynolds JD. Lymphocyte traffic associated with the gut: a review of in vivo studies in sheep. In: Husband AJ, editor. Migration and homing of lymphoid cells. 2. Boca Raton: CRC Press; 1988. pp. 113–36. [Google Scholar]
- 33.Pabst R, Fritz FJ. Comparison of lymphocyte production in lymphoid organs and their compartments using the metaphase arrest technique. Cell Tissue Res. 1986;245:423–30. doi: 10.1007/BF00213950. [DOI] [PubMed] [Google Scholar]
- 34.Hooper DC, Rubin DH, Cebra JJ. Spontaneous proliferation of Peyer's patch cells in vitro. Int Immunol. 1994;6:873–80. doi: 10.1093/intimm/6.6.873. [DOI] [PubMed] [Google Scholar]
- 35.Rothkötter HJ, Ulbrich H, Pabst R. The postnatal development of gut lamina propria lymphocytes: number, proliferation, and T and B cell subsets in conventional and germ-free pigs. Pediatr Res. 1991;29:237–42. doi: 10.1203/00006450-199103000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Rothkötter HJ, Möllhoff S, Pabst R. The influence of age and breeding conditions on the number and proliferation of intraepithelial lymphocytes in pigs. Scand J Immunol. 1999;49 doi: 10.1046/j.1365-3083.1999.00557.x. in press. [DOI] [PubMed] [Google Scholar]
- 37.Pabst R, Binns RM. Heterogeneity of lymphocyte homing physiology: several mechanisms operate in the control of migration to lymphoid and non-lymphoid organs in vivo. Immunol Rev. 1989;108:83–109. doi: 10.1111/j.1600-065x.1989.tb00014.x. [DOI] [PubMed] [Google Scholar]


