Abstract
We examined the therapeutic effect of heat-killed Lactobacillus casei (LC) on MRL/lpr mice. Ingestion of a diet containing 0.05% (w/w) LC from the weaning period prolonged the lifespan and tended to reduce the proportion of B220+ T cells in the spleen and mesenteric lymph nodes (MLN) of MRL/lpr mice. When LC was intraperitoneally injected once a week after the age of 8 weeks, I-A− macrophages accumulated in the spleen as well as the peritoneum and macrophage progenitors increased in the bone marrow. Moreover, the amount of IL-6 mRNA in peritoneal macrophages was reduced by LC injection. Splenocytes from LC-injected MRL/lpr mice exhibited lower proliferative responses to mitogens than those from control MRL/lpr mice and the increase in number of B220+ T cells in the spleen and MLN was prevented by LC injection. However, LC injection affected neither expression of interferon-gamma (IFN-γ) and IL-4 mRNAs nor proliferative capacities of splenic T cells. Our findings demonstrate that LC injection accelerates macrophage recruitment and prevents the expansion of B220+ T cells without affecting the functions of T cells in MRL/lpr mice. These immunological modulations induced by LC may lead to prolongation of the lifespan of MRL/lpr mice.
Keywords: B220+ T cells, Lactobacillus casei, macrophage, MRL/lpr mice
INTRODUCTION
MRL/Mp-lpr/lpr (MRL/lpr) mice develop autoimmune disease resembling human systemic lupus erythematosus (SLE). Moreover, B220+CD4−CD8− double-negative (DN) T cells increase progressively in peripheral lymphoid organs of MRL/lpr mice with ageing. The observation that MRL/lpr mice thymectomized within 24–48 h after birth survive much longer than non-treated MRL/lpr mice indicates that thymus-derived T cells are involved in the development of autoimmune disease of MRL/lpr mice [1,2]. It has been reported that T cells from MRL/lpr mice express higher level of interferon-gamma (IFN-γ) mRNA but fewer amount of IL-4 mRNA than MRL/+ mice [3]. As the clinical signs associated with nephritis are not detected in either MRL/lpr × IFN-γ−/− mice or MRL/lpr × IFN-γR−/− mice [4,5], IFN-γ produced by T cells is considered to play a critical role in the development of auto-immune disease in MRL/lpr mice.
On the other hand, it has been proposed that macrophages are related to the occurrence of nephropathy in MRL/lpr mice. I-A/I-E+ macrophages accumulate in the peritoneum of MRL/lpr mice [6,7], and the abilities of macrophages to produce IL-1β, IL-6, tumour necrosis factor-alpha (TNF-α), and IL-12 are dysregulated in MRL/lpr mice [8,9]. Moreover, macrophage proliferation at extramedullary sites such as liver, spleen, and kidney is augmented and macrophages activated by colory-stimulating factor-1 (CSF-1) infiltrate into the cortex of the kidney [10,11].
In order to keep MRL/lpr mice from having an attack of autoimmune disease, two categories of treatments were investigated. One trial was aimed at modulating the development of T cells. Survival of MRL/lpr mice was prolonged by injection of anti-Thy-1.2 MoAb [12], and intraperitoneal injection of FK506 prevented the progression of nephropathy and prolonged the lifespan of MRL/lpr mice [13]. Another strategy was focused on controlling the functions of macrophages. Intraperitoneal injection of a heat-killed Streptococcus pyogenes (OK-432) prevented the appearance of proteinuria and prolonged the lifespan of MRL/lpr mice [14]. Additionally, pentoxifylline has been demonstrated to reduce the production by macrophages of superoxide anion and TNF-α and to increase the survival rate of MRL/lpr mice [15].
Lactobacillus casei strain Shirota (LC) has been isolated from faeces of healthy humans. This bacterium has been found to enhance the phagocytic activity, carbon clearance ability, and anti-tumour activity of macrophages in mice [16,17]. In addition, LC injection induces the production of CSF and expands a pool of macrophage progenitors [18–20]. These results indicate that LC can modulate the functions of dysregulated macrophages in MRL/lpr mice.
In the present study, we examined the effect of LC on the immune system of MRL/lpr mice by giving LC orally or intraperitoneally. The results show that ingestion of LC prolongs the lifespan of MRL/lpr mice and LC modulates macrophage recruitment, resulting in the prevention of lymphadenopathy in MRL/lpr mice.
MATERIALS AND METHODS
Mice
MRL/lpr mice (female, 4 weeks old) were purchased from Charles River Japan Inc. (Atsugi, Japan). Mice were killed according to the legislation of animal experiments established by Yakult Central Institute.
Preparation of Lactobacillus casei
LC was cultivated in Rogosa's medium at 37°C for 24 h. After cultivation, LC was washed thoroughly with distilled water, heated at 100°C for 30 min, and lyophilized. To prepare the LC-containing diet, MM-3 powder (Funabashi Farm, Funabashi, Japan) was mixed with LC at 0.05% (w/w), changed into pellets, and sterilized by 60Co irradiation. For i.p. injection, LC was suspended in 0.15 m NaCl (saline).
Preparation of cells
Peritoneal cells were collected by injecting 10 ml of PBS in the peritoneum using a syringe with a 23 G needle. To collect adherent cells, peritoneal cells suspended in PBS were incubated in fetal calf serum (FCS)-coated dishes at 37°C for 30 min, and then non-adherent cells were removed. The same treatment was repeated two more times. After removing non-adherent cells, 5 mm EDTA/5% FCS/PBS was added into the dishes and they were incubated at 4°C for 40 min. Adherent cells were collected by gentle aspiration using a syringe with a 22 G needle.
Spleen was teased over gauze in Hanks' balanced salt solution (HBSS) to obtain single-cell suspensions. After cells were centrifuged, erythrocytes were lysed with a haemolysis buffer. Cells were washed with HBSS and suspended in RPMI 1640 supplemented with 10% FCS and 5 × 10−5m 2-mercaptoethanol (2-ME) (FCS–ME–RPMI). In order to deplete B cells and macrophages, splenocytes were applied to a nylon (NY) column (0.3 g/spleen) and cells passing through the NY column were collected. After the NY column was incubated in PBS at 4°C for 1 h, NY-adherent cells were recovered by squeezing it with forceps.
Mesenteric lymph nodes (MLN) and Peyer's patches were removed with scissors and teased over gauze in HBSS. Cells were washed with HBSS and suspended in FCS–ME–RPMI.
Bone marrow cells were prepared by flushing the bone marrow of femurs with Dulbecca's modified Eagle's medium (DME) using a syringe with a 26 G needle. Erythrocytes were depleted using a haemolysis buffer and cells were suspended in DME supplemented with 5% FCS.
Immunofluorescence analysis
Cells were stained with the following antibodies and analysed by an EPICS Elite flow cytometer: PE-conjugated anti-I-Ak (11-5.2; PharMingen, San Diego, CA), FITC-conjugated anti-CD11b (M1/70; PharMingen), unconjugated or PE-conjugated anti-B220 (RA3-6B2; PharMingen), biotin-conjugated anti-Thy-1.2 (30-H12; Becton Dickinson, San Jose, CA), FITC-conjugated anti-CD3 (145-2C11; PharMingen). Unconjugated anti-B220 antibody was detected by FITC-conjugated anti-rat IgG (Biosource Int., San Jose, CA) and biotin-conjugated anti-Thy-1.2 was detected by PE-conjugated streptavidin (Caltag, Burlingame, CA).
Reverse transcriptase-polymerase chain reaction analysis of cytokine mRNA expression
Total RNA was extracted using ISOGEN (Nippongene, Toyama, Japan). cDNA was synthesized from total RNA by first-strand cDNA synthesis kit (Pharmacia Biotech, Tokyo, Japan). Primer sets used for polymerase chain reaction (PCR) and product size were as follows: β-actin, ATG GAT GAC GAT ATC GCT and ATG AGG TAG TCT GTC AGG T, 570 bp; IL-1β, TGA TGA GAA TGA CCT GTT CT and CTT CTT CAA AGA TGA AGG AAA, 251 bp; IL-6, CTC TGC AAG AGA CTT CCA T and ATA GGC AAA TTT CCT GAT TAT A, 320 bp; TNF-α, ATG AGC ACA GAA AGC ATG ATC and AGA TGA TCT GAG TGT GAG GG, 249 bp; transforming growth factor-beta (TGF-β), CGG GGC GAC CTG GGC ACC ATC CAT GAC and CTG CTC CAC CTT GGG CTT GCG ACC CAC, 406 bp; IL-2, GTC AAC AGC GCA CCC ACT TCA AGC and GCT TGT TGA GAT GAT GCT TTG ACA, 451 bp; IFN-γ, TAC TGC CAC GGC ACA GTC ATT GAA and GCA GCG ACT CCT TTT CCG CTT CCT, 405 bp; IL-4, ACG GAG ATG GAT GTG CCA AAC GTC and CGA GTA ATC CAT TTG CAT GAT GC, 279 bp; IL-10, ATG CAG GAC TTT AAG GGT TAC TTG GGT T and ATT TCG GAG AGA GGT TAC AAA CGA GGT TT, 455 bp. After PCR products were separated by agarose gel electrophoresis and stained in 0.2 μg ethidium bromide/ml, the relative intensity of each band to β-actin band was analysed by the Bio-Profile Bio-1D System using Bio-1D V.96 Software (Vilber Lourmat, Marne la Vallée, France).
Proliferation assay
Responder cells suspended in FCS–ME–RPMI were added at 5 × 105 cells/well of a 96-well flat-bottomed microtitre plate and cultured in the absence or presence of concanavalin A (Con A) (at 5 μg/ml; Sigma, St Louis, MO), lipopolysaccharide (LPS) (at 5 μg/ml; Difco, Detroit, MI), or pokeweed mitogen (PWM) (at 0.2%; Difco) for 48 h. For stimulation with immobilized anti-T cell receptor (TCR) MoAbs, each well of a 96-well flat-bottomed microtitre plate was coated by adding 30 μl of the solution of anti-TCRβ (H57-597; PharMingen) and anti-TCRδ (GL-3; PharMingen) MoAbs (each at 10 μg/ml). After washing wells with PBS five times, 5 × 105 responder cells were added and cultured for 48 h. Cells were pulsed with 0.5 μCi 3H-thymidine for the last 8 h and the uptake was measured by a β-emission scintillation counter.
Measurement of macrophage colony-forming cells
Macrophage colony-forming cells (M-CFC) were measured by using clonal culture in semisolid agar medium [21]. An adequate number of bone marrow cells were suspended in 1 ml of DME supplemented with 20% FCS, l-asparagine at 20 μg/ml, 0.3% bacto agar (Difco), and 10% L929 cell-conditioned medium (crude M-CSF) [22] and poured in 35-mm tissue culture dishes. After cultivation for 7 days, the number of M-CFC was counted under an inverted microscope.
Statistical analysis
Difference of survival curve was assessed by the Mann–Whitney two-sample rank test. Statistical significance of difference in other experiments was evaluated by Student's t-test.
RESULTS
Growth and lifespan of MRL/lpr mice fed with control and LC-containing diets
MRL/lpr mice were allowed to take control diet or LC diet after the weaning time. The content of LC in the LC diet was fixed at 0.05% (w/w), because commercially available dairy products usually contain lactic acid bacteria at 0.05% in weight. Changes of body weight were almost comparable between control and LC groups (Fig. 1a). Mice in the control group started to die at 9 weeks old and half of them died by 34 weeks. In contrast, the onset of death was delayed by ingesting LC diet and the mean survival time of LC-fed mice (47 weeks) was significantly longer than that of control mice (Fig. 1b; P < 0.05).
Fig. 1.
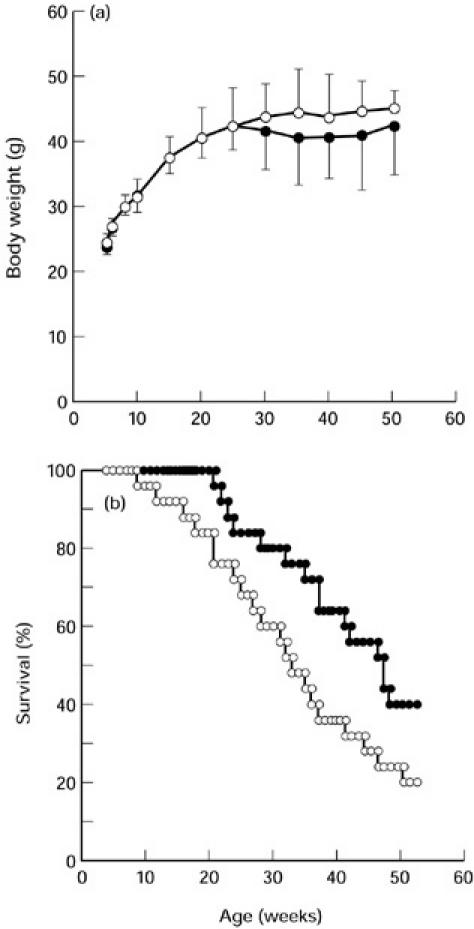
Growth (a) and survival (b) of MRL/lpr mice fed with control (○) or Lactobacillus casei(LC)-containing diets (•). Each group included 25 MRL/lpr mice. A representative result out of three experiments is shown.
The T cell constitution in various lymphoid organs was analysed at 22–24 weeks old when LC-fed mice started to die. Ingestion of LC diet tended to reduce the proportion of B220+ T cells in both spleen and MLN, although the T cell constitution in Peyer's patches was not affected by LC ingestion (Fig. 2). Moreover, the average titre of anti-ssDNA IgG in serum was 10-fold less at age 22–24 weeks in LC-fed MRL/lpr mice than in control MRL/lpr mice (data not shown).
Fig. 2.
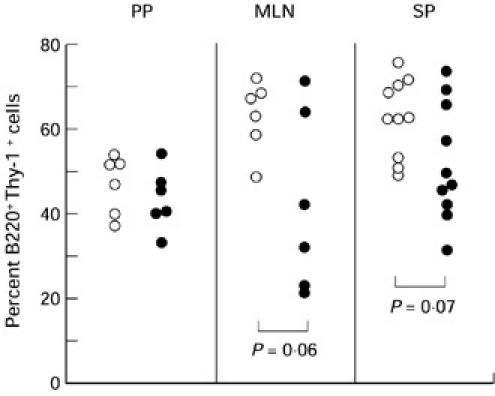
Effect of ingestion of Lactobacillus casei(LC)-containing diet on the proportion of B220+ T cells. Peyer's patches (PP), mesenteric lymph nodes (MLN), and spleen (SP) were individually isolated from MRL/lpr mice fed with control (○) or LC diet (•) at 22–24 weeks old.
Constitution of macrophages and T cells in saline- and LC-injected MRL/lpr mice
To unravel how LC prevents the expansion of B220+ T cells, LC was intraperitoneally injected into MRL/lpr mice. After LC was injected at 1 mg/kg, 5 mg/kg, or 20 mg/kg once a week from 8 weeks old, only the injection at a dose of 20 mg/kg decreased the proportion of B220+ T cells in the spleen at 16 weeks old (data not shown). Therefore, we determined to inject LC at 20 mg/kg intraperitoneally into MRL/lpr mice once a week from 8 weeks old in the following experiments.
LC injection induced the accumulation of I-A−CD11b+ cells in the peritoneum at 12 weeks old, and this change was still observed at 16 weeks old. While the number of splenocytes and MLN cells increased with ageing in saline-injected MRL/lpr mice, spleno-megaly and lymphadenopathy from 12 to 16 weeks old was completely prevented by LC injection. During this period, the proportion of B220+ T cells in the spleen was also maintained in LC-injected mice, while these T cells vigorously expanded in saline-injected mice. In contrast, LC injection slightly decreased the proportion of B220+ T cells in MLN (Fig. 3). The proportion of CD4−CD8− T cells in the spleen of LC-injected mice was also lower at 16 weeks old than that of saline-injected mice (data not shown).
Fig. 3.
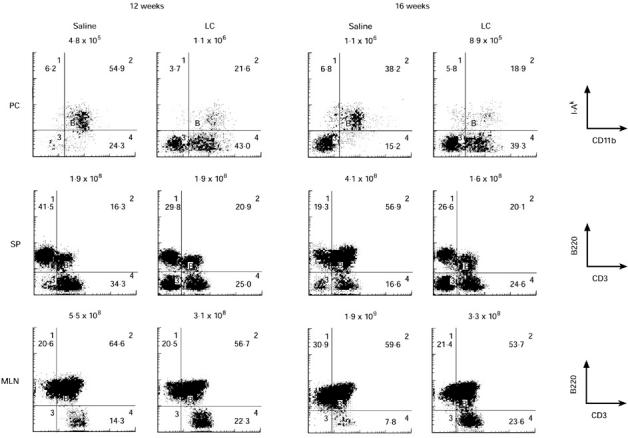
Immunofluorescence analysis of peritoneal adherent cells (PC), splenocytes (SP), and mesenteric lymph node cells (MLN) prepared from saline- or Lactobacillus casei(LC)-injected MRL/lpr mice at 12 and 16 weeks old. Numbers above each figure represent the absolute cell number per mouse. The results represent one of three experiments.
Immunological function of macrophages and T cells in saline- and LC-injected MRL/lpr mice
In order to examine the immunological competence of freshly isolated macrophages and T cells in saline- and LC-injected MRL/lpr mice, total RNAs were extracted from peritoneal adherent cells (PC), NY-passed fraction of splenocytes (including CD3+ cells by 86.5 ± 3.0% in saline-injected mice and by 84.9 ± 5.7% in LC-injected mice, respectively), and MLN cells of MRL/lpr mice at 12 and 16 weeks old, and then reverse transcriptase (RT)-PCR analysis was carried out. The amount of IL-6 mRNA in PC was consistently reduced by LC injection, while the relative intensity of mRNAs of IL-1β, TNF-α and TGF-β in PC was comparable between saline- and LC-injected groups. LC injection did not change the amount of IL-2, IFN-γ, and IL-4 mRNAs expressed by splenic T cells, but IL-10 mRNA expression by splenic T cells was slightly reduced by LC injection. In contrast, MLN cells from saline- and LC-injected mice expressed comparable amounts of IL-2, IFN-γ, IL-4, and IL-10 mRNAs (Fig. 4).
Fig. 4.
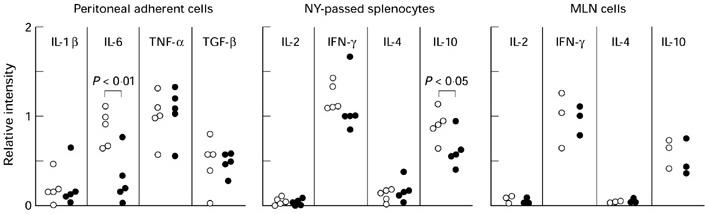
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of cytokine mRNA expression in macrophages and T cells from saline- (○) or Lactobacillus casei(LC)-injected (•) MRL/lpr mice. The relative intensity of each lymphokine band to β-actin band is shown. As the data on mice at 12 and 16 weeks old were indistinguishable, all the results obtained in five experiments (peritoneal adherent cells and nylon (NY)-passed splenocytes) and three experiments (mesenteric lymph node (MLN) cells) were collected.
The effect of LC injection on the proliferative capacities of peripheral lymphocytes in MRL/lpr mice was examined at 12 weeks old when the prevention of lymphadenopathy by LC injection began. Splenocytes from LC-injected mice cultured in the presence of Con A, LPS, PWM, or immobilized anti-TCR MoAbs exhibited markedly lower proliferative responses than those from saline-injected mice. In contrast, MLN cells from saline- and LC-injected MRL/lpr mice proliferated equivalently in response to these stimuli (Figs 5 and 6a).
Fig. 5.
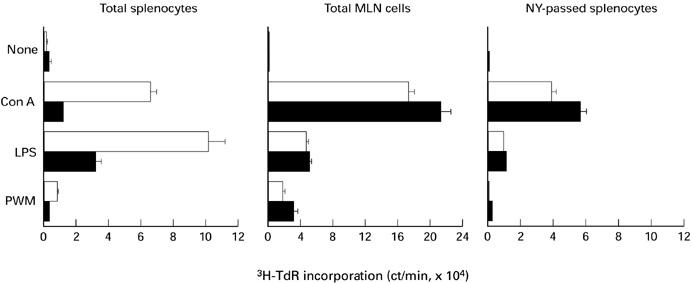
Proliferative response of splenocytes and mesenteric lymph node (MLN) cells from saline- (□) or Lactobacillus casei(LC)-injected (▪) MRL/lpr mice. Cells were cultured in the absence or presence of concanavalin a (Con A; 5 μg/ml), lipopolysaccharide (LPS; 5 μg/ml), or pokeweed mitogen (PWM; 0.2%) for 48 h and pulsed with 0.5 μCi of 3H-thymidine for the last 8 h. Data show mean ± s.d. of triplicate wells. The results represent one of three experiments.
Fig. 6.
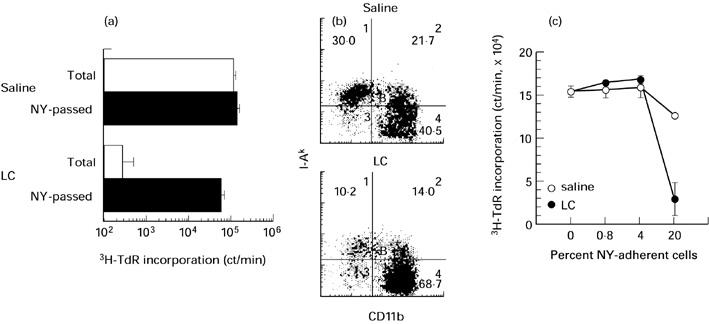
Down-modulation of the proliferative response of splenic T cells by nylon (NY)-adherent cells from Lactobacillus casei(LC)-injected MRL/lpr mice. (a) Proliferative response of total and NY-passed splenocytes to immobilized anti-T cell receptor (TCR)β plus anti-TCRδ MoAbs. (b) Immunofluorescence analysis of NY-adherent splenocytes from saline- and LC-injected MRL/lpr mice. (c) Effect of addition of NY-adherent splenocytes on anti-TCRβ plus anti-TCRδ MoAb-triggered proliferation of NY-passed splenocytes.
We checked the possibility that hyporesponsiveness of splenocytes in LC-injected MRL/lpr mice may have been due to the coexistence of immunoregulatory macrophages induced by LC injection. To address this issue, NY-adherent cells were removed from splenocytes and the proliferative response of NY-passed fraction was measured. NY-passed fraction of splenocytes from saline- and LC-injected MRL/lpr mice proliferated similarly when stimulated by either Con A or immobilized anti-TCR MoAbs (Figs 5 and 6a).
Immunofluorescence analysis of NY-adherent cells revealed that the proportion of I-A−CD11b+ cells was more in LC-injected mice than in saline-injected mice (Fig. 6b). Furthermore, NY-adherent cells from LC-injected mice down-regulated the proliferation of NY-passed cells more efficiently than the counterparts from saline-injected mice when added at the ratio of 20% (Fig. 6c).
Macrophage progenitors in bone marrow of saline- and LC-injected MRL/lpr mice
Finally, we investigated the effect of LC injection on a pool size of macrophage progenitors in the bone marrow. Saline-injected MRL/lpr mice included 4–7 × 104 M-CFC per femur when they were from 8 to 16 weeks old. LC injection increased the number of M-CFC by 1.5–1.7-fold during the period tested (Fig. 7).
Fig. 7.
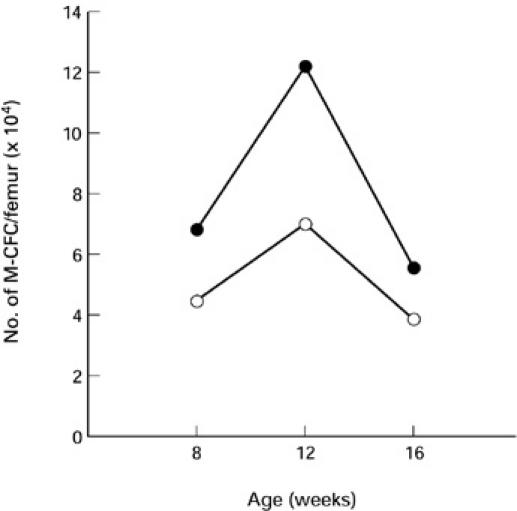
Enlargement of a pool size of macrophage colony-forming cells (M-CFC) in the bone marrow by Lactobacillus casei(LC) injection into MRL/lpr mice. Bone marrow cells from the femur were cultured in semisolid agar medium containing M-CSF for 7 days and the numbers of colonies formed were counted. ○, Saline; •, LC.
DISCUSSION
The results of this study show that ingestion of a diet containing LC prolongs the lifespan of MRL/lpr mice and tends to prevent the expansion of B220+ T cells. Intraperitoneal injection of LC into MRL/lpr mice down-modulated the proliferative capacities of splenocytes and arrested the accumulation of B220+ DN T cells with increasing I-A− macrophages and M-CFC. As hyporesponsiveness of splenocytes in LC-injected MRL/lpr mice was reversed by removal of NY-adherent cells, we propose that LC accelerates the recruitment of macrophages and then prevents the massive expansion of B220+ DN T cells in MRL/lpr mice.
Generation of macrophages in MRL/lpr mice is already accelerated in liver, spleen, and bone marrow at 4 weeks old when the signs of autoimmune disease are not discernible [10]. Additionally, most peritoneal macrophages in MRL/lpr mice express I-A/I-E molecules [6,7], and the production of inflammatory cytokines (IL-1β, IL-6, TNF-α) is augmented in MRL/lpr mice [9,23,24]. Injection of LC into BALB/c mice induces the increase of M-CFC and enhances I-A expression on macrophages [17–20,25]. In contrast, injection of LC into MRL/lpr mice induced the accumulation of I-A− macrophages in the peritoneum and spleen together with the increment of M-CFC. Therefore, I-A− macrophages are activated in response to LC to express I-A [17], but I-A+ macrophages decline and may be replaced by I-A− macrophages newly generated from M-CFC after LC injection. Moreover, the finding that the amount of IL-6 mRNA by peritoneal adherent cells in LC-injected MRL/lpr mice was consistently less than that in control mice indicates that I-A expression and IL-6 production by macrophages may be closely related in MRL/lpr mice. However, it is unclear whether LC can decrease IL-6 mRNA expression by I-A+ macrophages or LC induces I-A− macrophages expressing IL-6 mRNA at lower level.
B220+ DN T cells expanding in MRL/lpr mice express large amounts of IFN-γ mRNA, and analysis of MRL/lpr × IFN-γ−/− mice or MRL/lpr × IFN-γR−/− mice revealed that IFN-γ is critical in the development of nephritis in MRL/lpr mice [3–5]. However, LC injection did not affect the transcription of IFN-γ gene by T cells in MRL/lpr mice, although administration of LC prolonged the lifespan of MRL/lpr mice and prevented the expansion of B220+ DN T cells. Therefore, the therapeutic effect afforded by LC in MRL/lpr mice should not be caused by the down-modulation of IFN-γ gene expression by T cells. In contrast, LC injection reduced IL-10 mRNA expression by splenic T cells. In passing, B220+ DN T cells but not CD4+ T cells from MRL/lpr mice express IL-10 mRNA [3]. Since LC injection decreased the proportion of B220+ DN T cells in the spleen, it is likely that the reduction of IL-10 mRNA in total splenic T cells from LC-injected MRL/lpr mice is due to the decrease of the relative ratio of B220+ DN T cells. Taking all data together, we assume that LC injection into MRL/lpr mice prevents the expansion of B220+ DN T cells, leaving their capacities to express lymphokine genes unaltered.
Do macrophages induced by LC injection prevent the expansion of B220+ DN T cells? As the incubation of splenic T cells from MRL/lpr mice in the presence of LC in vitro did not decrease the proportion of B220+ T cells (M. Miyashita, unpublished data), it is unlikely that LC prevents the expansion of B220+ DN T cells directly. We have previously shown that LC injection into BALB/c mice induces an increase of Mac-2+ macrophages in the spleen, and bone marrow-derived Mac-2+ macrophages generated in the presence of CSF-1 down-modulate the growth of Meth A tumour cells [25]. Recently, it has been reported that Mac-2+ macrophages from tumour-bearing mice induce the loss of CD3ζ molecule in T cells and block antigen-triggered T cell proliferation [26,27]. Although the antigens recognized by B220+ DN T cells in MRL/lpr mice are still undefined, it is possible that they may be activated through TCR-mediated pathway in vivo and vigorously proliferate without apoptosis in MRL/lpr mice. This dysregulated proliferation of B220+ DN T cells may be down-modulated by macrophages induced by LC. Our results show that LC injection into MRL/lpr mice induces macrophages in the spleen capable of down-modulating TCR-triggered proliferation of T cells. LPS-activated macrophages secrete H2O2 which down-modulates the expression of CD3ζ molecule in T cells [27,28]. Whether macrophages induced by LC injection in MRL/lpr mice prevent the proliferation of B220+ DN T cells in the same way remains to be elucidated.
In this study we show that LC manifests a therapeutic effect on MRL/lpr mice through modulating the recruitment of macrophages. Other investigators have tried to rescue MRL/lpr mice from autoimmune disease by neonatal thymectomy, injection of anti-Thy-1.2 MoAb, or treatment with FK506 [2,12,13]. These T cell-specific treatments and LC injection exert therapeutic effects in a totally distinct way and may act synergistically. Our experiments suggest that LC in dairy products is worth investigating as a potential autoimmune disease modifier in man.
Acknowledgments
We would like to thank Drs Toshiaki Osawa, Yoshiaki Matsuoka, and Ikuo Kato for their critical reading of the manuscript and valuable suggestions, Dr Tamotsu Setoyama for his kind gift of LC, and Mrs Yuriko Nagata for her technical assistance.
REFERENCES
- 1.Theofilopoulos AN, Balderas RS, Shawler DL, Lee S, Dixon FJ. Influence of thymic genotype on the systemic lupus erythematosus-like disease and T cell proliferation of MRL/Mp-lpr/lpr mice. J Exp Med. 1981;153:1405–14. doi: 10.1084/jem.153.6.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hang L, Theofilopoulos AN, Balderas RS, Francis SJ, Dixon FJ. The effect of thymectomy on lupus-prone mice. J Immunol. 1984;132:1809–13. [PubMed] [Google Scholar]
- 3.Takahashi S, Fossati L, Iwamoto M, Merino R, Motta R, Kobayakawa T, Izui S. Imbalance towards Th1 predominance is associated with acceleration of lupus-like autoimmune syndrome in MRL mice. J Clin Invest. 1996;97:1597–604. doi: 10.1172/JCI118584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng SL, Moslehi J, Craft J. Roles of interferon-γ and interleukin-4 in murine lupus. J Clin Invest. 1997;99:1936–46. doi: 10.1172/JCI119361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haas C, Ryffel B, Hir ML. IFN-γ is essential for the development of autoimmune glomerulonephritis in MRL/lpr mice. J Immunol. 1997;158:5484–91. [PubMed] [Google Scholar]
- 6.Kelley VE, Roths JB. Increase in macrophage Ia expression in autoimmune mice: role of the lpr gene. J Immunol. 1982;129:923–5. [PubMed] [Google Scholar]
- 7.Kofler R, Schreiber RD, Dixon FJ, Theofilopoulos AN. Macrophage I-A/I-E expression and macrophage-stimulating lymphokines in murine lupus. Cell Immunol. 1984;87:92–100. doi: 10.1016/0008-8749(84)90133-3. [DOI] [PubMed] [Google Scholar]
- 8.Levine J, Hartwell D, Beller DI. Imbalanced cytokine production by macrophages from autoimmune-prone mice. Immunol Letters. 1991;30:183–92. doi: 10.1016/0165-2478(91)90023-4. [DOI] [PubMed] [Google Scholar]
- 9.Mustafa W, Zhu J, Deng G. Augmented levels of macrophage and Th1 cell-related cytokine mRNA in submandibular glands of MRL/lpr mice with autoimmune sialoadenitis. Clin Exp Immunol. 1998;112:389–96. doi: 10.1046/j.1365-2249.1998.00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Müller M, Emmendörffer A, Lohmann-Matthes M-L. Expansion and high proliferative potential of the macrophage system throughout life time of lupus-prone NZB/W and MRL lpr/lpr mice. Lack of down- regulation of extramedullar macrophage proliferation in the postnatal period. Eur J Immunol. 1991;21:2211–7. doi: 10.1002/eji.1830210932. [DOI] [PubMed] [Google Scholar]
- 11.Moore KJ, Naito T, Martin C, Kelley VR. Enhanced response of macrophages to CSF-1 in autoimmune mice. J Immunol. 1996;157:433–40. [PubMed] [Google Scholar]
- 12.Wofsy D, Ledbetter JA, Hendler PL, Seaman WE. Treatment of murine lupus with monoclonal anti-T cell antibody. J Immunol. 1985;134:852–7. [PubMed] [Google Scholar]
- 13.Takabayashi K, Koike T, Kurasawa K, et al. Effect of FK-506, a novel immunosuppressive drug on murine systemic lupus erythematosus. Clin Immunol Immunopathol. 1989;51:110–7. doi: 10.1016/0090-1229(89)90211-0. [DOI] [PubMed] [Google Scholar]
- 14.Mihara M, Ohsugi Y. Autoimmune kidney disease in MRL/Mp-lpr/lpr mice inhibited by OK-432, a streptococcal preparation. Clin Exp Immunol. 1989;78:102–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Hecht M, Müller M, Lohmann-Matthes M-L, Emmendörffer A. In vitro and in vivo effects of pentoxifylline on macrophages and lymphocytes derived from autoimmune MRL-lpr/lpr mice. J Leuk Biol. 1995;57:242–9. doi: 10.1002/jlb.57.2.242. [DOI] [PubMed] [Google Scholar]
- 16.Kato I, Yokokura T, Mutai M. Macrophage activation by Lactobacillus casei in mice. Microbiol Immunol. 1983;27:611–8. doi: 10.1111/j.1348-0421.1983.tb00622.x. [DOI] [PubMed] [Google Scholar]
- 17.Kato I, Yokokura T, Mutai M. Correlation between increase in Ia- bearing macrophages and induction of T-cell-dependent antitumor activity by Lactobacillus casei in mice. Cancer Immun Immunother. 1988;26:215–21. doi: 10.1007/BF00199932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yokokura T, Nomoto K, Shimizu T, Nomoto K. Enhancement of hematopoietic response of mice by subcutaneous administration of Lactobacillus casei. Infect Immun. 1986;52:156–60. doi: 10.1128/iai.52.1.156-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nanno M, Ohwaki M, Mutai M. Induction by Lactobacillus casei of increase in macrophage colony-forming cells and serum colony- stimulating activity in mice. Jpn J Cancer Res. 1986;77:703–10. [PubMed] [Google Scholar]
- 20.Nanno M, Shimizu T, Mike A, Ohwaki M, Mutai M. Role of macrophages in serum colony-stimulating factor induction by Lactobacillus casei in mice. Infect Immun. 1988;56:357–62. doi: 10.1128/iai.56.2.357-362.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradley TR, Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966;44:287–300. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- 22.Stanley ER, Heard PM. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977;252:4305–12. [PubMed] [Google Scholar]
- 23.Tang B, Matsuda T, Akira S, Nagata N, Ikehara S, Hirano T, Kishimoto T. Age-associated increase in interleukin 6 in MRL/lpr mice. Int Immunol. 1991;3:273–8. doi: 10.1093/intimm/3.3.273. [DOI] [PubMed] [Google Scholar]
- 24.Hamano H, Saito I, Haneji N, Mitsuhashi Y, Miyasaka N, Hayashi Y. Expression of cytokine genes during development of autoimmune sialadenitis in MRL/lpr mice. Eur J Immunol. 1993;23:2387–91. doi: 10.1002/eji.1830231002. [DOI] [PubMed] [Google Scholar]
- 25.Nanno M, Shimizu-Takeda T, Mike A, Ohwaki M, Togashi Y, Suzuki R, Kumagai K, Mutai M. Increased production of cytotoxic macrophage progenitors by Lactobacillus casei in mice. J Leuk Biol. 1989;46:89–95. doi: 10.1002/jlb.46.2.89. [DOI] [PubMed] [Google Scholar]
- 26.Aoe T, Okamoto Y, Saito T. Activated macrophages induce structural abnormalities of the T cell receptor–CD3 complex. J Exp Med. 1995;181:1881–6. doi: 10.1084/jem.181.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otsuji M, Kimura Y, Aoe T, Okamoto Y, Saito T. Oxidative stress by tumor-derived macrophages suppresses the expression of CD3ζ chain of T-cell receptor complex and antigen-specific T-cell responses. Proc Natl Acad Sci USA. 1996;93:13119–24. doi: 10.1073/pnas.93.23.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kono K, Salazar-Onfray F, Patersson M, et al. Hydrogen peroxide secreted by tumor-derived macrophages down-modulates signal- transducing zeta molecules and inhibits tumor-specific T cell- and natural killer cell-mediated cytotoxicity. Eur J Immunol. 1996;26:1308–13. doi: 10.1002/eji.1830260620. [DOI] [PubMed] [Google Scholar]


