Abstract
Chronic helminth infection induces a type-2 cellular immune response. In contrast to this, mycobacterial infections commonly induce a type-1 immune response which is considered protective. Type-2 responses and diminished type-1 responses to mycobacteria have been previously correlated with active infection states such as pulmonary tuberculosis and lepromatous leprosy. The present study examines the immune responses of children exposed to both the helminth parasite Onchocerca volvulus and the mycobacterial infections, Mycobacterium tuberculosis and M. leprae. Proliferation of peripheral blood mononuclear cells (PBMC) and production of IL-4 in response to both helminth and mycobacterial antigen (PPD) decreased dramatically with increasing microfilarial (MF) density. Although interferon-gamma (IFN-γ) production strongly correlated with cellular proliferation, it was surprisingly not related to MF density for either antigen. IL-4 production in response to helminth antigen and PPD increased with ascending children's age. IFN-γ and cellular proliferation to PPD were not related to age, but in response to helminth antigen were significantly higher in children of age 9–12 years than children of either the younger age group (5–8 years) or the older group (13–16 years). Thus, there was a MF density-related down-regulation of cellular responsiveness and age-related skewing toward type 2 which was paralleled in response to both the helminth antigen and PPD. This parasite-induced immunomodulation of the response to mycobacteria correlates with a previous report of doubled incidence of lepromatous leprosy in onchocerciasis hyperendemic regions. Moreover, this demonstration that helminth infection in humans can modulate the immune response to a concurrent infection or immunological challenge is of critical importance to future vaccination strategies.
Keywords: Onchocerca volvulus, helminth, mycobacterium, PPD, immune response
INTRODUCTION
The filarial nematode Onchocerca volvulus is a large multicellular parasite that can cause persistent and debilitating disease in individuals from endemic areas. The adult worms can reside in the dermis for up to 15 years [1] and a single female may produce 0.7–1.5 × 103 microfilariae per day [2]. The success of this physically conspicuous parasite relies upon its ability to modulate the host immune response to its favour. The peripheral blood mononuclear cells (PBMC) of onchocerciasis patients with generalized microfiladermia show minimal responsiveness to parasite antigens [3–6], suggesting specific T cell tolerance to parasite antigens. It has also been suggested that patent O. volvulus infection induces a parasite-specific immune response which is biased to a type-2 pathway [7,8]. However, the kinetics of the cellular response with respect to parasite intensity and host age has not been previously elucidated. To resolve this we examined the in vitro responses of PBMC from children resident in the Sanaga valley of Cameroon. This region is hyperendemic for onchocerciasis and the intensity of infection in such regions is known to increase from early childhood well into adulthood [9]. Thus, these children, aged 5–16 years, provided a key age profile for the examination of the dynamics of the immune response through the early development of O. volvulus infection. The study region is also endemic for the mycobacterial diseases tuberculosis and leprosy, and so provided the opportunity to examine how O. volvulus-driven immunomodulation affects the immune response to unrelated but co-endemic infectious agents.
SUBJECTS AND METHODS
Study population
In vitro cellular immune responses of PBMC from children (n = 50), aged 5–16 years, resident in or near Ntsan-Mendouga in the Sanaga valley of Cameroon were examined. This region is hyperendemic for onchocerciasis and is co-endemic for the mycobacterial diseases tuberculosis and leprosy, caused by Mycobacterium tuberculosis and M. leprae. All of the study individuals have lived in this hyperendemic area for their entire lives and none had previously received chemotherapy for onchocerciasis. Levels of microfilariae were assessed by bilateral standardized skin-snip taken with a sclerocorneal punch at the iliac crests. Examination of medical records and vaccination scars demonstrated that the study population was heterogeneous in terms of bacille Calmette–Guérin (BCG) vaccination status, with 46% vaccinated, 18% non-vaccinated and 36% of unknown status. All participants were offered appropriate ivermectin treatment.
Antigens and mitogen
A saline extract of adult female O. volvulus antigens (OvAg) was prepared as previously described [3]. Mycobacterium tuberculosis PPD was obtained from Connaught Laboratories (Willowdale, Ontario, Canada) and phytohaemagglutinin (PHA) from Burroughs Wellcome (Research Triangle Park, NC).
Cell proliferation and cytokine production
Whole blood (10–20 ml) was drawn from each individual and PBMC were separated on a Ficoll–diatrizoate gradient and cultured at 2 × 105 cells/0.2 ml per well essentially as described [7]. Cells were stimulated with OvAg 5 μg/ml, PPD 10 μg/ml, PHA 1:100 or medium alone for 5 days and proliferation was measured by 3H-thymidine incorporation [7]. Values of proliferation are expressed as Δct/min obtained by subtraction of 3H-thymidine incorporation of cells in medium alone from incorporation in stimulated cells.
For cytokine analysis, PBMC were cultured at 2 × 105 cells/0.2 ml per well with OvAg, PPD, PHA or medium alone and supernatants were harvested at 2 days for IL-4 and 5 days for interferon-gamma (IFN-γ). Measurement of IL-4 and IFN-γ levels was by capture ELISA on cell supernatants as described [10].
Statistical analysis
The data were found to be over-dispersed, which means the underlining error structure was not strictly Poisson. Consequently we assumed an error structure with an empirical scale parameter. The scale parameter is the ratio of residual deviance to the number of residual degrees of freedom. The scale parameter was approximately 70 with the residual deviance being in the order of 3500 with between 46 and 48 degrees of freedom depending on the model. Terms fitted to the model were host age, sex, BCG vaccination status (positive, negative and unknown) and mean number of skin microfilariae (MF) per snip. Age was considered in three ways: as a linear variate, a quadratic variate and a categorical variable (three levels: 5–8 years, 9–12 years and 13–16 years). No model could include age as both a continuous variable and a categorical variable. Age was considered as a categorical variable as well as a continuous variable in order to highlight any non-linear age effects. The levels of the categorical variable were chosen by fitting all ages as a categorical variable and then grouping ages by examination of the regression estimates. Up to second order interaction terms were included in the analyses with terms remaining in the model if significant at P < 0.05. The resultant predicted values have been displayed graphically as two-dimensional plots when no interaction terms were significant and three-dimensional plots when interaction terms were significant. Error bars represent s.e.m. around the predictions.
RESULTS
Proliferative responses
The proliferative response of PBMC stimulated with OvAg was influenced by both age and level of MF (Fig. 1a). Children of age 9–12 years had significantly higher proliferative responses than children of either the younger age group (5–8 years) or older group (13–16 years). There was also a down-regulatory effect of MF intensity on OvAg-stimulated proliferation and this was evident throughout the age range. Proliferation in response to PPD was not influenced by age, but it showed a strong inverse relationship to the level of MF (Fig. 1b). Mitogen-stimulated proliferative responses significantly increased with age but were unaffected by MF intensity (Fig. 1c), thus demonstrating the antigen-specific nature of the OvAg and PPD MF-related down-regulation.
Fig. 1.
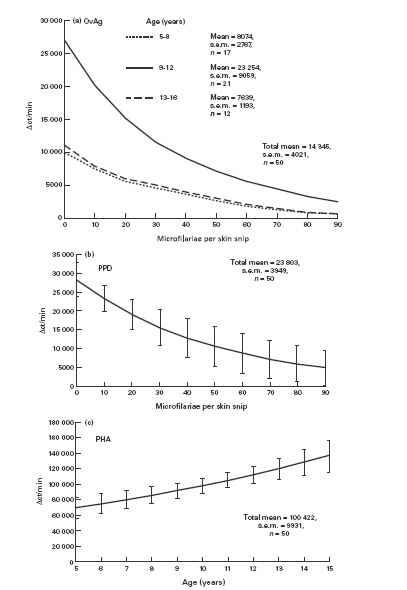
Proliferation of peripheral blood mononuclear cells (PBMC) from children resident in a region hyperendemic for onchocerciasis. PBMC were stimulated with: (a) adult female Onchocerca volvulus antigen (OvAg); (b) Mycobacterium tuberculosis PPD; (c) phytohaemagglutinin (PHA). (a) OvAg-stimulated cells were significantly influenced by age category and microfilariae (MF) (χ2 = 4.6, d.f. = 2 and χ2 = 4.5, d.f. = 2, respectively; P < 0.05 in both cases). (b) PPD-stimulated cells were significantly influenced by MF (χ2 = 4.18, d.f. = 1.48; P < 0.05). (c) PHA-stimulated cells were influenced by age as a linear variate (χ2 = 4.96, d.f. = 1; P < 0.05). Mean ct/min in medium alone = 652, s.e.m. = 98.
Neither BCG vaccination status nor sex had any significant influence on proliferative responses to any of the antigens/mitogen or on MF levels.
IL-4 and IFN-γ responses
IL-4 production by PBMC in response to OvAg was significantly influenced by age and MF intensity (Fig. 2a). IL-4 production was positively related to age group with no detectable production by children of the youngest age group (5–8 years) but detectable IL-4 production in age group 9–12 years and significantly higher production in the oldest group (13–16 years). There was a significant negative relationship between IL-4 production and level of MF. PPD-stimulated IL-4 production showed similar patterns of influence to OvAg-specific IL-4. Age as a linear variate had a positive relationship with PPD-specific IL-4 production but a negative relationship with MF intensity (Fig. 2b). Neither BCG vaccination status nor sex had any significant influence on OvAg- or PPD-stimulated IL-4 production. PHA-stimulated IL-4 was not influenced by age, MF or BCG vaccination status, but males produced significantly more IL-4 than females (χ2 = 5.17, d.f. = 1; P < 0.05).
Fig. 2.
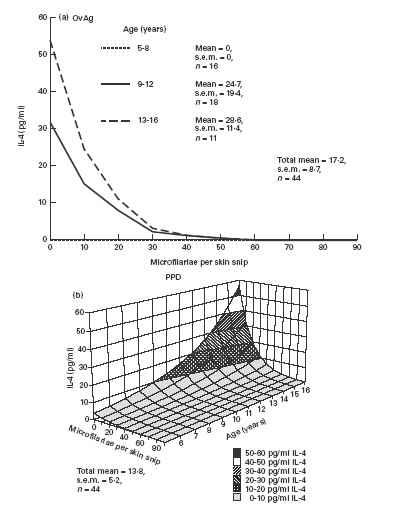
IL-4 production by peripheral blood mononuclear cells (PBMC) stimulated with: (a) adult female Onchocerca volvulus antigen (OvAg); (b) mycobacterial antigen (PPD). (a) Age as a categorical variable and intensity of microfilariae (MF) significantly influenced OvAg-stimulated IL-4 production (χ2 = 5.1, d.f. = 2 and χ2 = 7.3, d.f. = 2, respectively; P < 0.05 in both cases). (b) Both age as a linear variate and intensity of MF influenced PPD-stimulated IL-4 production (χ2 = 7.2, d.f. = 2 and χ2 = 5.3, d.f. = 2, respectively; P < 0.05 in both cases).
Age was influential to OvAg-stimulated IFN-γ production, and like the proliferative response to OvAg children of age 9–12 years had significantly higher responses than children of either the younger age group (5–8 years) or the older group (13–16 years) (Fig. 3a). BCG-vaccinated individuals produced higher levels of PPD-specific IFN-γ than non-vaccinated or unknown status children (Fig. 3b), but surprisingly there was no significant MF-related decrease in either PPD- or OvAg-specific IFN-γ. However, there was a downward trend in response to both antigens (0.05 < P < 0.1) and IFN-γ production positively correlated with proliferative responses for both worm and mycobacterial antigens (P < 0.01 in both cases; r2 = 0.9 and 0.39, respectively). Males produced more PPD-specific IFN-γ than females. PHA-stimulated IFN-γ was not influenced by age, MF, BCG vaccination status or sex.
Fig. 3.

IFN-γ production by peripheral blood mononuclear cells (PBMC) stimulated with: (a) adult female Onchocerca volvulus antigen (OvAg); (b) mycobacterial antigen (PPD). (a) Age was significantly influential to OvAg-stimulated IFN-γ production both as a quadratic variate (χ2 = 6.7, d.f. = 2, P < 0.05) and a categorical variable (χ2 = 6.4, d.f. = 2, P < 0.05). (b) PPD-stimulated IFN-γ production was significantly influenced by bacille Calmette–Guérin (BCG) vaccination status, b (χ2 = 4.2, d.f. = 1, P < 0.05), and also by sex with males producing higher levels of IFN-γ (χ2 = 7.3, d.f. = 1, P < 0.05). Error bars = s.e.m.
DISCUSSION
This study utilizes powerful generalized linear modelling [11] to analyse the immune responses of children infected with onchocerciasis and exposed to the mycobacterial infections tuberculosis and leprosy. It has previously been shown that individuals with patent onchocerciasis or lymphatic filariasis show cellular hyporesponsiveness to both parasite and non-parasite antigens in proliferative assays and defects in DTH skin tests [12–17]. Our findings concur with this, showing that in children with O. volvulus microfiladermia, the proliferation of PBMC in response to stimulation with both adult female parasite antigen (OvAg) and mycobacterial antigen (PPD) was down-regulated with increasing intensity of skin MF. It is of particular note that, although BCG-vaccinated individuals tended to have higher PPD-specific proliferative responses, the MF-related down-regulatory effect was independent of BCG vaccination status.
The present study dissected this down-regulation further by examining IL-4 and IFN-γ production in response OvAg and PPD stimulation. Upon antigen stimulation, murine [18,19] and human [20,21] CD4+ T cells polarize into subpopulations with characteristic profiles of cytokine production. Type-1 cells produce IFN-γ and IL-2 but little or no IL-4 or IL-5, whereas type-2 cells produce mainly IL-4 and IL-5. Onchocerca volvulus, like other helminth infections [22–24], will commonly induce an immune response to parasite antigens which is biased towards a type-2 response [7,8]. Areas that are endemic for onchocerciasis are often co-endemic for the mycobacterial infections tuberculosis and leprosy, which commonly induce a type-1 immune response [20,21]. However, it has been previously observed that the presence of a type-2 response to mycobacterial antigens and a diminished type-1 response is associated with the development of lepromatous leprosy [25,26] and pulmonary tuberculosis [27–29]. It is thus important to examine what happens when the two infections occur together, because evidence exists from murine models that a helminth-induced type-2 response can alter the nature of immune response to non-parasite or ‘third-party’ antigens. Indeed, murine infections of both Schistosoma mansoni and Brugia malayi induce a skewing of the immune response towards type 2 to antigens that normally promote a type 1 response [30–32], and S. mansoni infection induces an infection-resolving type-2 response to concomitant Trichuris muris infection [33]. In humans, however, the only evidence for similar immunomodulation of ‘third-party’ antigens is in S. mansoni-infected individuals who, following tetanus toxoid (TT) vaccination, produced less TT-specific IFN-γ [34].
The MF-related down-regulation of proliferation to PPD was paralleled by a significant decrease in both OvAg- and PPD-stimulated IL-4 production. There was no statistically significant MF-related decrease in either PPD- or OvAg-specific IFN-γ, but there was a downward trend in response to both antigens and IFN-γ production positively correlated with the down-regulated proliferative responses for both worm and mycobacterial antigens. This suggests that the cellular hyporesponsiveness may extend to both types 1 and 2 T cell subsets.
Concurrent with the MF-related cellular down-regulation was a host age effect on the balance of T cell subsets responding to both the parasite and mycobacterial antigens. OvAg-stimulated IFN-γ production reflected the proliferative response, with highest values in the middle (9–12 years) age group and negligible responses in the oldest (13–16 years) age group. This, in conjunction with the marked increase in OvAg-dependent IL-4 production through ascending age groups, is indicative of a down-regulation of type-1 responses in the older children accompanied by an age-related increase in type-2 responses. In the context of this study, the term age comprises two possible influences on the immune response, namely maturation of the childhood immune system, and duration of exposure to the parasite. However, as it is known that the adult female worms of B. malayi are the driving force of type 2 polarization in mouse infections [35], it seems likely that the age-related parasite-specific type 1 to type 2 shift is a function of duration of exposure to the female parasite.
Neither proliferative responses nor IFN-γ production by PBMC stimulated with mycobacterial antigen showed any relationship to age but IL-4 production increased markedly, with no difference between individuals of different BCG vaccination status. Unlike the response to parasite antigen, this suggests that there is no age-related down-regulation of the type-1 response to PPD, but there is an up-regulation of the PPD-stimulated type-2 cell subset. Although we do not have data relating to the immune response to PPD in children not exposed to O. volvulus, such a shift in the T cell subset bias could be caused by the parasite-induced IL-4-rich environment favouring the development of mycobacterial-specific T cells from a type-0 to a type-2 phenotype. In agreement with this, PPD immunization of B. malayi-infected mice produced a similar skewing of PPD-specific responses towards type 2 but with no change in IFN-γ production [31]. However, recent studies demonstrated that adult human infection with B. malayi [36] and in utero exposure to helminth antigens [37] did not influence the T helper cell response to PPD. This absence of an observed alteration in the response to PPD may simply reflect differences between human infection with O. volvulus and other helminth infections, or may result from methodological differences between the studies. Also, it may be that the parasite-induced up-regulation of type-2 responses to PPD is only evident in the developing immune system of children, and/or it may be that what are relatively subtle, yet meaningful changes in cellular responsiveness and cytokine production can only be revealed by the power of generalized linear modelling such as used in this study.
In mice infected with S. mansoni, a parasite-stimulated down-regulation of type-1 cytokine secretion and simultaneous increase in type-2 cytokines has been shown to extend not only to non-parasite antigen but also to mitogen, demonstrating a generalized imbalance in the T cell population [30,32,38]. In the present study, mitogen-specific proliferative responses significantly increased with age and were unaffected by MF intensity, and neither IFN-γ nor IL-4 production showed any relationship to MF intensity or age. This demonstrates that the parasite intensity-driven down-regulation of cellular responsiveness and the skewing of responses to type 2 are antigen-specific for OvAg and PPD and that the T cell population as a whole remains unaffected. To define further any changes in T cell subsets we had examined production of IL-2, IL-5 and IL-10. However, with the small sample size and low numbers of responders we were unable to reveal any significant relationships between production of these cytokines and age or MF.
The balance of type 1 and type 2 responses has a striking effect on the clinical manifestations of many diseases, including tuberculosis and leprosy. Tuberculin-positive healthy individuals have high PBMC proliferative responses to PPD and a strong PPD-specific type-1 cytokine profile [27,29]. Patients with pulmonary tuberculosis, however, exhibit low proliferative responses to PPD and show diminished production of type-1 cytokines but unaltered or elevated type-2 cytokine production [27–29]. Similarly, tuberculoid leprosy patients, who mount a resistant response, produce a type-1 cytokine expression pattern in skin lesions, whereas lepromatous leprosy patients express a type-2 cytokine profile [25,26]. Thus, in onchocerciasis patients we envisage that the down-regulation of cellular responsiveness to mycobacterial antigens and age-related ascension of a type-2 response observed in this study will affect the clinical manifestation of mycobacterial infection. Indeed, a previous study reported that the incidence of lepromatous leprosy in onchocerciasis hyperendemic communities was double that in areas with little or no onchocerciasis but a similar overall prevalence of leprosy [12]. To our knowledge there have been no controlled epidemiological studies to examine the incidence of active tuberculosis in onchocerciasis regions. The observed modulation of the immune response to mycobacterial antigens occurred irrespective of BCG vaccination status, suggesting that the efficacy of BCG vaccination may be altered. Indeed, it has been previously shown that PPD skin test-negative onchocerciasis patients are less likely than uninfected individuals to convert to skin test positivity following BCG vaccination [39]. Our study exposes the underlying mechanisms of this poor response to BCG and, further, may implicate for the first time the powerful immunomodulatory effect of helminthic infection in general as a contributory factor in the variable efficacy of BCG vaccination worldwide. It is notable that BCG offers the lowest protective efficacy in tropical regions [40], the very areas where helminth infections are most prevalent. It should also be considered that helminth infection may alter the response to other vaccines and the outcome of other concurrent infections.
Acknowledgments
We thank Dr A. Bilongo Manene, Secrétaire Général de l'OCEAC, and Drs F. J. Louis, P. Ringwald and N. Fievet from OCEAC. We thank Mr B. Bouchite and Drs J. Gardon, N. Gardon-Wendel and J. Kamgno from Antenne Orstom aupres du Centre Pasteur du Cameroun, Yaounde, and Judith Allen and Hazel Dockrell for constructive comments. Financial support was from The Royal Society, the Edna McConnell Clark Foundation and the INCO-DC programme of the European Economic Community.
REFERENCES
- 1.Duke BOL. Human onchocerciasis—an overview of the disease. Acta Leidensia. 1990;59:9–24. [PubMed] [Google Scholar]
- 2.Schulz-Key H. Observations on the reproductive biology of Onchocerca volvulus. Acta Leidensia. 1990;59:27–44. [PubMed] [Google Scholar]
- 3.Ward DJ, Nutman TB, Zea-Flores G, et al. Onchocerciasis and immunity in humans: enhanced T cell responsiveness to parasite antigen in putatively immune individuals. J Infect Dis. 1988;157:536–43. doi: 10.1093/infdis/157.3.536. [DOI] [PubMed] [Google Scholar]
- 4.Gallin M, Edmonds K, Ellner JJ, et al. Cell mediated immune responses in human infection with Onchocerca volvulus. J Immunol. 1988;140:1406–19. [PubMed] [Google Scholar]
- 5.Elkhalifa MY, Ghalib HW, Dafa'Alla T, et al. Suppression of human lymphocyte responses to specific and non-specific stimuli in human onchocerciasis. Clin Exp Immunol. 1991;83:433–9. doi: 10.1111/j.1365-2249.1991.tb02949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soboslay PT, Dreweck CM, Hoffmann WH, et al. Ivermectin-facilitated immunity in onchocerciasis. Reversal of lymphocytopenia, cellular anergy and deficient cytokine production after single treatment. Clin Exp Immunol. 1992;89:407–13. doi: 10.1111/j.1365-2249.1992.tb06971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elson L, Guderian R, Calvopena M, et al. Immunity to Onchocerciasis: putative immune individuals produce a Th1-like response to Onchocerca volvulus. J Infect Dis. 1995;171:652–8. doi: 10.1093/infdis/171.3.652. [DOI] [PubMed] [Google Scholar]
- 8.Mahanty S, King C, Kumaraswami V, et al. IL-4 and IL-5-secreting lymphocyte populations are preferentially stimulated by parasite-derived antigens in human tissue invasive nematode infections. J Immunol. 1993;151:3704–11. [PubMed] [Google Scholar]
- 9.Kirkwood B, Smith P, Marshall T, et al. Variations in the prevalence and intensity of microfilarial infections by age, sex, place and time in the area of the Onchocerciasis Control Programme. Trans R Soc Trop Med Hyg. 1983;77:857–61. doi: 10.1016/0035-9203(83)90307-3. [DOI] [PubMed] [Google Scholar]
- 10.Steel C, Guinea A, McCarthy JS, et al. Long-term effect of prenatal exposure to maternal microfilaraemia on immune responsiveness to filarial parasite antigens. Lancet. 1994;343:890–3. doi: 10.1016/s0140-6736(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 11.Wilson K, Grenfell B. Generalized linear modelling for parasitologists. Parasitol Today. 1997;13:33–38. doi: 10.1016/s0169-4758(96)40009-6. [DOI] [PubMed] [Google Scholar]
- 12.Prost A, Nebout M, Rougemont A. Lepromatous leprosy and onchocerciasis. Br Med J. 1979;1:589–90. doi: 10.1136/bmj.1.6163.589-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greene BM, Gbakima AA, Albiez EJ, et al. Humoral and cellular immune responses to Onchocerca volvulus infection in humans. Rev Infect Dis. 1985;7:789–95. doi: 10.1093/clinids/7.6.789. [DOI] [PubMed] [Google Scholar]
- 14.Rougemont A, Bobson-Pontal ME, Pontal PG, et al. Tuberculin skin tests and BCG vaccination in hyperendemic area of onchocerciasis. Lancet. 1977;2:309. doi: 10.1016/s0140-6736(77)91857-8. [DOI] [PubMed] [Google Scholar]
- 15.Ottesen EA, Weller PF, Heck L. Specific cellular immune unresponsiveness in human filariasis. Immunology. 1977;33:413–21. [PMC free article] [PubMed] [Google Scholar]
- 16.Piessens WF, McGreevy PW, Piessens M, et al. Immune responses in human infections with Brugia malayi: specific cellular unresponsiveness to filarial antigens. J Clin Invest. 1980;65:172–9. doi: 10.1172/JCI109648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sartono E, Kruize TC, Kurniawan A, et al. Elevated cellular immune responses and interferon-gamma release after long-term diethylcarbamazine treatment of patients with human lymphatic filariasis. J Infect Dis. 1995;17:1683–7. doi: 10.1093/infdis/171.6.1683. [DOI] [PubMed] [Google Scholar]
- 18.Mosmann TR, Cherwinski H, Bond MW, et al. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 19.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;9:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 20.Salgame P, Abrams JS, Clayberger C, et al. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T-cell clones. Science. 1991;254:279–82. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- 21.Kemp M, Kurtzhals JA, Bendtzen K, et al. Leishmania donovani-reactive Th1- and Th2-like T-cell clones from individuals who have recovered from visceral leishmaniasis. Infect Immun. 1993;61:1069–73. doi: 10.1128/iai.61.3.1069-1073.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearce EJ, Caspar P, Grzych J-M, et al. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173:159–66. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finkelman FD, Pearce EJ, Urban JF, Jr, et al. Regulation and biological function of helminth-induced cytokine responses. Immunol Today. 1991;12:A62–A66. doi: 10.1016/S0167-5699(05)80018-0. [DOI] [PubMed] [Google Scholar]
- 24.Williams ME, Montenegro S, Domingues AL, et al. Leukocytes of patients with Schistosoma mansoni respond with a Th2 pattern of cytokine production to mitogen or egg antigens but with a Th0 pattern to worm antigens. J Infect Dis. 1994;170:946–54. doi: 10.1093/infdis/170.4.946. [DOI] [PubMed] [Google Scholar]
- 25.Yamamura M, Uyemura K, Deans RJ, et al. Defining protective responses to pathogens—cytokine profiles in leprosy lesions. Science. 1991;254:277–9. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 26.Misra N, Murtaza A, Walker B, et al. Cytokine profile of circulating cells of leprosy patients reflects both indiscriminate and polarized T-helper subsets: T-helper phenotype is stable and uninfluenced by related antigens of Mycobacterium leprae. Immunology. 1995;86:97–103. [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez FO, Rodriguez JI, Agudelo G, et al. Immune responsiveness and lymphokine production in patients with tuberculosis and healthy controls. Infect Immun. 1994;62:5673–8. doi: 10.1128/iai.62.12.5673-5678.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Surcel H-M, Troye-Blomberg M, Paulie S, et al. Th1/Th2 profiles in tuberculosis, based on the proliferation and cytokine response of blood lymphocytes to mycobacterial antigens. Immunology. 1994;81:171–6. [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M, Lin Y, Iyer DV, et al. T-cell cytokine responses in human infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:3231–4. doi: 10.1128/iai.63.8.3231-3234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kullberg MC, Pearce EJ, Hieny SE, et al. Infection with Schistosoma mansoni alters Th1/Th2 cytokine responses to a non-parasite antigen. J Immunol. 1992;148:3264–70. [PubMed] [Google Scholar]
- 31.Pearlman E, Kazura JW, Hazlett FE, et al. Modulation of murine cytokine responses to mycobacterial antigens by helminth-induced T helper 2 responses. J Immunol. 1993;151:4857–64. [PubMed] [Google Scholar]
- 32.Actor JK, Shirai M, Kullberg MC, et al. Helminth infection results in decreased virus specific CD8+ cytotoxic T-cell and Th1 cytokine responses as well as delayed virus clearance. Proc Natl Acad Sci USA. 1993;90:948–52. doi: 10.1073/pnas.90.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curry AJ, Else KJ, Jones F, et al. Evidence that cytokine-mediated immune interactions induced by Schistosima mansoni alter disease outcome in mice concurrently infected with Trichuris muris. J Exp Med. 1995;181:769–74. doi: 10.1084/jem.181.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabin EA, Araujo MI, Carvalho EM, et al. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J Infect Dis. 1996;173:269–72. doi: 10.1093/infdis/173.1.269. [DOI] [PubMed] [Google Scholar]
- 35.Lawrence RA, Allen JE, Osborne J, et al. Adult and microfilarial stages of the filarial parasite Brugia malayi stimulate contrasting cytokine and Ig isotype responses in BALB/c mice. J Immunol. 1994;153:1216–24. [PubMed] [Google Scholar]
- 36.Sartono E, Kruize YCM, Kurniawan A, et al. In Th2-biased lymphatic filarial patients, responses to purified protein derivative of Mycobacterium tuberculosis remain Th1. Eur J Immunol. 1996;26:501–4. doi: 10.1002/eji.1830260233. [DOI] [PubMed] [Google Scholar]
- 37.Malhotra I, Ouma J, Wamachi A, et al. In utero exposure to helminth and mycobacterial antigens generates cytokine responses similar to that observed in adults. J Clin Invest. 1997;99:1759–66. doi: 10.1172/JCI119340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grzych J-M, Pearce E, Cheever A, et al. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol. 1991;146:1322–7. [PubMed] [Google Scholar]
- 39.Kilian HD, Nielson G. Cell-mediated and humoral immune responses to BCG and rubella vaccinations and to recall antigens in onchocerciasis patients. Trop Med Parasit. 1989;40:285–91. [PubMed] [Google Scholar]
- 40.Colditz GA, Brewer TF, Berkey CS, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]


