Abstract
Fifty-five serum samples from patients with reactive arthritis (ReA), 40 from patients with ankylosing spondylitis (AS) and three from patients with chronic sacroiliac joint arthritis were analysed for the presence of ANCA of IgG class by means of enzyme immunosorbent assay using lactoferrin (Lf), myeloperoxidase (MPO) and antigen extracted from azurophil granules (‘α-antigen’) containing proteinase 3 (PR3) as substrate. IgG-ANCA were found in 31 (56%) patients with ReA. Twenty-three (42%) had anti-Lf antibodies, nine (16%) had anti-MPO and eight (15%) had anti-α-antigen antibodies, none of which reacted with PR3. Only six (14%) AS or sacroiliac joint arthritis patients had ANCA (P < 0.001). Three (7%) had anti-Lf, two (5%) anti-MPO and two (5%) anti-α-antigen antibodies. Yersinia and Salmonella bacteria were separated by SDS–PAGE and blots were incubated with serum from rabbits immunized with human Lf. The hyperimmune serum recognized a band of 78 kD from both bacteria which was not seen when preimmune serum was used. The reaction to the 78-kD antigen could be completely inhibited when anti-Lf antibodies were absorbed on Lf coupled to cyanogen bromide-activated Sepharose, possibly indicating cross-reacting epitopes in Lf and enterobacterial antigen.
Keywords: anti-neutrophil cytoplasmic antibodies, reactive arthritis, ankylosing spondylitis
INTRODUCTION
The occurrence of ANCA in patients with various forms of vasculitis and crescentic necrotizing glomerulonephritis is well documented [1,2]. By indirect immunofluorescence (IIF) microscopy on ethanol-fixed human granulocytes, two distinct ANCA staining patterns are recognized. The pancytoplasmatic pattern (c-ANCA), corresponding to antibodies directed against proteinase 3 (PR3), is seen in Wegener's granulomatosis [3] and the artefactual perinuclear staining pattern (p-ANCA), representing autoantibodies directed against myeloperoxidase (MPO), occurs in other necrotizing small vessel vasculitides, e.g. microscopic polyangiitis and Churg–Strauss syndrome [1,2]. However, p-ANCA may also be due to antibodies against leucocyte antigens such as elastase, lysozyme, and lactoferrin (Lf) [4], and may occur in non-vasculitic conditions, e.g. rheumatoid arthritis, systemic lupus erythematosus and inflammatory bowel disease (IBD) [5–7].
Lf is a 78-kD cationic iron-binding protein residing in the specific granules of polymorphonuclear neutrophil leucocytes (PMNL) [8]. It is released upon neutrophil activation [9] and exerts anti-microbial effects both by depriving bacteria of iron essential for their growth and by direct bactericidal mechanisms [10,11]. Lf has been shown to bind to the lipid A part of lipopolysaccharides (LPS) [12]. It is also secreted for instance in tears, milk, and pancreatic juice, and may play an important role as a non-specific anti-phlogistic defence factor [13]. The generation of hydroxyl radicals at sites of inflammation requires iron. By iron-binding Lf can thus counteract the tissue-damaging effects of oxygen free radicals [13]. Anti-Lf antibodies have experimentally been shown to increase both the magnitude and duration of hydroxyl radical formation [14]. Hypothetically, Lf-ANCA could therefore have pathogenic importance by counteracting the anti-inflammatory effect of Lf, thereby aggravating and prolonging the inflammatory process.
Reactive arthritis (ReA) and ankylosing spondylitis (AS) belong to the group of seronegative spondyloarthropathies particularly seen in individuals possessing the histocompatibility antigen HLA-B27. Autoantibodies have not been considered features of these disorders, and in an early study only low frequencies of anti-leucocyte antibodies were found among patients with AS or psoriatic arthritis [15]. However, we found that ANCA was common among patients with ReA [16]. In the present study we confirmed this finding, especially the occurrence of anti-Lf antibodies in ReA, whereas ANCA was an infrequent finding in AS patients. A unique feature is that the HLA-B27-associated ReA is triggered by a few well known enteric or urogenital pathogens, all of which are facultative or obligate intracellular parasites, in most cases identified by culture or serology [17]. This prompted us to investigate whether Lf-ANCA could be the result of cross-immunization against epitopes on the arthritogenic bacteria Yersinia and Salmonella.
PATIENTS AND METHODS
Patients
Ninety-eight consecutive out-patients were enrolled in the study. A diagnosis of ReA was made in 55 patients (11 women, mean age 43 years, and 44 men, mean age 36 years) if they fulfilled the criteria of a seronegative mono- or oligo-arthritis following an enteric or urogenital infection. The triggering microorganisms were identified by culture and or serology, routinely performed at the Department of Clinical Microbiology. ReA patients were divided into acute or chronic (i.e. > 1 year of disease). In cases where no triggering infection could be determined, the diagnosis of ReA was made based on a combination of arthritis and one or more of the following manifestations: acute anterior uveitis/conjunctivitis, urethritis, sacroiliac joint arthritis, circinate balanitis, keratoderma blennorrhagia or dactylitis.
Forty patients were diagnosed with AS; five women (mean age 43 years) and 35 men (mean age 45 years). Mean duration of disease was 20.3 years (range 1–39 years). A diagnosis of AS was made when a patient presented with minimum 1 year of disease including bilateral arthritis in the sacroiliac joints proven by x-ray or computed tomographic scanning and characteristic involvement of at least the lumbar spine either shown on roentgenograms or judged clinically by a rheumatologist.
Three patients (two women, one man; mean age 37 years) presented with chronic bilateral sacroiliac joint inflammation (mean duration 16 years) without fulfilling our criteria of either ReA or AS; they were, however, included because, apart from being HLA-B27+, they shared the common characteristic features of the seronegative spondyloarthropathy.
Rabbit anti-Lf antibodies
Pooled rabbit hyperimmune serum against human milk Lf was produced as described elsewhere [7]. Western blot analysis confirmed anti-Lf specificity (see below). Anti-Lf antibodies were affinity-purified by passing the hyperimmune serum over a cyanogen bromide (CNBr)-activated Sepharose-4B column (Pharmacia Biotech, Uppsala, Sweden) to which Lf had been coupled according to the protocol provided with the kit. Specific antibodies were eluted from the column using 0.05 m glycine–HCl buffer pH 2.7. The pH of the eluate was immediately restored to neutral using solid Tris salt. Immunoglobulins were precipitated by mixing equal volumes of the eluate and super-saturated ammonium sulphate (SAS). The precipitate was pelleted by centrifugation, redissolved in PBS, and passed over a Sephadex G-25 column (Pharmacia) to a final concentration of 5.9 mg/ml. Rabbit anti-Lf antibodies were absorbed from rabbit hyperimmune serum by passing it twice over human Lf coupled to a CNBr-activated Sepharose column. Absence of anti-Lf antibody activity was confirmed by immunoblotting with Lf as antigen.
Hyperimmune rabbit serum containing IgG anti-dinitrophenyl (DNP) antibodies was used as a source of irrelevant control antibodies.
Monoclonal antibodies
A mouse monoclonal antibody preparation (ML 30 MoAb) recognizing mycobacterial heat shock protein 65 (hsp65) was a gift from Professor Ivanyi (MCR Tuberculosis and related infections Unit, Royal Postgraduate Medical School, London, UK).
Two different mouse anti-human Lf MoAbs were used; one was produced at the Department of Immunology, Statens Serum Institute (Copenhagen, Denmark) (2.88 mg/ml), and one purchased from American Research Products (Belmont, MA) (1 mg/ml).
Secondary antibodies
The following peroxidase-conjugated antibodies were used in immunoblotting assays: goat anti-rabbit antibodies (Dako, Glostrup, Denmark), γ-chain-specific rabbit anti-human IgG (Dako), and affinity-purified goat anti-mouse antibodies (Dako), all diluted 1:1000 in PBS–Tween with 1% bovine serum albumin (BSA). Alkaline phosphatase-conjugated γ-chain-specific rabbit anti-human IgG (Dako) diluted 1:1000 was used in the ELISA assays, and FITC-conjugated rabbit antibodies specific for human IgG γ-chain (Dako) were used as secondary antibodies for IIF microscopy.
Immunoblotting
Clinical isolates of Y. enterocolitica 0:3 and S. typhimurium were cultured, washed three times in PBS pH 7.3, suspended in reducing sample buffer at a concentration of 2 × 108 bacteria/ml and boiled for 5 min. Insoluble constituents were removed by centrifugation. Bacterial extract (20 μl) or 1 μg human milk Lf in sample buffer were loaded in each well and separated by SDS–PAGE, 4–12% linear gradient acrylamide gel (Novex, San Diego, CA) at 30 mA for 1 h. The gels were electroblotted for 3 h onto polyvinylidene diflouride (PVDF) membranes using a TRIS–glycine buffer system. The membrane was washed in PBS and blocked overnight at 4°C in PBS–Tween with 5% BSA, washed three times in PBS–Tween and incubated for 2 h at room temperature with primary antibodies diluted with PBS–Tween containing 1% BSA (patient sera were diluted 1:100, and rabbit immune sera 1:1000). The membranes were again washed three times in PBS–Tween and incubated for 1 h with peroxidase-conjugated secondary antibodies. After washing, the blots were developed with the ECL Western blotting detection system (Amersham, Aylesbury, UK).
IIF microscopy
Freshly isolated human neutrophils were ethanol-fixed on microscope slides and used for ANCA detection by IIF microscopy as described in detail elsewhere [4].
ELISA
ELISA for determination of MPO-ANCA, Lf-ANCA, or antibodies against a PR3-containing acid extract of PMNL azurophil granules (α-ANCA) have been described elsewhere [7]. Normal human serum served as a blank (0% value), and a known positive serum (Lf-ANCA, MPO-ANCA or PR3-ANCA) was included as a 100% reference. Sera producing optical density (OD) values > 3 s.d. above the mean of a normal reference material (218 healthy blood donors) were considered positive. Sera producing positive α-ANCA were further analysed for PR3 specificity (PR3-ANCA). In this assay, microtitre strips were coated with purified PR3 (Wieslab, Lund, Sweden) at a concentration of 1 μg/ml, and the subsequent procedures were essentially the same as those for the other ANCA-ELISA tests.
Inhibition ELISA
To investigate whether the anti-Lf antibodies in a serum sample from a patient with Yersinia-triggered ReA could be adsorbed by bacteria, serum was incubated at 37°C for 45 min with a clinical isolate of live and boiled Y. enterocolitica 0:3 in suspension. After centrifugation, the supernatant was diluted serially and analysed for Lf-ANCA by ELISA as described above. A non-incubated serum sample from the same patient was run in parallel.
Other laboratory analyses
Anti-nuclear antibodies (ANA) of IgG class (IF microscopy; HEp-2 cells), IgM rheumatoid factor, and HLA-B27 were also analysed. Blood samples to record haemoglobin, C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were drawn at the same time as those for the ANCA tests.
Statistical analysis
The χ2 test was used to compare the prevalence of ANCA between various disease groups.
RESULTS
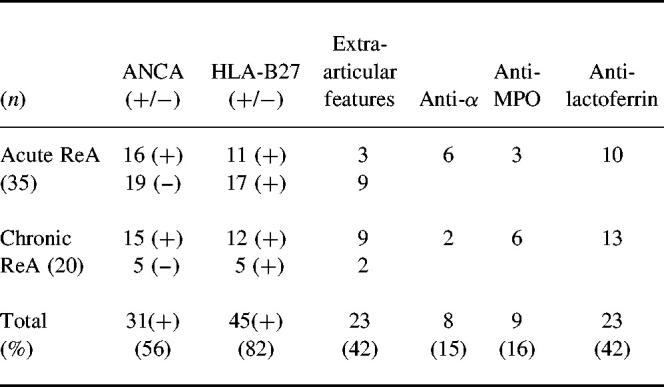
Thirty-five ReA patients had acute, and 20 had chronic or chronic-intermittent disease course (mean duration 10 years, range 2–26 years). The triggering infection was Y. enterocolitica in 12, Salmonella species in four, Campylobacter jejuni in eight and Chlamydia trachomatis in nine patients. In 22 cases no triggering infection was found. Overall 82% carried the HLA-B27 antigen, relatively more patients with chronic than with acute disease (85% versus 80%—see Table 1 for details). Forty-two percent had extra-articular manifestations, predominantly acute anterior uveitis (AAU) at some time point along the course of their disease.
Table 1.
ELISA results of IgG-ANCA among 55 patients with reactive arthritis (ReA)
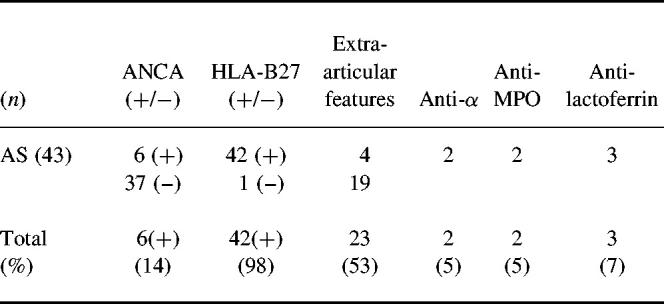
Among the 43 patients with either AS or chronic sacroiliac joint arthritis, 98% were HLA-B27+. Extra-articular features were found in 51%, mainly AAU (for details see Table 2). Overall, five patients were weekly positive in ANA testing and two had a low level of rheumatoid factor.
Table 2.
ELISA results of IgG-ANCA among 43 patients with ankylosing spondylitis (AS) and chronic sacroiliac joint arthritis
ANCA
IgG-ANCA as measured by ELISA was found in 31 out of 55 (56%) patients with ReA. Twenty-three (42%) had Lf-ANCA, nine (16%) had MPO-ANCA, and eight (15%) had antibodies against α-antigen, none of which reacted with PR3.
Among the 31 ANCA+ patients the triggering infections were Salmonella in two, Campylobacter in three, Yersinia in six and Chlamydia in seven. In the remaining 12 patients no triggering infection could be discovered.
Relatively more patients with chronic (75%) than acute disease (46%) were ANCA+ (P < 0.05) (Table 1). When sera were analysed by IIF, nine out of the 31 ELISA-positive (29%) showed a p-ANCA pattern, typically those with the highest serum ELISA values. Among the 43 patients with AS or chronic sacroiliac joint arthritis a total of six (14%) were positive in ANCA-ELISA. Two had anti-α-antigen antibodies (5%), although PR3-ANCA−. Two had anti-MPO antibodies (5%) and three had anti-Lf antibodies (7%). By IIF microscopy, one had c-ANCA (α-antigen+) and two had p-ANCA patterns.
One patient with long-standing AS contracted an acute episode of ReA due to Salmonella gastroenteritis. Prior to that he was tested and found positive for anti-MPO and anti-Lf. The titres did not change significantly during or after the Salmonella infection.
When CRP values, as indicators of inflammatory activity, drawn at the same time point as the ANCA tests, were compared, there were virtually no differences in mean CRP between the patients with acute or chronic ReA (38 ± 39 mg/lversus 31 ± 32 mg/l), nor between the ANCA+ patients from both groups. However, patients with AS had a slightly lower mean CRP of 26 ± 20 mg/l. Neither were there any differences between mean OD values for anti-Lf antibody titres among the three groups. Diagrams illustrating the individual ANCA absorbance values in percentage of the positive controls are shown in Figs 1 and 2. A number of patients from all groups had serial testing done in order to evaluate whether the ANCA titres varied according to inflammatory activity. Although in a few cases there was a tendency towards diminishing ANCA titres along with decreasing inflammation, the overall correlation was not obvious and therefore did not allow any conclusions (data not shown). Some patients were tested for ANCA in joint fluid aspirate (knee joints mostly) and the values obtained correlated well to serum titres (data not shown). When the ReA patients were divided into HLA-B27+ and HLA-B27−, it revealed that among the 45 HLA-B27+, 23 were also ANCA+ (51%). Among the eight HLA-B27−patients, seven were ANCA+ (88%). Two patients escaped HLA-B27 testing and were not included. For patients with acute ReA the mean disease duration was 6.5 ± 4 months, and no difference was seen between ANCA+ and ANCA− cases.
Fig. 1.
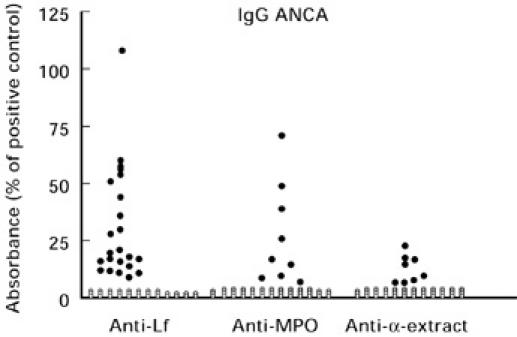
Negative (○) and positive (•) results of ELISA determinations of IgG antibodies against human lactoferrin (Lf), myeloperoxidase (MPO) and α-extract in 55 sera from patients with reactive arthritis. The lower limit for positive ELISA is the mean value + 3 s.d. of 218 blood donors.
Fig. 2.
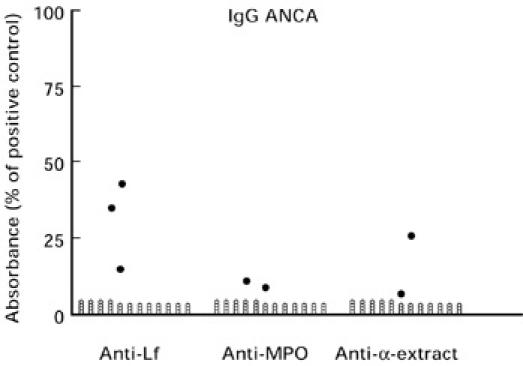
Negative (○) and positive (•) results of ELISA determinations of IgG antibodies against human lactoferrin (Lf), myeloperoxidase (MPO) and α-extract in 43 sera from patients with ankylosing spondylitis and chronic sacroiliac joint arthritis. The lower limit for positive ELISA is the mean value + 3 s.d. of 218 blood donors.
Immunoblotting
When preimmune rabbit serum was incubated with electroblotted extracts of Yersinia and Salmonella, each bacterial species produced three strong bands. Common for both species were bands of 18 and 35 kD. For Yersinia a further band was seen at 38 kD and for Salmonella a band at 40 kD (lanes 1, 2, Fig. 3).
Fig. 3.
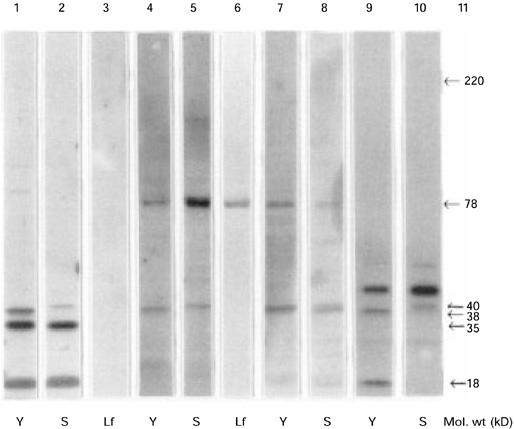
SDS–PAGE of human lactoferrin (Lf), bacterial extracts from Yersinia (Y) and Salmonella (S) incubated with rabbit and human serum. Lanes 1–3, rabbit preimmune serum; lanes 4 and 5, rabbit anti-Lf serum; lanes 6–8, human serum from a patient with Yersinia-triggered reactive arthritis positive for Lf-ANCA; lanes 9 and 10, mouse monoclonal antiserum against Mycobacterial 65-kD heat shock protein; lane 11, molecular weight marker.
Preimmune serum did not react with human Lf (lane 3). When affinity-purified rabbit anti-human Lf serum was used, two distinct bands showed up for each bacterium (lanes 4, 5), one being common and of approximately 78 kD, and the others were of 38 kD in Yersinia and 40 kD in Salmonella, similar to the bands seen with the preimmune serum. The reactivity to the 78-kD bacterial antigen was completely inhibited when rabbit hyperimmune serum was passed over a CNBr-activated Sepharose column to which Lf had been coupled.
When a Lf-ANCA+ serum from a patient with Yersinia-triggered ReA was applied to the same bacterial extracts, there was a strong reaction against human Lf (lane 6) as well as reaction to a 78-kD antigen in both bacteria. The human serum also recognized the two antigens of 38 kD and 40 kD in Yersinia and Salmonella, respectively (lanes 7, 8).
Rabbit preimmune serum bound to bands of the same molecular weight (18 and 35 kD) when incubated with extract of electroblotted Escherichia coli(not shown). The two mouse anti-Lf MoAbs failed to recognize the 78-kD enterobacterial antigen, when incubated with extracts of Yersinia and Salmonella. The mouse MoAb against mycobacterial hsp65 recognized the two bands of 18 and 38 kD in Yersinia and the single band of 40 kD in Salmonella(lanes 9,10), just as the rabbit preimmune serum. There was no reactivity to the 78-kD antigen. Neither did rabbit anti-DNP immune serum react with the 78-kD band.
Inhibition ELISA
An Lf-ANCA+ serum from a Yersinia ReA patient was preincubated with live and heat-killed Yersinia bacteria in an attempt to block the anti-Lf antibodies. When a dilution series was run on an Lf-coated ELISA plate in parallel with non-preincubated serum from the same patient, the two curves obtained were virtually identical, and did not indicate that Lf antibodies could be blocked by means of cross-reacting epitopes expressed on the Yersinia bacteria.
DISCUSSION
In this study we found that ANCA was prevalent among patients with ReA, occurring in 31 out of 55 sera (56%) when tested by ELISA for reactivity against Lf, or MPO, or an acid extract of azurophil granules (α-antigen). By contrast, we found significantly less ANCA among the AS sera, in that only six out of 43 (14%) were positive (P < 0.001). In ReA, Lf-ANCA dominated (42%), followed by MPO-ANCA (16%) and α-ANCA (15%). The corresponding figures for AS were 7%, 5% and 5%, respectively. The α-antigen preparation allows detection of PR3-ANCA but not MPO-ANCA [4]. Neither does it permit the detection of anti-elastase antibodies (unpublished observations). Analysis of α-ANCA+ sera for reactivity against purified PR3 consequently turned out negative.
The highest proportion of ANCA was found among ReA patients with a chronic persistent disease course (75%). This did not seem to be correlated to degree of inflammatory activity, as there was only a slight difference in mean CRP between the acute and chronic group. The AS patients were found to be less inflammatorily active, as judged by the CRP levels, although great individual differences were noted. Just as in the case of ReA, there was no obvious correlation between high disease activity and ANCA positivity. Neither was there any connection between ANCA and the presence of extra-articular manifestations, HLA-B27 status or triggering infection. Although there have been occasional reports on the presence of ANCA in sera from patients with various infections [18–20], this does not seem to be a common phenomenon.
A problem often encountered in analysing ANCA, as well as other autoantibodies, is the inconsistency in results obtained by different techniques of antibody detection. Thus, not all sera positive for ANCA by IIF microscopy turn out positive in solid-phase assays and vice versa. This may depend on several factors such as the avidity of the autoantibodies, epitope exposure, conformational changes/denaturation of the antigens, etc. Several proteins, apart from PR3, MPO and Lf, have been characterized as target antigens for ANCA, many of which are detected by IIF microscopy. Still, it is highly likely that many ANCA target antigens remain to be identified. Only nine out of 35 (26%) ELISA-positive ReA sera gave rise to positive immunofluorescence staining (p-ANCA pattern), all of which had either Lf or MPO specificity. Three AS sera were ANCA+ as tested by IIF microscopy, one c-ANCA (α-ANCA+) and two p-ANCA (one Lf-ANCA+).
p-ANCA is a common finding in patients with IBD, mainly in ulcerative colitis (UC) and to a lesser extend in Crohn's disease [21–23]. Several candidate antigens for the p-ANCA reactivity have been suggested. Some investigators have reported a high prevalence of Lf-ANCA in UC [7,24,25]. There is a strong link between IBD and the seronegative spondyloarthropathies, and gut inflammation (mainly subclinical) resembling IBD has been reported in about 70% of patients with ReA or AS [26]. Taking this into account, it is not surprising that Lf-ANCA is a common finding not only in UC but also in spondyloarthropathies, as shown in the present study.
The driving forces for the generation of autoantibodies are obscure. One possibility may be immunological cross-reactivity with microbial antigens. Human Lf has been found to cross-react with mycobacterial hsp65, and T cells from rats with adjuvant arthritis induced by heat-killed mycobacteria have been found to proliferate upon exposure to human Lf [27,28]. As hsp are highly conserved throughout nature and found in virtually all life forms, great sequence homologies exist between human and microbial hsp [29]. Comparisons between amino acid sequences of microbial/human hsp60 and of autoantigens have revealed similarities in many instances, e.g. MPO [30]. This gives at least a theoretical possibility of cross-reaction between immunodominant regions on microbial hsp and self antigens. However, immunization with mycobacterial hsp65 does not result in the production of anti-Lf antibodies [31,32].
To analyse possible antigenic cross-reactions between human Lf and enterobacterial antigens, pooled rabbit preimmune serum, pooled rabbit anti-Lf hyperimmune serum, and affinity-purified rabbit anti-human Lf antibodies were analysed by Western blot on extracts from Y. enterocolitica and S. typhimurium. The hyperimmune serum, but not the preimmune serum, recognized an enterobacterial antigen of approximately 78 kD in both bacterial extracts. This antigen was not recognized by two separate MoAbs against human Lf. However, reactivity with the 78-kD bacterial antigen was completely inhibited by absorbing the hyperimmune serum with human Lf coupled to CNBr-activated Sepharose. The 78-kD reactivity was recovered after elution of the absorbed antibodies from the affinity column. Reactivity with the 78-kD enterobacterial antigen was not seen with an irrelevant rabbit hyperimmune serum containing anti-DNP antibodies. An anti-hsp65 MoAb recognized several antigens from both bacterial strains, among other bands of 38 kD in Yersinia and 40 kD in Salmonella(lanes 9, 10) indicating cross-reacting epitopes between mycobacterial hsp65 and enterobacterial antigens. However, the MoAbs against mycobacterial hsp65 did not stain the 78-kD antigen. Interestingly, a human Lf-ANCA serum from a patient with long-standing Yersinia-triggered ReA recognized the 38/40-kD antigens as well as the 78-kD bands. The reactivity of rabbit preimmune serum with 18 and 35 kD of E. coli probably represents reactions to antigens normally found in the intestinal flora of the animal (not shown).
We were not able to absorb the Lf-ANCA reactivity in this serum with live or heat-killed Yersinia bacteria.
Although cross-immunization against bacterial antigens is a possible explanation for the occurrence of Lf-ANCA, it cannot be excluded that prolonged exposure to neutrophil antigens is the driving force for autoantibody production. Massive neutrophil infiltration, either in the intestinal mucosa or in the synovium, is a common feature of the clinical conditions where Lf-ANCA occur, e.g. UC and ReA. The more indolent low active inflammation in the enthesis of the axial skeleton characteristic of AS could possibly explain the lower frequency of ANCA in this condition.
In conclusion, we have shown that ANCA, especially Lf-ANCA, is a frequent finding in patients with ReA, whereas it occurs only occasionally in AS. Lf-ANCA may possibly be generated by cross-immunization against arthritogenic enterobacteria.
REFERENCES
- 1.Schultz DR, Tozman EC. Antineutrophil cytoplasmic antibodies. Major autoantigens, pathophysiology and disease associations. Sem Arthritis Rheum. 1995;25:143–59. doi: 10.1016/s0049-0172(95)80027-1. [DOI] [PubMed] [Google Scholar]
- 2.Falk RJ, Jennette JC. Antineutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988;318:1651–7. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- 3.Gross WL, Csernok E, Flesch K. Classic anti-neutrophil cytoplasmic autoantibodies (cANCA), ‘Wegener's autoantigen’ and their immonopathogenetic role in Wegener's granulomatosis. J Autoimmun. 1993;3:171–84. doi: 10.1006/jaut.1993.1015. [DOI] [PubMed] [Google Scholar]
- 4.Skogh T, Dahlgren C, Holmgren K, Peen E, Stendahl O. Anti-granulocyte antibodies (C-ANCA, P-ANCA, GS-ANA) studied by confocal scanning laser fluorescence microscopy, ELISA and chemiluminescence techniques. Scand J Immunol. 1991;34:137–45. doi: 10.1111/j.1365-3083.1991.tb01530.x. [DOI] [PubMed] [Google Scholar]
- 5.Coremans IEM, Hagen EC, Daha MR, et al. Antilactoferrin antibodies in patients with rheumatoid arthritis are associated with vasculitis. Arthritis Rheum. 1992;35:1466–75. doi: 10.1002/art.1780351210. [DOI] [PubMed] [Google Scholar]
- 6.Schnabel A, Csernok E, Isenberg D, Mrowka C, Gross WR. Antineutrophil cytoplasmic antibodies in systemic lupus erythematosus. Arthritis Rheum. 1995;38:633–7. doi: 10.1002/art.1780380509. [DOI] [PubMed] [Google Scholar]
- 7.Peen E, Almer S, Bodemar G, Ryden B-O, Sjölin C, Tejle K, Skogh T. Antilactoferrin antibodies and other types of ANCA in ulcerative colitis, primary sclerosing cholangitis, and Crohn's disease. Gut. 1993;34:56–62. doi: 10.1136/gut.34.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cramer E, Pryzwansky KB, Villeval JL, Testa U, Breton Gorius J. Ultrastructual localization of lactoferrin and myeloperoxidase in human neutrophils by immunogold. Blood. 1985;65:423–32. [PubMed] [Google Scholar]
- 9.Lash JA, Coates D, Lfuze J, Baehner RL, Boxer LA. Plasma lactoferrin reflects granulocyte activation in vivo. Blood. 1983;61:885–8. [PubMed] [Google Scholar]
- 10.Akin DT, Lu MQ, Lu SJ, Kendall S, Arnold RR. Bactericidal activities of different forms of lactoferrin. Adv Exp Med Biol. 1994;357:61–70. doi: 10.1007/978-1-4615-2548-6_7. [DOI] [PubMed] [Google Scholar]
- 11.Ellison RT. The effect of lactoferrin on gram negative bacteria. Adv Exp Med Biol. 1994;357:71–90. doi: 10.1007/978-1-4615-2548-6_8. [DOI] [PubMed] [Google Scholar]
- 12.Appelmelk BJ, An YQ, Geerts M, et al. Lactoferrin is a lipid A binding protein. Infect Immun. 1994;62:2628–32. doi: 10.1128/iai.62.6.2628-2632.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Britigan BE, Jonathan S, Serody S, Cohen MS. The role of lactoferrin as an anti-inflammatory molecule. Adv Exp Med Biol. 1994;357:143–56. doi: 10.1007/978-1-4615-2548-6_14. [DOI] [PubMed] [Google Scholar]
- 14.Britigan BE, Hassett DJ, Rosen GM, Hamill DR, Cohen MS. Neutrophil degranulation inhibits potential hydroxyl-radical formation. Biochem J. 1989;264:447–55. doi: 10.1042/bj2640447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg JN, Johnson GD, Holborow EJ. Antinuclear antibodies in ankylosing spondylitis, psoriatic arthritis, and psoriasis. Ann Rheum Dis. 1979;38:526–8. doi: 10.1136/ard.38.6.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Locht H, Peen E, Skogh T. Antineutrophil cytoplasmic antibodies in reactive arthritis. J Rheumatol. 1995;22:2304–6. [PubMed] [Google Scholar]
- 17.Sieper J, Kingsley G. Recent advances in the pathogenesis of reactive arthritis. Immunol Today. 1996;17:160–3. doi: 10.1016/0167-5699(96)80612-8. [DOI] [PubMed] [Google Scholar]
- 18.Davenport A. ‘False positive’ perinuclear and cytoplasmic anti-neutrophil cytoplasmic antibody results leading to misdiagnosis of Wegener's granulomatosis and/or microscopic polyarteritis. Clin Nephrol. 1992;37:124–30. [PubMed] [Google Scholar]
- 19.Klaassen RJL, Goldschmeding R, Dolman KN, et al. Antineutrophil cytoplasmic autoantibodies in patients with systemic HIV infection. Clin Exp Immunol. 1992;87:24–30. doi: 10.1111/j.1365-2249.1992.tb06408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yahya TM, Benedict S, Shalabi A, Bayoumi R. Anti-neutrophil cytoplasmic antibody (ANCA) in malaria is directed against cathepsin G. Clin Exp Immunol. 1997;110:41–44. doi: 10.1046/j.1365-2249.1997.4981395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellerbroek PM, Oudkerk Pool M, Ridwan BU, et al. Neutrophil cytoplasmic antibodies (pANCA) in ulcerative colitis. J Clin Pathol. 1994;47:257–62. doi: 10.1136/jcp.47.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abad E, Tural C, Mirapeix E, Cuxart A. Relationship between ANCA and clinical activity in inflammatory bowel diseases: variation in prevalence of ANCA and evidence of heterogeneity. J Autoimmun. 1997;10:175–80. doi: 10.1006/jaut.1996.0114. [DOI] [PubMed] [Google Scholar]
- 23.Broekroelofs J, Mulder AH, Nelis GF, Westerveld BD, Cohen Tervaert JW, Kallenberg CGM. Anti-neutrophil cytoplasmic antibodies (ANCA) in sera from patients with inflammatory bowel diseases (IBD). Relation to disease pattern and disease activity. Dig Dis Sci. 1994;39:545–9. doi: 10.1007/BF02088340. [DOI] [PubMed] [Google Scholar]
- 24.Kossa K, Coulthart A, Ives CT, Pusey CD, Hodgson HJF. Antigen specificity of circulating anti-neutrophil cytoplasmic antibodies in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1995;7:783–9. [PubMed] [Google Scholar]
- 25.Roozendal C, Zhao MH, Horst G, Lockwood CM, Kleibeuker JH, Limburg PC, Nelis GF, Kallenberg CGM. Catalase and a-enolase: two novel autoantigens in inflammatory bowel disease (IBD) Clin Exp Immunol. 1998;112:10–16. doi: 10.1046/j.1365-2249.1998.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mielants H, Veys EM, Cuvelier C, et al. The evolution of spondylarthropathies in relation to gut histology. II. Histological aspects. J Rheumatol. 1995;22:2273–8. [PubMed] [Google Scholar]
- 27.Esagui N, Arguas AP, van Embden JDA, Silva MT. Mycobacteria and human autoimmune disease: direct evidence of cross-reactivity between human lactoferrin and the 65-kilodalton protein of tubercle and leprosy bacilli. Infect Immun. 1991;59:1117–25. doi: 10.1128/iai.59.3.1117-1125.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esagui N, Freire O, van Embden JDA, Arguas AP. Lactoferrin triggers in vitro proliferation of T-cells of Lewis rats submitted to mycobacteria-induced adjuvant arthritis. Scand J Immunol. 1993;38:147–52. doi: 10.1111/j.1365-3083.1993.tb01706.x. [DOI] [PubMed] [Google Scholar]
- 29.Schultz DR, Arnold PI. Heat-shock (stress) proteins and autoimmunity in rheumatic diseases. Sem Arthritis Rheum. 1993;22:357–74. doi: 10.1016/s0049-0172(05)80028-9. [DOI] [PubMed] [Google Scholar]
- 30.Jones DB, Coulson AFW, Duff GW. Sequence homologies between hsp 60 and autoantigens. Immunol Today. 1993;14:115–8. doi: 10.1016/0167-5699(93)90210-C. [DOI] [PubMed] [Google Scholar]
- 31.Hajeer AH, Bernstein RM. Antibody to mycobacterial 65-kD heat shock protein in commercial antisera. Clin Exp Immunol. 1993;94:544–7. doi: 10.1111/j.1365-2249.1993.tb08232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peen E, Skogh T, Eneström S. Distribution of lactoferrin and 60/65 heat shock protein in normal and inflamed human intestine and liver. Gut. 1996;38:135–40. doi: 10.1136/gut.38.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]


