Abstract
HIV-1 in adults changes the proportion of mitogen-stimulated lymphocytes expressing the CD69 activation molecule, but little is known about this molecule expression on lymphocytes of HIV-1-infected (HIV-1+) children. Freshly isolated CD3+, CD4+, CD8+ and CD19+ and phytohaemagglutinin (PHA)-stimulated CD3+, CD4+ and CD8+ lymphocytes co-expressing CD69 were investigated cross-sectionally (adopting a MoAb double-staining technique) in 24 HIV-1+ children with severe disease and given anti-retroviral therapy and in 24 age-matched healthy children. CD69 results in HIV-1+ children were correlated with plasma HIV-1 RNA load prospectively determined. HIV-1+ compared with healthy children had higher frequencies of freshly isolated CD3+CD69+ (2.4 ± 2.2% versus 0.9 ± 0.5%; P = 0.002) and CD8+CD69+ (1.5 ± 1.1% versus 0.5 ± 0.2%; P < 0.0001) lymphocytes. The frequencies of CD4+CD69+ and CD19+CD69+ lymphocytes were similar. High viral load correlated with an elevated proportion of freshly isolated CD3+CD69+ and CD8+CD69+ lymphocytes. HIV-1+ children showed reduced frequencies of PHA-stimulated CD3+CD69+ (60.7 ± 7.6% versus 86.1 ± 7.6%; P < 0.001), CD4+CD69+ (73.6 ± 18.2% versus 92.6 ± 5.1%; P < 0.001), and CD8+CD69+ (51.0 ± 19.1% versus 65.3 ± 15.4%; P = 0.007) lymphocytes. Virologic worsening within 6 months correlated with a low proportion of PHA-stimulated CD3+CD69+ and CD8+CD69+ lymphocytes. CD69 molecule expression reflected the coexistence of immune activation and immune deficiency in HIV-1 infection. Changes partly differed from those observed in HIV-1+ adults. CD8+CD69+ (but not CD4+CD69+) lymphocyte proportion correlated with virologic course, and an impaired ability of CD8+ lymphocytes to express CD69 upon PHA stimulation preceded a virologic worsening.
Keywords: HIV-1, perinatal HIV-1 infection, HIV-1 load, CD69, activation molecules
INTRODUCTION
The CD69 activation molecule [1,2], a possible receptor for positive costimulatory signals, is a phosphorylated 28–32-kD disulphide-linked homodimer constitutively expressed on the membrane of thymocytes, and rapidly induced upon activation on the membrane of T and B lymphocytes through the stimulated antigen receptor/CD3 complex or cross-linking of surface immunoglobulins, respectively. Adults infected with HIV-1 have reduced proportions of T lymphocytes co-expressing CD69 upon mitogen stimulation, but normal proportions in freshly isolated lymphocytes [1]. Little is known about CD69 molecule expression on lymphocytes of HIV-1-infected (HIV-1+) children. We have already shown that the expression of CD38 and human leucocyte antigen class II activation molecules differs on T lymphocyte subsets of HIV-1+ children and adults [3]. The present study investigates the frequencies of CD69+ cells in freshly isolated and phytohaemagglutinin (PHA)-stimulated lymphocyte subsets of HIV-1+ children and their relationships with the virologic course.
PATIENTS AND METHODS
Definitions
The Centers for Disease Control (CDC) and Prevention 1994 criteria were used in defining the children's infection status and clinical condition [3,4]. High viral load was defined as ≥ 5 (children aged ≤ 30 months) or ≥ 4.3 (children aged > 30 months) log10 HIV-1 RNA copy numbers/ml [4]. Virologic worsening (confirmed on two consecutive occasions 2 months apart) was an increase of ≥ 0.7 log10 HIV-1 RNA copy numbers/ml in 6 months [4].
Study groups
Twenty-four children (13 girls and 11 boys aged 84 months (median; range 15–153 months)) with perinatal HIV-1 infection and severe disease, and given two nucleoside reverse transcriptase inhibitors at the time of study entry, were studied. Twenty-four healthy HIV-1− children (10 girls and 14 boys aged 84 months (median; range 15–153 months)), hospitalized before undergoing minor elective surgery, without any known immune diseases, and free of clinically evident infections and medication for at least 1 month prior to the blood sample, were the control group. Blood was taken during routine examinations after the parents' or guardians' informed consent had been obtained. The study received local Ethical Committee approval.
Phenotype of freshly isolated unstimulated and cultured mitogen-stimulated lymphocytes
Peripheral blood mononuclear cells (PBMC) were separated from EDTA-treated blood by centrifugation over Ficoll–Hypaque (δ: 1.077 g/cm3; Pharmacia Biotech, Uppsala, Sweden). One part of the cells was immediately double-stained with CD69 MoAb directly conjugated with PE (Becton Dickinson, San Jose, CA) and CD3, CD4, CD8, or CD19 MoAb directly conjugated with FITC (Dako, Glostrup, Denmark) [3]. Another part of cells was resuspended in RPMI 1640 medium (HyClone Europe, Cramlington, UK) containing 20 μg/ml PHA (Sigma, St Louis, MO), 10% heat-inactivated fetal calf serum (FCS; HyClone Europe), 0.5% penicillin, 0.5% streptomycin, and 1% glutamine [1]. Viable cell numbers were determined by trypan blue exclusion. Cells (105 in 200 μl of medium/well) were cultured in 96-well round-bottomed plates (Costar Corp., Cambridge, MA) at 37°C in a humidified atmosphere of 5% CO2 in air. After a 24-h culture, cells were double-stained with PE-conjugated CD69 and FITC-conjugated CD3, CD4, or CD8 MoAb.
Both freshly isolated and cultured PHA-stimulated cells were incubated with MoAb for 30 min at room temperature, washed twice by centrifugation at 250 g for 10 min at room temperature, and analysed immediately using a FACScan flow cytometer. The cell population was gated using forward and 90° angle light scatter. A minimum of 104 cells/sample was acquired in list mode. Cells staining positive for FITC or both FITC and PE were quantified using the FACScan Lysis II analysis program (Becton Dickinson) [3]. Absolute numbers of circulating lymphocyte subsets were calculated by leucocyte and differential counts performed on EDTA-treated peripheral blood.
Viral load
The plasma viral load (log10HIV-1 RNA copies/ml) was measured quantitatively by the Amplicor HIV Monitor test (Roche Diagnostic System, Inc., Branchburg, NJ). A 142 base pair in the gag gene of HIV-1 was amplified by reverse transcription and polymerase chain reaction in a single reaction with rTth DNA polymerase. A synthetic RNA molecule (with known number of copies) was used as a standard. Biotinylated HIV-1 and standard amplicons were detected by ELISA with five-fold serial dilutions of amplicons. HIV-1 RNA copy numbers were calculated from the known input copy number of standard RNA, the optical densities of sample and standard wells, and the dilution factors were associated with the selected wells [3].
Experimental design and statistical analysis
At the first blood test the viral load and freshly isolated CD3+, CD4+, CD8+, and CD19+ and PHA-stimulated CD3+, CD4+, and CD8+ lymphocytes co-expressing CD69 were determined. The viral load was again determined at three subsequent blood tests carried out 2 months apart. Data were processed through the SPSSX (SPSS Inc., Chicago, IL) statistical package. Lymphocyte subset results were reported as mean and s.d. and the differences were evaluated by Student's t-test. Values of P > 0.05 were defined as not significant.
RESULTS
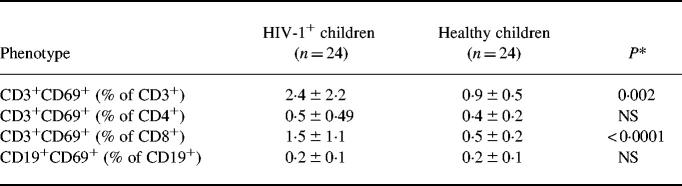
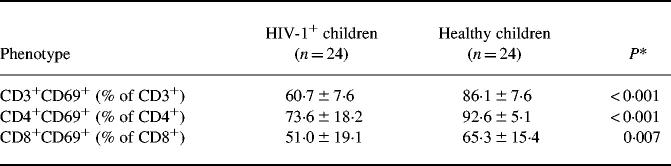
HIV-1+ children, compared with healthy children, had lower absolute numbers of circulating CD3+ (1440 ± 910 versus 2719 ± 305 cells/μl; P < 0.0001) and CD4+ (483 ± 342 versus 1839 ± 321 cells/μl; P < 0.0001) lymphocytes; differences in CD8+ (780 ± 520 versus 814 ± 157 cells/μl) and CD19+ (376 ± 180 versus 523 ± 123 cells/μl) were not significant. The proportion of freshly isolated CD3+ and CD8+ (but not CD4+ and CD19+) lymphocytes co-expressing CD69 was significantly higher in HIV-1+ than in healthy children (Table 1), whereas all T lymphocyte subsets of HIV-1+ children showed a significantly reduced ability to co-express CD69 upon PHA stimulation (Table 2).
Table 1.
Frequency (mean and s.d.) of freshly isolated CD3+, CD4+, CD8+, and CD19+ lymphocytes co-expressing the CD69 molecule
*Student's t-test; NS, not significant.
Table 2.
Frequency (mean and s.d.) of phytohaemagglutinin-stimulated CD3+, CD4+, and CD8+ lymphocytes co-expressing the CD69 molecule
*Student's t-test.
Nine children with high viral load, compared with 15 children without high viral load, had similar frequencies of CD4+ and CD19+ but higher frequencies of CD3+ (3.9 ± 3.06% versus 1.5 ± 1.2%; P = 0.012) and CD8+ (2.1 ± 1.7% versus 0.6 ± 0.2%; P = 0.002) freshly isolated lymphocytes co-expressing CD69. No significant difference according to viral load was observed in PHA-stimulated lymphocytes. No child was lost to follow up, changed anti-retroviral treatment, or died during the 6-month follow up. Ten children showed a virologic worsening at the end of follow up. These children, compared with 14 who did not show virologic worsening, had similar frequencies of CD4+ but reduced frequencies of CD3+ (42.5 ± 14.1% versus 70.1 ± 10.8%; P < 0.0001) and CD8+ (35.8 ± 9.9% versus 64.1 ± 5.8%; P < 0.0001) lymphocytes co-expressing CD69 upon PHA stimulation. Children with or without subsequent virologic worsening did not differ in their frequencies of freshly isolated lymphocyte subsets co-expressing CD69.
DISCUSSION
HIV-1+ children present modified proportions of freshly isolated and PHA-stimulated CD69+ T lymphocytes. These changes partly differ from those observed in HIV-1+ adults, particularly involve the CD8+ subset, and (as far as the CD8+CD69+ lymphocytes are concerned) relate to viral load. It is known that HIV-1 can modify the surface expression of some cell receptors, which may be up- or down-regulated through interference in molecule synthesis, recycling, or shedding [5,6].
Increased proportions of freshly isolated lymphocytes expressing CD69 coexisted with reduced proportions of lymphocytes able to express this molecule upon PHA activation. This apparently paradoxical finding probably reflects the coexistence of immune activation and immune deficiency in HIV-1 infection. It has long been recognized that increased HLA-DR and CD38 molecule expression, elevated IL-6 synthesis, high neopterin and β2-microglobulin serum levels, and polyclonal hypergammaglobulinaemia associate during the course of HIV-1 infection together with CD4+ lymphocyte loss, a reduced IL-2 synthesis, an impaired T lymphocyte function and a poor antibody response [3,7].
Different to adults [1], HIV-1+ children had a proportion of freshly isolated CD8+ lymphocytes co-expressing CD69 at a higher level than normal. These cells are probably activated cytotoxic lymphocytes, as in the case of CD8+ lymphocytes bearing the HLA-DR or CD38 activation molecules [3]. Since the membrane CD69 disappears a few hours after signal extinction [1,2], CD8+ lymphocytes of HIV-1+ children seem to be engaged in some continuous signalling. The relationship between HIV-1 load and freshly isolated CD8+CD69+ lymphocyte proportion seems to suggest a role of HIV-1 in such signalling. An HIV-1 load which is commonly higher in children than adults, even during anti-retroviral treatment [4], might explain the different findings in children and adults. No previous data exist concerning the relationships between HIV-1 load and CD8+CD69+ lymphocytes in humans, but elevated proportions of these lymphocytes correlate in animals with high load of simian immunodeficiency virus [8].
On the other hand, the frequencies of freshly isolated CD4+ and CD19+ co-expressing CD69 did not differ in HIV-1+ and healthy children, suggesting that the CD69 activation pathway is not necessarily involved in CD4+ lymphocyte apoptosis and B lymphocyte hyperactivation. B lymphocyte hyperactivation in HIV-1+ children seems to relate to the CD62L and CD23 rather than CD69 molecules [9].
CD69 expression upon PHA stimulation was impaired in both CD4+ and CD8+ subsets. Such generalized impairment has been already observed in HIV-1+ adults, but the mechanisms behind it remain unknown [1]. Cell infection could account for the impairment of CD4+ lymphocytes, but a minority of these cells are actually infected in HIV-1+ individuals. Exposure to gp120 in vivo inhibits T lymphocytes in expressing CD69 upon subsequent mitogen stimulation in vitro, but the ability to bind gp120 is limited to the CD4+ subset [1]. Since lymphocytes were double-stained using CD69 and CD4 or CD8 MoAb, the frequencies of CD4+ or CD8+ lymphocytes co-expressing CD69 upon PHA stimulation could not be influenced by altered CD4+/CD8+ lymphocyte ratios in HIV-1+ children.
Virologic worsening was preceded by reduced proportions of PHA-stimulated CD8+CD69+ lymphocytes. CD69 expression upon mitogen stimulation is a measure of T lymphocyte functionality in HIV-1-infected individuals [1,2], and T lymphocytes, particularly CD8+CD69+ T lymphocytes, sustain an effective response against HIV-1 [10]. Maybe CD69 expression on PHA-stimulated CD8+ lymphocytes is an immune activation marker which predicts positively during the course of HIV-1 infection.
New changes in the phenotype of circulating lymphocytes of children with HIV-1 perinatal infection are described. Our phenomenological report could spur further studies to define whether these changes have a pathogenic meaning and whether CD69 molecule expression on CD8+ lymphocytes may be an assay to predict and monitor disease progression and treatment response in HIV-1+ children.
Acknowledgments
This study was partly supported by grant no. 9405.18 issued by the Italian Ministero della Sanità, Istituto Superiore di Sanità, Rome, Italy and European shared cost project no BMH4-97-2262.
REFERENCES
- 1.Krowka JF, Cuevas B, Maron DC, Steimer KS, Asher MS, Sheppard HW. Expression of CD69 after in vitro stimulation: a rapid method for quantitating impaired lymphocyte responses in HIV-infected individuals. J Acquir Immune Defic Syndr Hum Retrovir. 1996;11:95–104. doi: 10.1097/00042560-199601010-00013. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen SD, Afzelius P, Ersboll AK, Nielsen JO, Hansen J-ES. Expression of the activation antigen CD69 predicts functionality of in vitro expanded peripheral blood mononuclear cells (PBMC) from healthy donors and HIV-infected patients. Clin Exp Immunol. 1998;114:66–72. doi: 10.1046/j.1365-2249.1998.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Martino M, Rossi ME, Azzari C, Gelli MG, Galli L, Vierucci A. Different meaning of CD38 molecule expression on CD4+ and CD8+ cells of children perinatally infected with human immunodeficiency virus type 1 infection surviving longer than five years. Pediatr Res. 1998;43:752–8. doi: 10.1203/00006450-199806000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Italian Register for HIV Infection in Children. Italian guidelines for the use of antiretroviral treatment in children. Acta Paediatr. 1999;88:228–32. [PubMed] [Google Scholar]
- 5.Kerkau T, Bacik I, Bennink JR, Yewdell JW, Hunig T, Schimpl A, Schubert U. The human immunodeficiency virus type 1 (HIV-1) vpu protein interferes with an early step of the biosynthesis of major histocompatibility complex (MHC) class I molecules. J Exp Med. 1997;185:1295–305. doi: 10.1084/jem.185.7.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trimble LA, Lieberman J. Circulating CD8 T lymphocytes in human immunodeficiency virus-infected individuals have impaired function and downmodulate CD3 zeta, the signaling chain of the T-cell receptor complex. Blood. 1998;91:585–94. [PubMed] [Google Scholar]
- 7.Fahey JL, Taylor JMG, Manna B, Nishanian P, Aziz N, Giorgi JV, Detels R. Prognostic significance of plasma markers of immune activation, HIV viral load and CD4 T-cell measurements. AIDS. 1998;12:1581–90. doi: 10.1097/00002030-199813000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Akari H, Mori K, Otani I, Terao K, Ono F, Adachi A, Yoshikawa Y. Induction of MHC-IIDR expression on circulating CD8(+) lymphocytes in macaques infected with SIVmac239 nef-open but not with its nef-deletion mutant. AIDS Res Hum Retrovir. 1998;14:619–25. doi: 10.1089/aid.1998.14.619. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez C, Thomas JK, O'Rourke S, Stiehm ER, Plaeger S. HIV disease in children is associated with a selective decrease of CD23+ and CD62L+ B cells. Clin Immunol Immunopathol. 1996;81:191–9. doi: 10.1006/clin.1996.0176. [DOI] [PubMed] [Google Scholar]
- 10.Agostini C, Trentin L, Sancetta R, et al. Interleukin-15 triggers activation and growth of the CD8 T-pool in extravascular tissues of patients with acquired immunodeficiency syndrome. Blood. 1997;90:1115–23. [PubMed] [Google Scholar]