Abstract
Bacterial infection coincides with migration of leucocytes from the circulation into the bacterium-infected tissue. Recently, we have shown that endothelial cells, upon binding and ingestion of Staphylococcus aureus, exhibit proinflammatory properties including procoagulant activity and increased intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) expression on the cell surface, resulting in hyperadhesiveness, mainly for monocytes. The enhanced extravasation of monocytes to bacterium-infected sites is facilitated by the local production of chemotactic factors. From another study we concluded that the locally produced chemokine MCP-1 is important in the recruitment of monocytes to the peritoneal cavity in a model of bacterial peritonitis. In the present study we investigated whether cultured human endothelial cells after infection with bacteria produce and release MCP-1, which in turn stimulates monocyte chemotaxis. We observed that endothelial cells released significant amounts of MCP-1 within 48 h after ingestion of S. aureus. This was dependent on the number and the virulence of the bacteria used to infect the endothelial cells. The kinetics as well as the amount of MCP-1 released by S. aureus-infected endothelial cells differed markedly from that released by endothelial cells upon stimulation with IL-1β. Supernatant from S. aureus-infected or IL-1β-stimulated cells promoted monocyte chemotaxis which was almost entirely abrogated in the presence of neutralizing anti-MCP-1 antibody, indicating that most of the chemotactic activity was due to the release of MCP-1 into the supernatant. Our findings support the notion that endothelial cells can actively initiate and sustain an inflammatory response after an encounter with pathogenic microorganisms, without the intervention of macrophage-derived proinflammatory cytokines.
Keywords: MCP-1, endothelial cells, Staphylococcus aureus, chemotaxis, monocytes, intravascular infection
INTRODUCTION
Staphylococcus aureus is one of the causative pathogens of Gram-positive sepsis, which can occur, for example, in intravenous drug abusers [1]. Also, particular clinical procedures such as i.v. needle implantation are known to be a risk factor for S. aureus bacteraemia that may lead to endovascular or endocardial diseases such as vasculitis or acute infective endocarditis [2]. Staphylococcus aureus is also able to colonize intra-abdominal catheters [3] and to invade the mesothelium of the peritoneal cavity of peritoneal dialysis patients causing an acute peritonitis [4,5]. Several studies, including our own, have shown that S. aureus can adhere to and subsequently are internalized by endothelial cells in vitro [1,6,7]. Internalization of S. aureus provokes endothelial cell activation with aspects of inflammation such as increased production of cytokines [8–10], expression of tissue factor-dependent procoagulant activity [11], up-regulation of cell surface adhesion molecules and subsequent hyperadhesiveness for phagocytes, in particular monocytes [6].
In agreement with previous in vitro studies [9,10], we have shown that the chemotactic cytokine IL-8 is secreted by human endothelial cells in response to internalization of S. aureus [12]. This response also resembled our observations with S. aureus-infected human mesothelial cells [13]. IL-8 belongs to a family of structurally related chemotactic cytokines, referred to as chemokines, that are produced and secreted by many different cell types, including stromal cells such as endothelial and mesothelial cells, in response to stimulation with proinflammatory mediators [14–16]. A major action of chemokines is to elicit the selective extravasation of circulating leucocytes and their directional migration into inflamed tissue [16–18]. Production of IL-8 by S. aureus-infected endothelial cells suggests that endothelial cells can develop an efficient mechanism for bacterial elimination through the production of a potent neutrophil chemoattractant, and the subsequent recruitment of the cell population that is specialized in bacterial killing.
However, in reaction to bacterial infection, besides granulocytes also monocytes are recruited to the inflamed tissue. These monocytes usually provide for a competent immune reaction, but excessive accumulation of monocytes may coincide with extensive tissue damage, mainly due to mediators released by activated monocytes [19,20]. From a previous study of an ex vivo bacterial inflammation process, it was concluded that MCP-1 is the main chemokine responsible for the influx of monocytes in the peritoneal cavity during peritonitis [21]. Several other studies revealed that MCP-1 is one of the most potent chemoattractants for monocytes and that activation of monocytes, that are bound to the surface of endothelial cells, by endothelium-derived MCP-1 may be an essential step in the cascade of events that ultimately leads to the emigration of monocytes into inflamed tissue [17,18,22,23]. We surmise that, in addition to up-regulation of adhesion molecules [6], endothelial-derived MCP-1 may contribute to the observed hyperadhesiveness of S. aureus-infected endothelial cells for monocytes. Therefore, the present study was undertaken to determine whether endothelial cells after infection with S. aureus are induced to express MCP-1, which in turn facilitates monocyte chemotaxis. Staphylococcus aureus strains which differ in virulence were used to assess the impact of bacterial colonization on the response of infected endothelial cells.
MATERIALS AND METHODS
Bacterial strains
Two strains of S. aureus were used: one relatively avirulent strain of S. aureus (strain 42D) and a virulent strain of S. aureus (strain CAPD), isolated from spent dialysis fluid of a continuous ambulatory peritoneal dialysis (CAPD) patient suffering from peritonitis [13]. The efficiency of strain 42D to infect endothelial cells can be compared with that of the avirulent S. aureus strain ATCC 25923. The infection characteristics of strain CAPD strongly resembles those of the virulent S. aureus strain RN4220 [24] (unpublished observation). Both strains were stored on agar slants at 4°C. Before infection they were grown overnight in nutrient broth no. 2 (Oxoid Ltd, London, UK) giving an average of 7 × 107 (strain 42D) or 1.5 × 108 (strain CAPD) colony-forming units (CFU)/ml. The bacteria were harvested by centrifugation at 1500 g for 10 min, washed once in PBS and once in M199 (Gibco Labs, Grand Island, NY) with 0.1% (w/v) gelatin. Next, bacteria were opsonized by incubation in M199 supplemented with 0.1% (w/v) gelatin and 10% human serum (HS) for 30 min under rotation (4 rev/min). One wash step followed in M199 with 0.1% (w/v) gelatin before bacteria were suspended in M199 with 5% heat-inactivated HS at the desired concentration.
Human umbilical vein endothelial cell cultures
Endothelial cells were isolated from human umbilical cord veins by collagenase digestion as described by us previously [25]. Cells were resuspended in culture medium, consisting of M199 supplemented with 10% HS, 1 mml-glutamine, 0.1 mg/ml streptomycin, 5 U/ml heparin, 0.1 mg/ml endothelial cell growth factor, 100 U/ml penicillin G and 100 U/ml amphotericin B. Endothelial cells were grown to confluence in 0.75% (w/v) gelatin-coated culture dishes. The cells were then harvested by trypsinization, washed and cultured until confluence in 24-well tissue culture plates on glass cover slips coated with 0.75% (w/v) gelatin (passage 1). A confluent culture contained about 2 × 105 endothelial cells per well. Some experiments were performed with monolayers of endothelial cells that had been exposed to different concentrations of recombinant human IL-1β (specific activity 5 × 108 U/mg; R&D Systems, Abingdon, UK) or to 5 ng/ml recombinant human IL-1α (a gift from Dr P. Lomedico, Hoffmann-La Roche, Nutley, NJ).
Monocyte isolation
Mononuclear cells were isolated from human heparinized blood by Lymphoprep (Nycoprep, Oslo, Sweden) density gradient centrifugation for 30 min at 900 g. The cells were washed and suspended in RPMI 1640 (Gibco BRL Life Technologies, Breda, The Netherlands) containing 20% newborn calf serum (NBCS) to a concentration of 2–4 × 106 cells/ml. The monocytes were further purified by adherence to plasma-treated, gelatin-coated culture flasks according to the method of Freundlich & Avdalovic [26]. Monocyte-enriched suspensions containing > 90% monocytes were recovered by incubation with 10 mm EDTA in PBS and suspended in RPMI 1640 supplemented with 2% NBCS at a concentration of 4 × 105 cells/ml.
Infection assay
Infection assays were performed as described earlier [6]. Briefly, confluent cultures of endothelial cells were washed with warm culture medium without antibiotics. Next, various inocula of S. aureus were added in culture medium without antibiotics. After incubation for 1 h at 37°C the cells were washed twice with warm M199 and incubated with 10 U/ml lysostaphin (Sigma Chemical Co., St Louis, MO) for 5 min to lyse extracellular bacteria. After washing in M199, monolayers of endothelial cells with intracellular bacteria were cultured with M199 with 2% fetal calf serum (FCS), 0.1 mg/ml endothelial cell growth factor and 5 U/ml heparin for different periods of time in a 5% CO2 incubator at 37°C. The supernatants of these cultures were filtered through a 0.2-μm pore filter and analysed in the MCP-1 ELISA. Alternatively, the monolayers of infected endothelial cells were fixed in methanol and stained with Giemsa dye. The efficiency of S. aureus in infecting endothelial cells was determined by light microscopic examination of the stained glass cover slips with infected endothelial cell layers. The number of endothelial cells that had internalized one or more bacteria as well as the mean number of internalized bacteria per infected endothelial cell were counted.
For the dose–response experiments, endothelial cell cultures were incubated with different numbers of bacteria. The bacterial concentrations used to infect endothelial cells were confirmed retrospectively by colony counts after plating the suspensions on diagnostic sensitivity test (DST)-agar. In some experiments, monolayers of endothelial cells were exposed to S. aureus in the presence of 30 ng/ml recombinant human IL-1 receptor antagonist (IL-1Ra; R&D Systems). Control experiments were performed in which endothelial cell monolayers were incubated with UV-killed S. aureus [6], with lysostaphin alone or with cell wall fragments obtained from 2 × 108 lysostaphin-treated S. aureus.
MCP-1 ELISA
The concentration of MCP-1 released in the supernatant of S. aureus-infected or IL-1β-stimulated endothelial cells was measured using a sandwich ELISA as described previously [21]. Briefly, Maxisorb 96-well plates were coated overnight at room temperature with a mouse anti-human MCP-1 catching MoAb 5D3-F7 [27], a kind gift from Dr A. Mantovani. Remaining binding sites were blocked with 0.3% (w/v) gelatin in PBS containing 0.05% (v/v) Tween 20 and the wells were incubated for 60 min at room temperature with a polyclonal goat anti-human MCP-1 detecting antibody (R&D Systems). The wells were washed five times with PBS–0.05% (v/v) Tween 20, incubated for 60 min with peroxidase-conjugated swine anti-goat immunoglobulin (Tago Inc., Burlingame, CA) and washed again. The peroxidase substrate, i.e. 0.11 m NaAc buffer with 0.1 mg/ml 3,3′,5,5′-tetramethyl benzidine and 0.0375 mg/ml H2O2, was then added. The conversion reaction was stopped after 15 min by adding 2 m H2SO4 and the optical density (OD) was measured at 450 nm in a spectrophotometer. The standard concentration curve for MCP-1, using recombinant human MCP-1 (R&D Systems), measured by this ELISA was linear from 0.3 to 3 ng/ml.
Monocyte chemotaxis assay
Monocyte chemotaxis assays were performed as described earlier [21,28]. Briefly, monocytes (2 × 104 cells/well) were placed in the top wells of a standard 48-well chemotaxis chamber (NeuroProbe Inc., MD), which were separated from the bottom wells by a polyvinylpyrrolidone-free 5-μm pore filter (Costar Europe Ltd, Badhoevedorp, The Netherlands). The bottom wells contained either medium alone or 25:l of culture supernatant from S. aureus-infected, non-infected or IL-1β-stimulated endothelial cells. The chamber was incubated for 1 h at 37°C and 5% CO2 to allow monocyte chemotaxis through the filter. Thereafter, the non-migrated cells were scraped off and the filters were dried. The cells on the filters were fixed and stained in coomassie brilliant blue. To elicit maximal monocyte chemotaxis, 25 μl of 10−8 mn-formyl-methionyl-leucyl-phenylalanine (fMLP; Sigma) were used as a positive control. Optimal MCP-1-dependent monocyte chemotaxis was achieved by adding 25 μl of 30 ng/ml human recombinant MCP-1 (R&D Systems) in the bottom wells. Chemotaxis was measured by counting the number of migrated cells in 10 microscopic high-powered fields using a scored eyepiece. Each supernatant was tested in triplicate. Monocyte chemotaxis was standardized to chemotaxis in response to non-stimulated endothelial cell culture supernatant and expressed as the ratio between chemotaxis induced by supernatant from stimulated endothelial cells and chemotaxis induced by supernatant from non-stimulated endothelial cells. To study the contribution of MCP-1 to monocyte chemotaxis, endothelial cell culture supernatants were preincubated with 75 μg/ml of a neutralizing polyclonal goat anti-human MCP-1 antibody (R&D Systems) for 30 min before use in the chemotaxis assay.
Statistical analysis
The MCP-1 concentrations in the supernatants of IL-1β-stimulated or S. aureus-infected endothelial cell cultures were compared mutually and with the MCP-1 concentration in supernatants of non-infected endothelial cells using the Wilcoxon signed rank test. Supernatant concentrations of MCP-1 were considered significantly increased when P < 0.05.
RESULTS
Efficiency of S. aureus strain 42D and CAPD to infect endothelial cells
For both strains of S. aureus the percentage of endothelial cells with intracellularly localized bacteria increased in a concentration-dependent manner (Fig. 1a). For each inoculum of the strain CAPD, the percentage of infected endothelial cells and the mean number of internalized bacteria per infected endothelial cell (Fig. 1b) was higher in comparison with that of cells infected with the less virulent strain 42D. Internalization of strain CAPD was already observed at bacterial numbers of about 104/ml, i.e. one bacterium per 20 endothelial cells. At an inoculum of 5 × 107 bacteria per ml, endothelial cell infection reached almost 100% with a mean of about 75 intracellular bacteria per infected endothelial cell. Internalization of strain 42D started at inocula of at least 106 bacteria per ml and reached a maximum of 86% infected endothelial cells with 20–30 bacteria per cell at 108 bacteria per ml, which confirms our earlier findings [6].
Fig. 1.

Infection of endothelial cells with different Staphylococcus aureus strains. Monolayers of endothelial cells were incubated for 60 min at 37°C with the indicated number of S. aureus strain 42D (•) or strain CAPD (▴). After washing and lysostaphin treatment, the percentage of endothelial cells with internalized bacteria (a) and mean number of intracellular bacteria per infected endothelial cell (b) were determined. Data are expressed as means ± s.d. of three to nine experiments with endothelial cells from different donors.
For both strains of S. aureus, exposure of endothelial cells for 1 h to more than 108 bacteria per ml resulted in detachment of infected endothelial cells and loss of the monolayer integrity.
MCP-1 production
Culture supernatants derived from non-infected, S. aureus-infected or IL-1β-stimulated endothelial cells were analysed for the presence of MCP-1. The kinetic studies presented in Fig. 2 were performed with a fixed number of bacteria or a single dose of IL-1β. In order to achieve a comparable degree of infection, endothelial cells were incubated with either 1 × 107S. aureus strain CAPD or 5 × 107S. aureus strain 42D. This resulted in about 75% infected endothelial cells (see Fig. 1). Supernatant from endothelial cell monolayers cultured up to 48 h in the absence of bacteria contained about 3 ng/ml of MCP-1 (Fig. 2a). A time-dependent statistically significant induction of MCP-1 production was observed in response to activation of endothelial cells with IL-1β (P < 0.01) or infection with either strain 42D or strain CAPD S. aureus (P < 0.02 and P = 0.01, respectively). Stimulation of endothelial cells by IL-1β induced secretion of significant amounts of MCP-1 within 4 h (Fig. 2b), confirming findings of others [29]. MCP-1 production gradually increased to about 6 ng/ml at 48 h after IL-1β stimulation. The kinetics of the release of MCP-1 from S. aureus-infected endothelial cells, however, differed markedly from that of cells stimulated with IL-1β (Fig. 2a). No significant increase in MCP-1 release was observed in the first 11 h after infection. Between 11 h and 23 h after infection the amount of MCP-1 released in the supernatant increased rapidly and consistently increased, although at a lower rate, up to 48 h of culture. After 2 days of culture, maximal MCP-1 concentrations of 21–29 ng/ml were achieved depending on the type of bacterium. Although the degree of endothelial cell infection was similar (about 75%) for both strains of S. aureus at each time point, the amount of MCP-1 released from endothelial cells infected with strain CAPD was higher compared with strain 42D-infected endothelial cells (Fig. 2a). This difference, however, was not statistically significant (P = 0.05). The amount of MCP-1 secreted by endothelial cells at 23 h after infection increased with increasing concentrations of bacteria (Fig. 3). This was seen for both S. aureus strains. For each bacterial inoculum, the amount of MCP-1 produced in response to infection with the strain CAPD was higher compared with strain 42D. No MCP-1 secretion was observed at 3 h after infection.
Fig. 2.
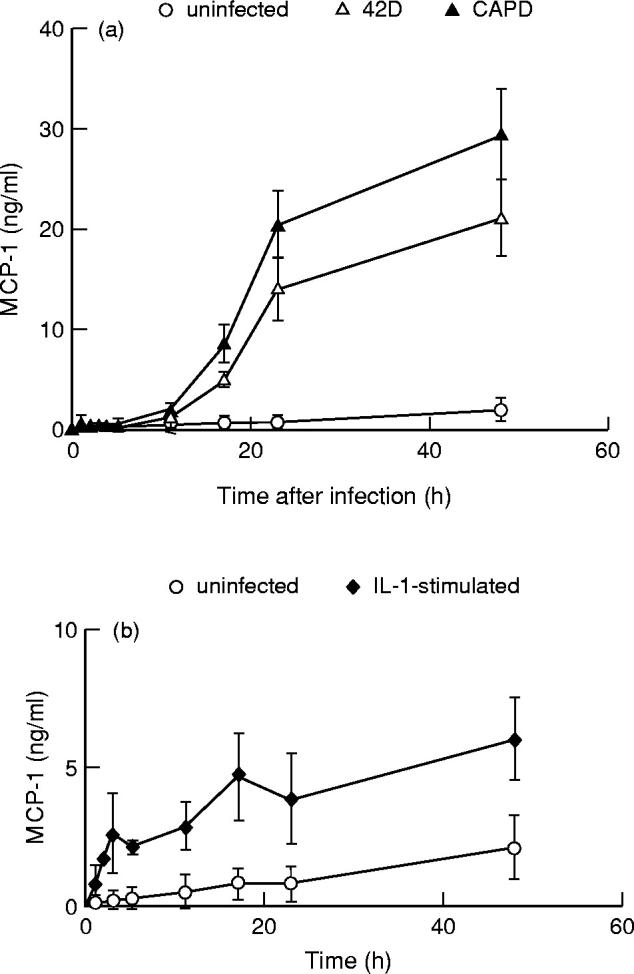
Time course of MCP-1 production by endothelial cells. Monolayers of endothelial cells were incubated for 60 min at 37°C with medium alone, with 5 × 107Staphylococcus aureus strain 42D, with 1 × 107S. aureus strain CAPD (a) or with 100 U/ml recombinant human IL-β (b), washed and treated with lysostaphin and cultured for different time periods. The release of MCP-1 into the culture medium was measured and plotted against time of culture. Values represent means ± s.e.m. of three experiments with endothelial cells from different donors.
Fig. 3.
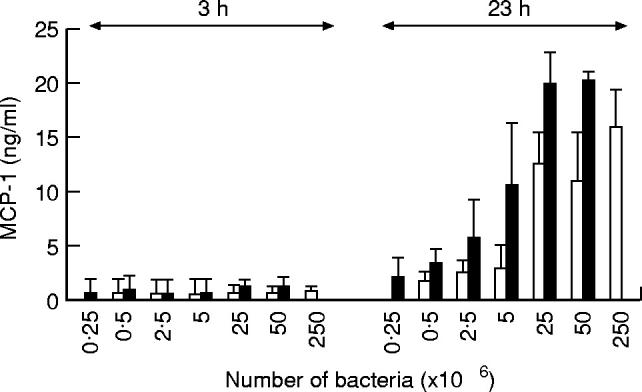
Dose dependence of the MCP-1 secretion by Staphylococcus aureus-infected endothelial cells. Monolayers of endothelial cells were incubated with increasing numbers of S. aureus strain 42D (□) or strain CAPD (▪), for 60 min at 37°C, washed with lysostaphin and cultured for an additional 3 h or 23 h and the amount of MCP-1 secreted in the culture supernatant was measured. Values represent means ± s.d. of four experiments with endothelial cells from different donors.
Control experiments in which endothelial cells were incubated with lysostaphin alone or with cell wall fragments obtained from 2 × 108 lysostaphin-treated S. aureus (strain 42D) did not show MCP-1 production above control level, i.e. MCP-1 produced by untreated endothelial cells (data not shown). Incubation of endothelial cells with UV-killed S. aureus induced a small but significant (P < 0.05, n = 3) increase in endothelial MCP-1 production (18% above the control level).
Influence of endogenous IL-1
To elucidate whether the enhanced production of MCP-1 by S. aureus-infected endothelial cells is the result of endothelial stimulation by endogenous IL-1, the infection assay with strain 42D was performed in the presence of 30 ng/ml IL-1Ra, a potent IL-1α and IL-1β antagonist. The data depicted in Table 1 show that IL-1Ra did not affect MCP-1 secretion by infected endothelial cells, whereas it completely hampered the IL-1α-induced MCP-1 production by these cells.
Table 1.
Contribution of endogenous IL-1 to the production of MCP-1 by Staphylococcus aureus-infected endothelial cells

* Monolayers of 2 × 105 endothelial cells were treated as indicated in the presence or absence of 30 ng/ml IL-1Ra, followed by an additional 7-h or 24-h culture period. Supernatants were analysed for the presence of MCP-1 as described in Materials and Methods.
† Values represent mean ± s.d. of three experiments with endothelial cells from different donors.
‡ Significantly different (P < 0.01) compared with IL-1 in the absence of IL-1Ra.
Bioactivity of MCP-1: monocyte chemotaxis
The chemotactic bioactivity of culture supernatant of endothelial cells stimulated with IL-1β or infected with S. aureus strain 42D or CAPD was measured. Supernatants were harvested from cultures of infected endothelial cells that showed a similar degree of infection (about 75%) for both S. aureus strains and secreted comparable levels of MCP-1 (19–22 ng/ml). Spontaneous migration of monocytes in response to supernatant from untreated endothelial cell cultures was about 6%. Supernatant from IL-1β-stimulated endothelial cells as well as from endothelial cells infected with either strain of bacteria showed markedly increased chemotactic activity towards monocytes compared with control supernatant from untreated endothelial cell cultures (Fig. 4). Significant migration of monocytes (more than four times above control level) towards supernatants from IL-1β-stimulated endothelial cells was already detectable within 1 h after stimulation, whereas chemotaxis induced by supernatant from bacteria-infected endothelial cells was markedly increased at 11 h after infection (Fig. 4). A neutralizing anti-human MCP-1 antibody (Fig. 4) effectively blocked chemotactic activity of these supernatants for monocytes. The percentage of monocytes that migrated in response to 30 ng/ml recombinant human MCP-1 was greater than 50% of that migrating in response to the reference chemoattractant 10−8 fMLP (data not shown), confirming previous results [21,30]. The anti-MCP-1 antibody completely abolished the increased monocyte migration towards recombinant human MCP-1, but had no effect on the fMLP-induced chemotaxis (not shown).
Fig. 4.

Monocyte chemotactic activity in supernatants of endothelial cells. Monolayers of endothelial cells were stimulated with 100 U/ml recombinant human IL-β, or incubated with 5 × 107 strain 42D or with 1 × 107 strain CAPD for 60 min at 37°C. After washing and lysostaphin treatment, the endothelial cells were subsequently cultured. At different time points, culture supernatants were harvested and tested for chemotactic activity towards human monocytes. A representative experiment is shown. Data are expressed as the ratio between the number of migrated monocytes towards culture supernatant of infected or IL-1-β-stimulated endothelial cells and the number of migrated monocytes towards culture supernatant of untreated endothelial cells. Chemotaxis was tested in the presence (□) or absence (▪) of neutralizing anti-MCP-1 antibody.
DISCUSSION
In this study we demonstrate that infection of endothelial cells with S. aureus is accompanied by a significantly increased production of the monocyte chemoattractant MCP-1. The amount of MCP-1 released from infected endothelial cells was dependent on the degree of infection and the time of culture after infection. The kinetics of the release of MCP-1 from S. aureus-infected endothelial cells was clearly different from that released by IL-1β-stimulated endothelial cells. Endothelial cell-derived MCP-1, present in the supernatants of S. aureus-infected or IL-1β-stimulated endothelial cells, accounted almost entirely for the chemotactic activity of human monocytes.
The ability of endothelial cells to produce MCP-1 after exposure to proinflammatory cytokines, in particular IL-1β and tumour necrosis factor-alpha (TNF-α), has been described by others [15,22,29]. The present data, however, demonstrate that endothelial cells produce MCP-1 also in response to infection with S. aureus pathogens. This response is probably elicited by the process of bacterial binding and internalization. This is illustrated by our findings that live as well as intact UV-killed S. aureus, both being bacteria that are efficiently internalized by endothelial cells [6], enhanced endothelial MCP-1 production, whereas endothelial exposure to bacterial fragments from lysed S. aureus did not. It should be noted that endothelial cells in response to their exposure to UV-killed S. aureus produced less MCP-1 compared with similar numbers of live S. aureus. This may indicate that, in addition to bacterial binding and internalization, possible intracellular bacterial proliferation or intracellular release of bacterial factors contribute to elicit MCP-1 production. This, however, awaits further investigation.
The amount of MCP-1 released in the supernatant of endothelial cell cultures infected with the virulent S. aureus strain CAPD is more pronounced and is detectable at a lower inoculum compared with that released by endothelial cells infected with the relatively non-virulent strain 42D. This is explained, at least in part, by the enhanced invasive capacity of strain CAPD and implies that the number of infected endothelial cells and ingested bacteria define the MCP-1 concentration in the supernatant. However, the data depicted in Fig. 2 suggest that certain characteristics specific for the virulent strain CAPD may also contribute to MCP-1 induction. In these experiments, the percentage of infected endothelial cells was similar for either bacterial strain, whereas the amount of MCP-1 released by strain CAPD-infected cells tended to be slightly higher compared with cells infected with strain 42D. Statistical analysis of these data revealed a P value of 0.05, indicating that this difference should be interpreted as non-significant.
The present data further demonstrate that supernatants from cultures of endothelial cells stimulated with IL-1β or infected with S. aureus contained monocyte chemotactic activity. At time points less than 23 h after stimulation or infection, this chemotactic activity was attributable solely to the increased MCP-1 production. However, such was not the case when endothelial supernatants, obtained at later time points after stimulation or infection, were analysed. This was demonstrated by the finding that neutralizing anti-MCP-1 antibodies did not completely inhibit the chemotaxis of monocytes observed in the presence of these endothelial supernatants. This may indicate that at these later time points other molecules with chemotactic activity towards monocytes are produced. Indeed, our preliminary experiments with S. aureus-infected endothelial cells demonstrated a modest release of the chemokine regulated on activation normal T cell expressed and secreted (RANTES) at 23 h and 48 h after infection which was preceded by induction of RANTES mRNA (unpublished observations). Besides endothelial cell-derived chemokines, chemotactic activity for monocytes caused by bacterial degradation products can also not be excluded.
The difference in chemotactic activity in supernatants of 23 h IL-1β-stimulated endothelial cells in comparison with that of 11 h IL-1β-stimulated cells was not expected, since both supernatants contained similar quantities of MCP-1. We assume that endothelial cells after prolonged IL-1β stimulation release factors that can interfere with MCP-1-induced monocyte chemotaxis. A possible candidate for such a factor could be IL-1. This cytokine is produced by endothelial cells after stimulation with IL-1β for at least 20 h (this study, data not shown) and has recently been identified as a cytokine that decreases monocyte chemotaxis through inhibition of expression of the monocyte CCR2 receptor for MCP-1 [31].
It has been reported that endothelial cells express and secrete cytokines including IL-1β upon bacterial infection [8]. Therefore it is plausible to suggest that such endogenous IL-1 could activate the cells in an auto- or paracrine manner. A contribution of endogenous IL-1 with respect to the present results can be excluded, since MCP-1 production by S. aureus-infected endothelial cells was not affected by the presence of IL-1Ra. In addition, mRNA for IL-1 in S. aureus-infected endothelial cells could not be detected up to at least 20 h after infection using polymerase chain reaction (PCR) (data not shown). Analogously, S. aureus-infected endothelial cells do not express mRNA for TNF-α (data not shown), arguing against a possible contribution of endogenous TNF-α in the MCP-1 release by infected endothelial cells.
In our previous studies [6,12] it was shown that internalization of S. aureus induced endothelial cell activation that resulted in surface expression of the adhesion receptors intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) and enhanced binding of monocytes, indicating the important role of endothelial cells in the recruitment of these phagocytes to sites of infection. Endothelial-derived MCP-1 may play an additional regulatory role in this migratory process. Randolph & Furie [32] showed that MCP-1, when secreted by IL-1-activated endothelium, promoted the transendothelial migration of monocytes in vitro by a concentration gradient-dependent chemotactic mechanism. These findings were confirmed by others (e.g. [33]) and agree with the general assumption that soluble chemotactic molecules released by activated endothelial cells induce leucocyte activation and adhesion to endothelial cells and guide their emigration into tissue [16,34]. Alternatively, MCP-1, like the structurally related chemokine macrophage inflammatory protein-1β (MIP-1β), could be captured by proteoglycans on the apical endothelial surface [35,36]. Monocytes that adhere to endothelial cells could then be directly exposed to the immobilized chemokines and be activated to promote their transendothelial migration.
In conclusion, the current study shows that endothelial cells upon ingestion of S. aureus are actively involved in the recruitment of monocytes from the circulation. These data, together with evidence from recent studies with vascular endothelial cells [6–12] or mesothelial cells, i.e. cells that line the body cavities [5,13], suggest that stromal cells can actively initiate and sustain an inflammatory response after encounter with invasive pathogenic microorganisms. For endothelial cells we surmise this can proceed without the intervention of macrophages or macrophage-derived proinflammatory cytokines.
Acknowledgments
Part of this study was supported by the Institute for Radiopathology and Radiation Protection, J. A. Cohen Institute, Leiden, The Netherlands, Grant 3.2.13.
REFERENCES
- 1.Lowy FD. Staphylococcus aureus infections (review) N Engl J Med. 1998;339:520–32. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Libman H, Arbeit RD. Complications associated with Staphylococcus aureus bacteremia. Arch Intern Med. 1984;144:541–5. [PubMed] [Google Scholar]
- 3.Herrmann M, Vaudaux PE, Pittet D, Auckenthaler R, Lew PD, Schumacher-Perdreau F, Peters G, Waldvogel FA. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical Staphylococcal isolates to foreign material. J Infect Dis. 1988;158:693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- 4.Haagen IA, Heezius HC, Verkooyen RP, Verhoef P, Verbrugh HA. Adherence of peritonitis causing Staphylococci to human peritoneal mesothelial cell monolayers. J Infect Dis. 1990;161:266–73. doi: 10.1093/infdis/161.2.266. [DOI] [PubMed] [Google Scholar]
- 5.Visser CE, Brouwer-Steenbergen JJE, Schadee-Eestermans IL, Meijer S, Krediet RT, Beelen RHJ. Ingestion of Staphylococcus aureus, Staphylococcus epidermidis, and Escherichia coli by human peritoneal mesothelial cells. Infect Immun. 1996;64:3425–8. doi: 10.1128/iai.64.8.3425-3428.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beekhuizen H, van de Gevel JS, Olsson B, van Benten IJ, van Furth R. Infection of human vascular endothelial cells with Staphylococcus aureus induces hyper-adhesiveness for human monocytes and granulocytes. J Immunol. 1997;158:774–82. [PubMed] [Google Scholar]
- 7.Ogawa SK, Yurberg ER, Hatcher VB, Levitt MA, Lowy FD. Bacterial adherence to human endothelial cells in vitro. Infect Immun. 1985;50:218–24. doi: 10.1128/iai.50.1.218-224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao L, Bengualid V, Lowy FD, Gibbons JJ, Hatcher VB, Berman JW. Internalization of Staphylococcus aureus by endothelial cells induces cytokine gene expression. Infect Immun. 1995;63:1835–9. doi: 10.1128/iai.63.5.1835-1839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soderquist B, Kallman J, Holmberg H, Vikerfors T, Kihlstrom E. Secretion of IL-6, IL-8 and G-CSF by human endothelial cells in vitro in response to Staphylococcus aureus and staphylococcal exotoxins. APMIS. 1998;106:1157–64. [PubMed] [Google Scholar]
- 10.Yao L, Lowy FD, Berman JW. Interleukin-8 gene expression in Staphylococcus aureus-infected endothelial cells. Infect Immun. 1996;64:3407–9. doi: 10.1128/iai.64.8.3407-3409.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veltrop MHAM, Beekhuizen H, Thompson J. Procoagulant properties of endothelial cells after infection with bacteria. Endothelium. 1997;5:358. [Google Scholar]
- 12.van de Gevel JS, Veltrop MHAM, Thompson J, Tekstra J, Beekhuizen H. Vascular endothelium displays pro-inflammatory-like characteristics upon binding and ingestion of bacteria. Immunol Letters. 1997;56:257–8. [Google Scholar]
- 13.Visser CE, Steenbergen JJE, Betjes MHG, Meijer S, Arisz L, Hoefsmit ECM, Krediet RT, Beelen RHJ. Interleukin-8 production by human mesothelial cells after direct stimulation with Staphylococci. Infect Immun. 1995;63:4206–9. doi: 10.1128/iai.63.10.4206-4209.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Adv Immunol Rev. 1994;55:97–179. [PubMed] [Google Scholar]
- 15.Sica A, Wang JM, Colotta F, et al. Monocyte chemotactic and activating factor gene expression induced in endothelial cells by IL-1 and tumor necrosis factor. J Immunol. 1990;144:3034–8. [PubMed] [Google Scholar]
- 16.Taub DD. The role of chemokines in leukocyte recruitment in inflammation. In: Peltz G, editor. Leukocyte recruitment in inflammatory disease. R G Landes Co.; 1993. pp. 43–68. [Google Scholar]
- 17.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 18.Taub DD. Chemokine–leukocyte interactions. The Voodoo that they do so well. Cytok Growth Factor Rev. 1996;7:355–76. doi: 10.1016/s1359-6101(97)89237-4. [DOI] [PubMed] [Google Scholar]
- 19.Fujita H, Morita I, Murota S. A possible mechanism for vascular endothelial cell injury elicited by activated leukocytes: a significant involvement of adhesion molecules, CD11/CD18, and ICAM-1. Arch Biochem Biophys. 1994;309:62–69. doi: 10.1006/abbi.1994.1085. [DOI] [PubMed] [Google Scholar]
- 20.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 21.Tekstra J, Visser CE, Tuk CW, Brouwer-Steenbergen JJE, Burger CW, Krediet RT, Beelen RHJ. Identification of the major chemokines that regulate cell influxes in peritoneal dialysis patients. J Am Soc Nephrol. 1996;7:2379–84. doi: 10.1681/ASN.V7112379. [DOI] [PubMed] [Google Scholar]
- 22.Yi-Shuan L, Yeun-Jund S, Wright JG, Valente AJ, Cornhill JF, Kolattukudy PE. The expression of monocyte chemotactic protein (MCP-1) in human vascular endothelium in vitro and in vivo. Mol Cell Biochem. 1993;126:61–68. doi: 10.1007/BF01772208. [DOI] [PubMed] [Google Scholar]
- 23.Rollings BJ. Monocyte chemoattractant protein 1: a potential regulator of monocyte recruitment in inflammatory disease. Mol Med Today. 1996;2:198–204. doi: 10.1016/1357-4310(96)88772-7. [DOI] [PubMed] [Google Scholar]
- 24.Lee JC. Electrotransformation of Staphylococci. Methods Mol Biol. 1995;47:209–16. doi: 10.1385/0-89603-310-4:209. [DOI] [PubMed] [Google Scholar]
- 25.Beekhuizen H, van Furth R. Growth characteristics of cultured human macrovascular venous and arterial and microvascular endothelial cells. J Vasc Res. 1994;31:230–9. doi: 10.1159/000159048. [DOI] [PubMed] [Google Scholar]
- 26.Freundlich B, Avdalovic N. Use of gelatin/plasma coated flasks for isolating human peripheral blood monocytes. J Immunol Methods. 1983;62:31–37. doi: 10.1016/0022-1759(83)90107-2. [DOI] [PubMed] [Google Scholar]
- 27.Peri G, Milanese C, Matteucci C, Ruco L, Zhou D, Sozzani S, Coletta I, Mantovani A. A new monoclonal antibody (5D3-F7) which recognizes human monocyte-chemotactic protein-1 but not related chemokines. Development of a sandwich ELISA and in situ detection of producing cells. J Immunol Methods. 1994;174:249–57. doi: 10.1016/0022-1759(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 28.Martinet Y, Martinet N, Vignaud JM, Plenat F. Blood monocyte chemotaxis. J Immunol Methods. 1994;174:209–14. doi: 10.1016/0022-1759(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi M, Masuyama J-I, Ikeda U, Kasahara T, Kitagawa S-I, Takahashi Y-I, Shimada K, Kano S. Induction of monocyte chemoattractant protein-1 synthesis in human monocytes during transendothelial migration in vitro. Circ Res. 1995;76:750–7. doi: 10.1161/01.res.76.5.750. [DOI] [PubMed] [Google Scholar]
- 30.Tekstra J, Tuk CW, Beelen RHJ. Detection of CD14 on migrated monocytes by specific antibody: a possible quantification for blood monocyte chemotaxis. Immunobiol. 1996;195:491–8. doi: 10.1016/S0171-2985(96)80018-0. [DOI] [PubMed] [Google Scholar]
- 31.Penton-Rol G, Polentarutti N, Liuni W, Borsatti A, Mancinelli R, Sica A, Sozzanni S, Mantovani A. Selective inhibition of expression of the chemokine receptor CCR2 in human monocytes by IFN-γ. J Immunol. 1998;160:3869–73. [PubMed] [Google Scholar]
- 32.Randolph GJ, Furie MB. A soluble gradient of endogenous monocyte chemoattractant protein-1 promotes the transendothelial migration of monocytes in vitro. J Immunol. 1995;155:3610–8. [PubMed] [Google Scholar]
- 33.Takahashi M, Masuyama J-I, Ikeda U, Kitagawa S-I, Kasahara T, Saito M, Kano S, Shimada K. Suppressive role of endogenous endothelial monocyte chemoattractant protein-1 on monocyte transendothelial migration in vitro. Atherioscler Thromb Vasc Biol. 1995;15:629–36. doi: 10.1161/01.atv.15.5.629. [DOI] [PubMed] [Google Scholar]
- 34.del Pozo MA, Sánchez-Mateos P, Sánchez-Madrid F. Cellular polarization induced by chemokines: a mechanism for leukocyte recruitment? Immunol Today. 1996;17:127–31. doi: 10.1016/0167-5699(96)80604-9. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka Y, Adams DH, Hubscher S, Hirano H, Siebenlist U, Shaw S. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1β. Nature. 1993;361:79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka Y, Adams DH, Shaw S. Proteoglycans on endothelial cells present adhesion-inducing cytokines to leukocytes. Immunol Today. 1993;14:111–5. doi: 10.1016/0167-5699(93)90209-4. [DOI] [PubMed] [Google Scholar]


