Abstract
CSF-1 plays an important role in female reproduction and normal embryo development. To understand further CSF-1 function in normal and, especially, in compromised pregnancy, we studied the pattern of its mRNA expression as well as expression of its receptor (c-fms) in the uteroplacental units of mice with induced (cyclophosphamide (CY)-treated) and spontaneous (CBA/J × DBA/2J mating combination) pregnancy loss. RNase protection analysis demonstrated the presence of two forms of CSF-1 mRNA in the uteroplacental unit corresponding to 1400- and 263-bp protective fragments. Densitometric analysis demonstrated that the level of 1400-bp mRNA form was decreased by 40% in the uteroplacental units of mice with CY-induced pregnancy loss compared with the control mice. About 20% decrease in 263-bp protective fragment was registered in resorbing versus non-resorbed placenta of CBA/J females mated to DBA/2J males. As judged by in situ hybridization assay, CSF-1 mRNA transcripts were localized in the uterine epithelium and stroma, while c-fms mRNA was found mainly in the trophoblast. The number of metrial gland cells as well as the number of uterine leucocytes expressing CSF-1 and c-fms mRNAs was substantially lower in the uteroplacental unit of mice with pregnancy loss than in control animals. Maternal immunostimulation, while significantly decreasing the resorption rate in mice with CY-induced pregnancy loss, also strengthened CSF-1 mRNA expression at the fetomaternal interface and resulted in reconstitution in the number of CSF-1+ uterine leucocytes and metrial gland cells. These data suggest a role for uterine CSF-1 in the physiology of normal and compromised pregnancy and demonstrate a possible involvement of CSF-1-associated signalling in mechanisms of placenta and endometrium repair following immunopotentiation.
Keywords: cytokines, mouse resorptions, c-fms, immunopotentiation
INTRODUCTION
Allorecognition of the embryo by the maternal immune system is associated with production of cytokines at the fetomaternal interface, which support placental growth and function and play an important role in protective mechanisms determining the embryo's survival and normal development [1,2].
Macrophage colony-stimulating factor (CSF-1 or M-CSF) regulates the proliferation and differentiation of mononuclear phagocytes [3] and elevation of CSF-1 expression in the pregnant uterus is associated with a substantial increase in the number of uterine macrophages [4]. CSF-1 bioactivity is high in the mouse uterus, placenta and amniotic fluid [5,6]. Thus, the expression of CSF-1 mRNA transcripts in the uterine epithelium of mice was detected on day 3 and it reached a peak at days 14–15 of pregnancy [7]. In humans, the cellular pattern of CSF-1 expression resembles that in the mouse. However, human trophoblast expresses CSF-1 mRNA transcripts, which are not found in the mouse [7,8].
Two alternative spliced forms of CSF-1 mRNA in the uterus, 2.3 kb and 4.6 kb, give rise to both soluble and membrane-bound forms of the cytokine [9]. The synthesis of uterine CSF-1 is regulated by the female reproductive hormones. Administration of oestrogen or progesterone to ovariectomized mice increases CSF-1 production by the uterine epithelium [10]. CSF-1 is also differentially expressed by various organs and tissues of the developing embryo, suggesting the existence of a tissue-specific mechanism regulating its expression [11].
The biological activity of CSF-1 is dependent on binding to its receptor on target cells. The receptor is encoded by the c-fms proto-oncogene [12,13]. In haematopoietic cell lineages the c-fms antigen may serve as a macrophage differentiation marker [14]. Its transcripts have also been found in the trophoectoderm of the blastocyst, and, its expression intensifies in the trophoblast as the placenta develops [9,15].
Functions of CSF-1 in pregnancy are still unclear. Correlation of the temporal pattern of CSF-1 mRNA expression at the fetomaternal interface with both placental growth and c-fms expression by the trophoblast suggests that CSF-1 may be involved in the regulation of placental growth and differentiation [9,16]. It has been reported that CSF-1 may increase the rate of proliferation of placenta-derived cells [17,18], and stimulates the differentiation of the human cytotrophoblast in vitro [19]. CSF-1 has also been shown to regulate cell division in the blastocyst before implantation [20].
The role of CSF-1 in female reproduction has been clarified by studying mice lacking functional CSF-1. Homozygous females with a mutated CSF-1 gene (op/op) mutation have macrophage deficiency, skeletal anomalies and absence of teeth [21]. Fertility is also impaired in these females when mated with homozygous op/op males [21]. In these mice, exogenous CSF-1 administration partially prevents skeletal anomalies, but does not improve the fertility rate [22].
Since maintenance of the local immune microenvironment at the fetomaternal interface is required for healthy pregnancy [2,23], modulation of maternal immune responses may be beneficial for embryo survival in compromised pregnancies [24]. Indeed, it has repeatedly been shown that stimulation of the maternal immune response may protect the fetus and improve reproductive outcome in both humans and animals [23,25]. This protective effect of immunopotentiation is accompanied by alterations in cytokine production and activities at the fetomaternal interface [26–28]. However, the pattern of CSF-1 expression at the fetomaternal interface following maternal immunostimulation has not yet been characterized.
This study delineates the pattern of CSF-1 and c-fms mRNA expression in the uteroplacental unit of mice with a high rate of pregnancy loss, and assesses changes in their expression following maternal immunostimulation.
MATERIALS AND METHODS
Animals
Six-to-eight-week old ICR and C57Bl/6 mice and Long Evans rats were obtained from the Tel Aviv University breeding colonies. CBA/J females and DBA/2J males were obtained from The Jackson Laboratory (Bar Harbor, ME). The animals were maintained on a 14-h light/10-h dark cycle with food and water ad libitum. To obtain pregnancies, females were caged with males overnight and the presence of a vaginal plug was designated as day 1 of pregnancy.
Animal models of pregnancy loss
Two mouse models of pregnancy loss were used in this study.
The CBA/J × DBA/2J mouse combination, which is well known for its high level of post-implantation loss (approximately 30%), was used as a model of spontaneous abortions [29]. Cyclophosphamide (CY)-treated ICR × ICR and CBA/J × C57Bl/6 mouse combinations were used as models of induced pregnancy loss.
CY was injected on day 12 of pregnancy at a dose of 40 mg/kg as described elsewhere [30]. Control females were injected with saline.
CBA/J females mated to DBA/2J males were killed on day 12 or 19 of gestation, while CY-treated mice were killed on days 15 or 19 of pregnancy. The numbers of implantation sites, resorptions and live fetuses were recorded in these animals and the incidence of post-implantation loss was calculated as described elsewhere [30].
Immunopotentiation
CBA/J and ICR females were treated with either allogeneic paternal (C57Bl/6) or xenogeneic rat splenocytes, respectively, 21 days before mating, as described elsewhere [31]. Briefly, splenocytes at 25–30 × 106/0.04 ml saline were injected under Nembutal anaesthesia into each uterine horn. Mice injected with saline or syngeneic splenocytes served as controls.
Tissue processing
Placentas together with the adjacent uterus were collected from mice with spontaneous resorptions on day 12 and from CY-treated mice on day 15 of pregnancy. Two types of placenta, the non-resorbed and resorbing placenta, were tested in the present study. The term ‘resorbing placenta’ refers to a placenta from both CY-treated and CBA/J mice mated to DBA/2J having dead embryo or remnants of extra-embryonic tissues, but which could still be identified macroscopically as a placenta. The histological examination of these placentas showed that trophoblast tissue was mainly injured, and necrotic zones in placental tissue were infiltrated by numerous leucocytes. The term ‘non-resorbed placenta’ refers to a placenta of CY-treated mice that had live embryos and was visibly indistinguishable from placentas of control non-treated mice. Microscopically, these placentas demonstrated a normal placental morphology, excepting some trophoblast cells located underneath of decidua and displaying highly fragmented and picnotic nuclei.
Probe construction
The 1400-bp SmaI-XhoI fragment of CSF-1 cDNA (a gift from Dr B.Tartakovsky, Weizmann Institute of Science, Israel) was subcloned into the pBluescript SK (+) vector (Stratagene, La Jolla, CA). The 2500-bp c-fms cDNA was in the EcoRI-HindIII site of the pGEM2 vector (kindly provided by Dr L. Rohrscheinder, Fred Hutchinson Cancer Research Center, Seattle, WA). After linearization with SmaI for CSF-1 and HindIII for c-fms cDNA, the cDNA templates served for generation of digoxigenin (DIG)-11-UTP-labelled (Boehringer, Mannheim, Germany) anti-sense RNA probes using T7 RNA polymerase for CSF-1 riboprobe and the SP6 RNA polymerase (Stratagene) for c-fms probe. RNA probes for β-actin (360 bp) and the procaryotic neo gene (760 bp) were synthesized as described above. The length of the generated RNA probes was controlled by electrophoresis on denatured 5% polyacrylamide/urea gel.
RNase protection analysis
Total RNA was extracted from placentas and uteri by the method of Chomzynski & Sacchi [32] using the TRI reagent (Molecular Research Center Inc., Cincinnati, OH). RNA integrity was monitored by electrophoresis in 1% agarose/2.2 m formaldehyde gel. The following procedures are those described earlier [28]. Briefly, 30–50 μg total RNA were co-precipitated with 30 ng of anti-sense DIG-labelled RNA probe, incubated overnight in 20 μl of hybridization buffer (80% formamide, 1 mm EDTA, 0.2 m sodium acetate and 40 mm PIPES, pH 6.4) at 45°C and then digested with 16 U RNase ONE (Promega, Madison, WI) for 1 h at room temperature. Following RNase inactivation, RNA was precipitated, resuspended in gel loading buffer and protected fragments were resolved by electrophoresis through 5% polyacrylamide/8 m urea gel. Then the RNA protected fragments were transferred to Nytran nylon membranes (Schleicher and Schuell, Dassel, Germany). Hybridization bands were visualized by chemiluminescent method by incubating the blots in alkaline phosphatase-conjugated anti-DIG antibodies according to the manufacturer's instructions (Boehringer) with CSPD chemiluminescent substrate (Tropix, Bedford, MA) followed by exposure to x-ray film. The molecular weights of specific mRNA were calculated using the DIG-labelled VIII type DNA marker (Boehringer). As a negative control, a sample of tissue RNA was replaced by yeast tRNA. Additionally, undigested full length probe was electrophoresed in parallel with the tested samples. Equivalence of RNA loading on the gel was controlled by hybridization of the same quantity of tissue RNA with β-actin riboprobe (a 250-bp protected fragment). The quantitative character of the RNase protection assay was confirmed by titration of tissue RNA with the labelled probe and generation of a titration curve (data not shown). Films were scanned using a B.I.S. 202D densitometer (Renium, Rehovot, Israel) and results were analysed by TINA software.
In situ hybridization
For in situ hybridization, placentas were fixed in 4% paraformaldehyde and embedded in paraffin. The 7-μm tissue sections were further used after histological examination. Only specimens of resorbing placentas containing regions with morphologically unaffected tissues were chosen for further analysis. Tissue sections were deparaffinized and processed as described [28]. Briefly, the sections were washed and heated for 30 min at 70°C in 2× SSC, treated with 10 μg/ml proteinase K (IBI, New Haven, CT) for 15 min at 37°C and fixed in 4% paraformaldehyde. Prehybridization was performed for 1 h at 45°C in 50% formamide, 6 × SSPE (150 mm sodium chloride, 10 mm sodium phosphate, and 1 mm EDTA, pH 7.4), 5× Denhardt's solution (Sigma, Rehovot, Israel) and 0.5% SDS. The sections were overlaid with 30 μl of hybridization mixture (50% formamide, 5× Denhardt's solution, 10% dextran sulphate, 6 × SSPE and 0.5% SDS) containing 0.5 ng/μl DIG-labelled anti-sense RNA probe. Hybridization was carried out overnight at 45°C in a humidified chamber. The slides were washed twice for 15 min in 2× SSC, followed by incubation with 20 μg/ml RNase A (Sigma) for 30 min at 37°C. High stringency washes were performed by incubating the slides twice for 15 min at 50°C in 0.1 × SSC followed by a 10-min wash in 0.1 × SSC at room temperature. The hybridization signal was detected by alkaline phosphatase-conjugated anti-DIG antibodies followed by incubation in the NBT/BCIP colour substrate solution (Boehringer), containing 1 mm levamisole according to the manufacturer's recommendations. Finally, sections were lightly counterstained with neutral red and the positive signal was indicated by a deep purple staining.
As a control for hybridization, a non-homologous RNA probe synthesized from a prokaryotic Neo cDNA (760 bp) was substituted for the specific probes. Tissue sections pretreated before hybridization with 100 μg/ml RNase A (Sigma) for 30 min at 37°C served as an additional control.
Reverse transcriptase-polymerase chain reaction analysis
For reverse transcription 1 μg of total RNA was treated with 5 U of RQ1 DNase (Promega) following by denaturation of the RNA at 68°C for 10 min. cDNA was synthesized with 200 U Superscript reverse transcriptase (Gibco BRL, Gaithersburg, MD) in a total volume of 20 μl containing 0.5 μg oligo (dT) primer, 50 mm Tris–HCl pH 8.3, 75 mm KCl, 3 mm MgCl2, 10 mm DTT, dATP, dTTP, dGTP and dCTP at 0.5 mmol each, and 4 U RNasin (Promega). The reaction was carried out at 41°C for 1 h followed by denaturation at 95°C and rapid cooling on ice. cDNA (2 μl) from each sample was amplified with 100 pmol of either c-fms- or β-actin-specific primers in a total volume of 50 μl and 2.5 U KlenTaq polymerase (Ab Peptides Inc., St Louis, MO). The PCR reaction was performed for 35 cycles in a MiniCycler (MJ Research Inc., Watertown, MA). Hot start of polymerase chain reaction (PCR) was performed at 94°C for 5 min. The thermal cycle conditions for all primer pairs included 1 min of denaturation at 94°C, 2 min of annealing at 55°C and 1 min of primer extension at 72°C. Oligonucleotide primers used for amplification of c-fms cDNA were 5′-CTGAGTCAGAAGCCCTTCCGACAAAG-3′ and 5′-CTTTGCCCAGACCAAAGGCTGTAGC-3′, corresponding to the 1441–1456 bp and 1865–1889 bp of c-fms cDNA. Up-stream and down-stream primers, namely 5′-TGAACCCTAAGGC CAACCGTG-3′ and 5′-GCTCATAGCTCTTCTCCAGGG-3′, corresponding to 409–429 bp and 784–804 bp, respectively, were used for amplification of β-actin cDNA. Primers were chosen using Oligos software (kindly gifted by Dr P. Green, Washington University, School of Medicine).
The PCR products were loaded on a 2% agarose gel following by staining with 0.5 μg/ml ethidium bromide. The gel was photographed by a B.I.S. 202D imaging system (Renium). The molecular weight of amplified products was estimated by comparison with the pBR322/AluI DNA molecular weight marker (MBI, Vilnius, Lithuania). Two types of controls were used for reverse transcriptase (RT)-PCR analysis. First, a negative control for cDNA synthesis in which RT was omitted confirmed the absence of contamination by genomic DNA. Second, PCR reaction performed without cDNA template proved the lack of external contamination. The specificity of the PCR products was validated by sequencing analysis.
Statistical analysis
Each tested sample of total RNA was obtained by combining four or five placentas in a tested litter. To evaluate results of the RNase protection assay statistically, four or five samples obtained from different litters were analysed and compared by Student's t-test. The two-tailed level of significance of differences was α = 0.05.
For in situ hybridization analysis, four or five resorbing and/or non-resorbed uteroplacental units collected from four mice were analysed for each experimental group. In situ hybridization experiments were repeated three times. In each experiment, four or five tissue sections of each uteroplacental unit were processed and analysed by two independent readers. Results characterizing signal intensity were averaged.
RESULTS
Pregnancy loss in tested animal models
Two mouse models demonstrating a high level of either spontaneous or CY-induced induced pregnancy loss were used in this study. As well as in our earlier studies [28], CBA/J females mated to DBA/2J (the resorption-prone mouse combination) demonstrated an increased level of spontaneous pregnancy loss (approximately 28%) by day 12 of pregnancy. In CBA/J females mated to C57Bl/6 males that were used as a control, the incidence of post-implantation loss did not exceed 9% at this time point The resorption rate in both mouse combinations did not change much when tested on day 19 of pregnancy.
In ICR × ICR mice treated with CY on day 12 of pregnancy, the incidence of pregnancy loss reached approximately 28% by day 15 of pregnancy and exceeded 85% by day 19 of pregnancy. In non-treated ICR mice, the resorption rate did not exceed 10%. A similar dynamics of pregnancy loss was observed in CY-treated CBA/J females mated to C57Bl/6 males (approximately 30% and 81%, respectively).
In immunized CY-treated ICR females tested on day 19 of pregnancy, the incidence of resorptions (approximately 52%) was significantly lower (P < 0.05) than that in non-immunized CY-treated mice (approximately 86%). This index was found to be lower in immunized females tested on day 15 of pregnancy (19% versus 29%, respectively). These values did not however differ statistically.
Similar results were obtained in CY-treated CBA/J females immunized with paternal lymphocytes. Thus, the incidence of post-implantation embryonic loss in immunized CBA/J females tested on day 19 of pregnancy was 46% versus 81% in non-immunized ones (P < 0.05).
Immunopotentiation itself had no effect on reproductive performance of tested mouse strains.
The above results clearly demonstrate that embryos of ICR × ICR and CBA/J × C57Bl/6 mouse combinations have practically equal sensitivity to CY-induced pregnancy loss. Both mouse combinations also responded equally to CY after maternal immunostimulation. Therefore, we used the ICR × ICR mouse combination to evaluate the patterns of expression and localization of CSF-1 and c-fms at the fetomaternal interface of mice with induced pregnancy loss.
Expression of CSF-1 in the uteroplacental unit of mice with pregnancy loss
CSF-1 mRNA expression in the uteroplacental unit was estimated using RNase protection analysis. Hybridization of CSF-1-specific RNA probe with RNA isolated from the control or from non-resorbed and resorbing uteroplacental units of CY-treated mice revealed two protected fragments of 1400 bp and 263 bp (Fig. 1), corresponding to 3.0-kb and 1.6-kb CSF-1 mRNA transcripts, respectively. The 1400-bp mRNA form was shown to be expressed at a lower level than the 263-bp variant in all tested samples. Semiquantitative densitometric analysis of RNA blots demonstrated a 40% decrease in the expression of the 1400-bp CSF-1 mRNA form in both non-resorbed and resorbing uteroplacental units of CY-treated compared with control mice (P < 0.05), while no difference in the expression of the 263-bp form was detected (Fig. 1).
Fig. 1.
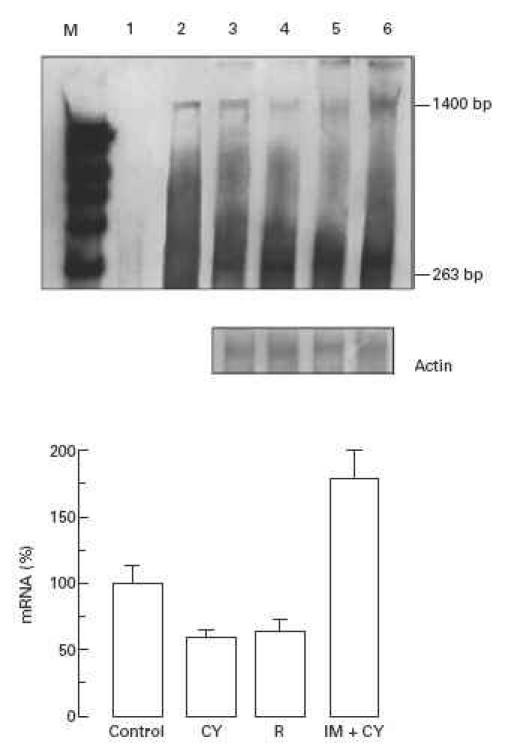
RNase protection analysis of CSF-1 mRNA in the uteroplacental unit of cyclophosphamide (CY)-treated ICR mice. Top: hybridization with CSF-1-specific anti-sense RNA probe. Lane 1, undigested CSF-1 riboprobe; lane 2, yeast tRNA used as negative control; lane 3, uteroplacental unit from control mice; lane 4, non-resorbed uteroplacental unit from CY-treated mice; lane 5, resorbing uteroplacental unit from CY-treated mice; lane 6, non-resorbed uteroplacental unit from immunopotentiated CY-treated mice. Middle: hybridization with β-actin-specific riboprobe (250 bp protected fragment). Bottom: densitometry analysis of protected fragment corresponding to 1400 bp. CY, Non-resorbed uteroplacental unit from mice treated with CY; R, resorbing uteroplacental unit from mice treated with CY; IM + CY, non-resorbed uteroplacental unit from immunopotentiated CY-treated mice. To evaluate results of RNase protection assay statistically, four samples obtained from different litters were analysed.
In the uteroplacental unit of the CBA/J × DBA/2 J mouse combination only the 263-bp mRNA fragment was found (Fig. 2). In this mouse model of pregnancy loss a 20% decrease in CSF-1 mRNA expression was demonstrated in the resorbing compared with the non-resorbed uteroplacental units (P < 0.05) (Fig. 2).
Fig. 2.
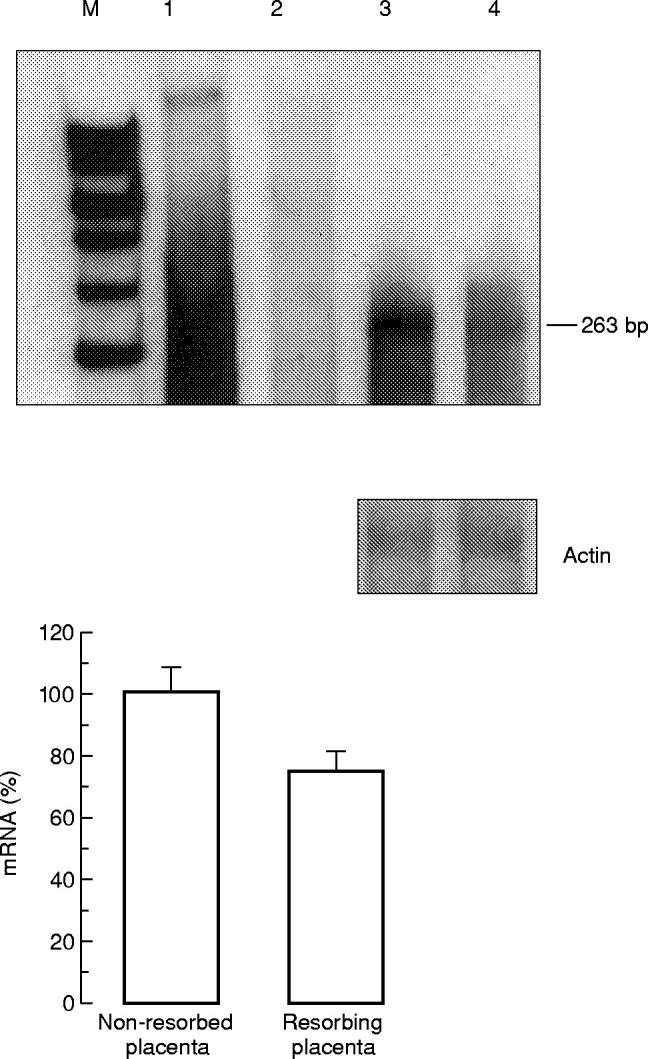
RNase protection analysis of CSF-1 mRNA in the uteroplacental unit of CBA/J mice. Top: hybridization with CSF-1-specific RNA probe. Lane 1, undigested CSF-1 riboprobe; lane 2, yeast tRNA negative control; lane 3, non-resorbed uteroplacental unit from CBA/J × DBA/2J mouse combination; lane 4, resorbing uteroplacental unit from CBA/J × DBA/2J mouse combination. Middle: hybridization with β-actin-specific riboprobe (250 bp protected fragment). Bottom: densitometry analysis of RNA blots. Five samples were analysed to evaluate results of RNase protection assay statistically.
Localization of CSF-1 mRNA at the fetomaternal interface
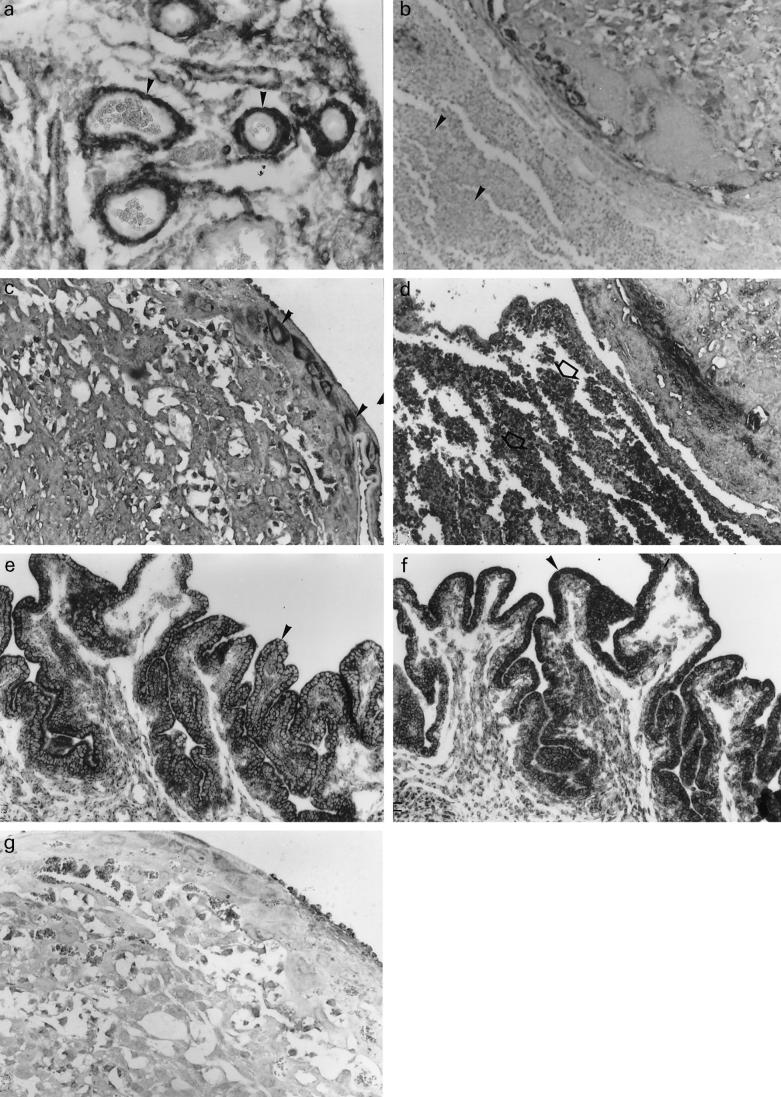
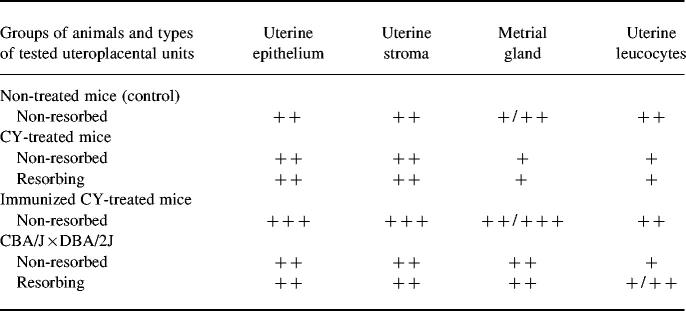
The cellular pattern of CSF-1 mRNA expression in the uteroplacental unit was studied by in situ hybridization analysis. In the mice with CY-induced resorptions, CSF-1 mRNA was shown to be expressed in the uterine epithelium, and especially in cells of the uterine vasculature (Fig. 3a,e and Table 1). A specific hybridization signal was found also in the metrial gland cells and in the uterine leucocytes (Table 1).
Fig. 3.
Distribution of CSF-1 and c-fms mRNAs in placentas and uteri of cyclophosphamide (CY)-treated ICR mice. (a) Expression of CSF-1 mRNA in the uterus of CY-treated mice The positive signal is detected in stromal cells and muscle cells of uterine vasculature (b) Leucocytes in necrotic zone in the resorbing uteroplacental unit of CY-treated mice demonstrate no expression of CSF-1 mRNA. (c) c-fms mRNA expression in giant cells in the placenta of CY-treated mice. (d) Strong positive signal for c-fms mRNA in leucocytes infiltrating necrotic zone of the resorbing uteroplacental unit of CY-treated mice. (e,f) CSF-1 mRNA expression in the uterine epithelium of non-immunopotentiated (e) and immunopotentiated (f) CY-treated mice. (g) Negative control—a placental section of CY-treated mice hybridized with non-homologous RNA (× 100).
Table 1.
Tissue distribution of CSF-1 mRNA in the uteroplacental unit of mice with pregnancy loss*
*In situ hybridization signals were scored as follows: +, weak signal; + +, moderate signal; + + +, high intensity signal.
In total, the cellular pattern of CSF-1 mRNA expression in uteroplacental units of CY-treated mice was similar to that observed in the control mice (Table 1).
The number of uterine leucocytes expressing CSF-1 mRNA was substantially lower in CY-treated than in control animals. At the same time, leucocytes infiltrating zones of necrosis in the resorbing placenta did not contain the CSF-1 transcripts (Fig. 3b).
The cellular localization and signal intensity in uteroplacental units of CBA/J × DBA/2J mice were similar to those observed in mice with CY-induced pregnancy loss (Table 1).
No hybridization signal could be detected when specific riboprobes were replaced by non-homologous RNA or when tissues were pretreated with RNase.
Expression of c-fms in placenta of mice with compromised pregnancy
Using RT-PCR analysis it was observed that amplification of cDNA from the control or non-resorbed uteroplacental unit of CY-treated mice with specific c-fms primers resulted in generation of 448-bp PCR product (Fig. 4a). No c-fms-specific bands were identified in RNA samples from resorbing placenta of CY-treated mice (Fig. 4a). In CBA/J females mated with DBA/2J males, RT-PCR analysis revealed expression of c-fms mRNA in the non-resorbed uteroplacental unit only (Fig. 4b).
Fig. 4.
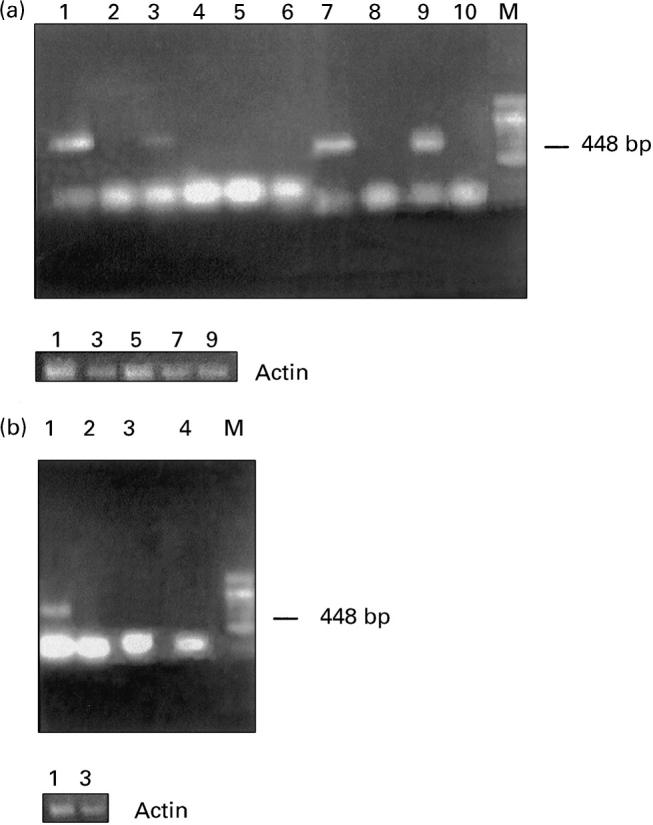
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of c-fms mRNA. Top: (a) Lanes 1 and 2, uteroplacental unit of control mice. Cyclophosphamide (CY)-treated mice: lanes 3 and 4, non-resorbed placenta; lanes 5 and 6, resorbing placenta; lanes 7 and 8, placenta of immunopotentiated control mice; lanes 9 and 10, placenta of immunopotentiated and CY-treated mice. Lanes 2, 4, 6, 8 and 10 demonstrate negative controls for RT-PCR in which reverse transcriptase was omitted. (b) CBA/J × DBA/2J mating combination. Lanes 1 and 2, non-resorbed placenta; lanes 3 and 4, resorbing placenta. Lanes 2 and 4 represent negative controls. Bottom: (a,b) RT-PCR with β-actin-specific primers.
The presence of RNA in the tested samples was confirmed by use of actin primers, which generated a 395-bp PCR product (Fig. 4a, b bottom). Control samples in which either RT was omitted during cDNA synthesis (Fig. 4a, b) or PCR reaction was performed in the absence of cDNA (data not shown) demonstrated no PCR products.
Localization of c-fms mRNA at the fetomaternal interface
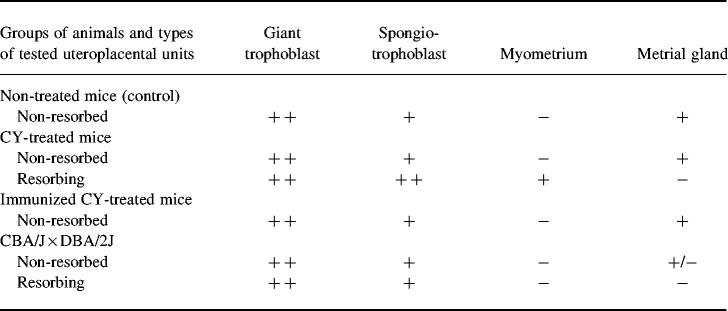
In mice with CY-induced resorptions, a strong hybridization signal was detected in giant trophoblast cells (Fig. 3c and Table 2), while spongiotrophoblast cells also demonstrated a certain level of c-fms mRNA expression (Fig. 3c and Table 2). In labyrinthine zone of trophoblast signal was weak or absent completely. A relatively weak specific signal was found in the metrial gland cells (Table 2).
Table 2.
Tissue distribution of c-fms mRNA in the uteroplacental unit of mice with pregnancy loss*
*In situ hybridization signals were scored as follows: −, not detectable; +/−, detectable but patchy; +, weak signal; + +, moderate signal.
Neither the pattern nor the intensity of the hybridization signal demonstrated in the non-resorbed uteroplacental unit of CY-treated mice differed from those of control mice (Table 2). In the resorbing uteroplacental unit, CY treatment resulted in a complete loss of specific c-fms hybridization signals in metrial gland cells (Table 2). Tissue areas undergoing necrosis in the resorbing placenta were infiltrated with numerous leucocytes abundant with c-fms mRNA transcripts (Fig. 3d).
The cellular pattern of c-fms mRNA expression in the uteroplacental unit of CBA/J females from the CBA × DBA/2 mouse combination was similar to that observed in mice with induced pregnancy loss (Table 2).
Effect of immunopotentiation on CSF-1 and c-fms mRNA expression
Immunopotentiation of ICR females with xenogeneic rat splenocytes substantially decreased the level of CY-induced post-implantation embryonic loss (52% versus 86% in non-immunopotentiated female mice). In parallel, immunopotentiation also modulated the level of CSF-1 mRNA expression at the fetomaternal interface. Thus, densitometric analysis of RNA blots revealed that immunopotentiation caused up to 120% increase in CSF-1 mRNA expression in the uteroplacental unit of mice with CY-induced resorptions compared with non-immunized animals (P < 0.05) (Fig. 1, lanes 4 and 6). In situ hybridization analysis also demonstrated an enhancement in hybridization signal intensity in the uterine epithelium and stroma cells of immunized CY-treated compared with non-immunized mice (Fig. 3e,f and Table 1).
In situ hybridization analysis suggested that neither the cellular pattern nor the intensity of c-fms RNA expression were altered by immunopotentiation (Table 2). Also, RT-PCR analysis resulted in generation of the same PCR product in the uteroplacental unit of immunopotentiated mice as was observed in non-immunopotentiated control and CY-treated mice (Fig. 4a).
DISCUSSION
This study reports on data characterizing CSF-1 and its receptor (c-fms) mRNA expression at the fetomaternal interface in mice with a high rate of pregnancy loss. The model used for spontaneous pregnancy loss was the resorption rate in the CBAJ × DBA 2J mouse combination. The resorption rate on day 12 of pregnancy was 27.7%, which is similar to the expected rate in this mating combination [33,34]. In CY-treated mice the resorption rate was approximately 30% on day 15 of pregnancy and reached 83% by day 19. This model allows us to evaluate the pattern of CSF-1 mRNA expression in placentas which are not resorbed at day 15 of pregnancy, but most of which are destined to be resorbed by the end of pregnancy.
Two major forms of CSF-1 mRNA, representing protected fragments of 1400 and 263 bp, have been demonstrated in the uteroplacental units of CY-treated mice, whereas in uteroplacental units of the CBA/J × DBA/2J mouse mating combination only the 263-bp mRNA transcripts were found. The absence of the 1400-bp mRNA transcript in CBA/J females may be due to differential regulation of the expression of CSF-1 mRNA variants in the uterus at different stages of gestation [11].
Semiquantitative densitometric analysis of RNase protection assay results has shown a 40% decrease in the expression of 1400-bp CSF-1 mRNA form not only in the resorbing but also in non-resorbed uteroplacental unit of mice with CY-induced pregnancy loss, compared with control mice. At the same time, in mice with spontaneous pregnancy loss (CBA/J × DBA/2J), a 20% decrease in the expression of the 263-bp CSF-1 mRNA form was demonstrated in the resorbing compared with non-resorbed placentas. The temporal pattern of embryonic death observed in CY-treated mice suggests that non-resorbed placentas tested on day 15 of pregnancy were destined to be resorbed by the end of pregnancy. In contrast, the pattern seen in CBA/J females mated to DBA/2J suggests that non-resorbed placentas tested on day 12 of pregnancy were destined to survive. Taking all data together, the above results strongly suggest that down-regulation of CSF-1 mRNA expression at the fetomaternal interface may precede and be associated with pregnancy loss. This concept is supported by data that women with recurrent spontaneous abortions have a low systemic level of M-CSF [35].
In situ hybridization assay has shown that the cellular pattern of CSF-1 mRNA expression at the fetomaternal interface is similar in both mouse models of pregnancy loss, and practically no differences were observed in the localization of CSF-1 transcripts between the non-resorbed and resorbing uteroplacental unit. CSF-1 transcripts have mainly been localized to the uterine epithelium and stroma. A specific hybridization signal has been found in uterine and placental leucocytes and in the resident cells of metrial glands. CSF-1 mRNA expression in the uterine epithelium of non-resorbed uteroplacental units of CY-treated mice did not differ from control mice. However, there were fewer leucocytes expressing CSF-1 mRNA in uteroplacental units of CY-treated mice.
CSF-1 expression in the uterine stroma and vasculature is believed to be important for endometrial remodelling and repair [36]. The endometrium might otherwise be damaged by toxins and oxidative enzymes released from the dying embryo and impaired placental tissues. Moreover, CSF-1 may contribute to the local cytokine microenvironment which determines the trafficking and behaviour of uterine macrophages [36]. Indeed, we have demonstrated that necrotic resorbing placental tissue is infiltrated by numerous leucocytes expressing c-fms mRNA, which may be a marker for differentiated macrophages [14]. These macrophages have been shown to express tumour necrosis factor-alpha (TNF-α) [28], but not CSF-1 mRNA. The number of uterine leucocytes expressing both CSF-1 and c-fms mRNA and the number of CSF-1+ cells in metrial glands has been found to be lower in CY-treated than control mice.
These data suggest that CSF-1 produced by uterine macrophages and by metrial gland cells may be implicated in the regulation of local immune responses protecting the fetus. We therefore studied the expression of CSF-1 mRNA in mice with pregnancy loss after immunostimulation of the maternal immune system. It has been reported that maternal alloimmunization with lymphocytes of paternal haplotype mice significantly decreases pregnancy loss in the CBA/J × DBA/2J mouse combination [23]. Also, non-specific immunopotentiation with Freund's complete adjuvant (FCA) or xenogeneic lymphocytes may improve the reproductive performance of mice with spontaneous pregnancy loss [28,31,34,37]. Although the precise mechanisms whereby immunostimulation prevents embryo loss are still unknown, it has been suggested that immunostimulation may result in modulation of the cytokine milieu in the embryonic microenvironment, leading to enhanced production of Th2 cytokines and suppression of Th1-type immune responses [38,39]. Indeed, the protective effect of immunopotentiation on reproductive performance in mice with pregnancy loss is associated with a strong decrease in TNF-α expression at both mRNA and protein levels [28], and elevated transforming growth factor-beta 2 (TGF-β2) mRNA expression in the uteroplacental unit compared with non-immunized mice [40]. The pregnancy rate is also improved in CBA/J × DBA/2J mice following immunopotentiation. This is accompanied by a dramatic shift towards reduced interferon-gamma (IFN-γ) and increased IL-10 expression in the uteroplacental unit [27,41]. It has also been demonstrated that immunization of mice with stress-triggered pregnancy loss protects the embryo, and that this protection is associated with elevation of TGF-β2-mediated immunosuppression and a simultaneous decrease in TNF-α-associated cytotoxic activity in decidual tissue [26].
This study demonstrates that a decreased resorption rate in immunostimulated females is accompanied by both elevation in the CSF-1 mRNA expression in the uterine epithelium, and an increased number of CSF-1+ leucocytes in the endometrium. Cellular signals promoted by CSF-1 have been shown to be associated with increased embryo survival and placental growth [42]. The in vitro experiments demonstrate that CSF-1 may influence proliferation of the trophoblast, stimulate trophoblast differentiation and the production of placental hormones [17–19]. The observation that CSF-1 mRNA is expressed at a high level in the uterus and that transcripts of its receptor (c-fms) are abundant in the trophoblast indicates that CSF-1 may influence trophoblast growth and differentiation in a paracrine manner. Finally, there is also evidence that expression of cellular proto-oncogenes, early response genes such as c-myc, c-fos and c-jun and expression of D-type cycline are required for the realization of the mitogenic and trophic effects of CSF-1 [43]. Therefore, augmentation of CSF-1 expression at the fetomaternal interface following immunostimulation may result in improved trophoblast and embryo cell proliferation and thereby fetal survival. Increased CSF-1 production may be important for down-regulation of MHC-Ia antigens on placental cells, thereby reducing the risk of pregnancy loss [44], or could result in activation of local immunosuppression [45], which may be an important mechanism preventing aggressive maternal responses towards the embryo [46].
Along with the above data, suggesting a positive role of CSF-1 in embryo development, there are studies demonstrating that a distorted expression of this cytokine in the uterus may result in death of preimplantation or early implantation embryos. Thus, CSF-1 injection to C57Bl/6 mice for 5 consecutive days from day 1 of pregnancy onward was shown to cause a significant decrease in the number of viable implantations [47,48]. It seems that appropriate CSF-1 expression in the pregnant uterus is important for the pregnancy outcome—its over-production in the preimplantation stage may be destructive, whereas an elevated CSF-1 level after implantation appears to be beneficial for the maintenance of normal pregnancy.
Also, it is worth nothing that the role of CSF-1 in pregnancy can not be completely understood without understanding the inter-relationships between CSF-1 and other cytokines. TNF-α and IFN-γ may be associated with placental death and a high risk of fetal loss [28,49]. It has been demonstrated that these cytokines may alter the CSF-1 proliferative effect by down-regulation of its receptor expression [50,51], thereby inhibiting DNA synthesis and cell cycle progression [43]. Other cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF), TGF-β and IL-10 may promote placental growth and may induce the secretion of placental lactogen and hCG [18,19]. These findings indicate that CSF-1 acts at the fetomaternal interface in conjunction with other cytokines, and that alterations in the local cytokine balance may lead to pregnancy termination.
Acknowledgments
This work was supported by grants from The Basic Science Foundation administrated by the Israel Academy of Science and Humanities, Recanaty Foundation, the Israel Ministry of Health and the Israel Ministry of Science and Technology. This work is in partial fulfilment of the requirements for the PhD degree of M.G. from the Sackler School of Medicine at Tel Aviv University.
REFERENCES
- 1.Wegmann TG. The cytokine basis for cross-talk between the maternal immune and reproductive systems. Curr Opin Immunol. 1990;2:765–9. doi: 10.1016/0952-7915(90)90048-l. [DOI] [PubMed] [Google Scholar]
- 2.Toder V, Shomer B. The role of lymphokines in pregnancy. Immunol Allerg Clin N Am. 1990;10:65–69. [Google Scholar]
- 3.Stanley ER, Guilbert LJ, Tushinsky RJ, Bartelmez SH. CSF-1—a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem. 1983;21:151–9. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- 4.De M, Sanford T, Wood GW. Relationship between macrophage colony-stimulating factor production by uterine epithelial cells and accumulation and distribution of macrophages in the uterus of pregnant mice. J Leukoc Biol. 1993;53:240–8. doi: 10.1002/jlb.53.3.240. [DOI] [PubMed] [Google Scholar]
- 5.Bartocci A, Pollard JW, Stanley ER. Regulation of colony stimulating factor 1 during pregnancy. J Exp Med. 1986;164:956–61. doi: 10.1084/jem.164.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daiter E, Pampfer S, Yeung Y, Barad D, Stanley ER, Pollard JW. Expression of colony-stimulating factor-1 in the human uterus and placenta. J Clin Endocrinol Metab. 1992;74:850–8. doi: 10.1210/jcem.74.4.1548350. [DOI] [PubMed] [Google Scholar]
- 7.Arceci RJ, Shanahan F, Stanley ER, Pollard JW. The temporal expression and location of Colony Stimulating Factor-1 (CSF-1) and its receptor in the female reproductive tract are consistent with CSF-1 regulated placental development. Proc Natl Acad Sci USA. 1989;86:8818–22. doi: 10.1073/pnas.86.22.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanzaki H, Yui J, Iwai M, et al. The expression and localization of mRNA for colony-stimulating factor (CSF)-1 in human term placenta. Hum Reprod. 1992;7:563–7. doi: 10.1093/oxfordjournals.humrep.a137691. [DOI] [PubMed] [Google Scholar]
- 9.Stanley ER, Berg KL, Einstein DB, Lee PSW, Pixley FJ, Wang Y, Yeung Y-G. Biology and action of colony-stimulating factor-1. Mol Reprod Dev. 1997;46:4–10. doi: 10.1002/(SICI)1098-2795(199701)46:1<4::AID-MRD2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 10.Azuma C, Saji F, Kimura T, Tokugawa Y, Takemura M, Samejima Y, Tanizawa O. Steroid hormones induce macrophage colony-stimulating factor (MCSF) and MCSF receptor mRNAs in the human endometrium. J Mol Endocrinol. 1990;5:103–8. doi: 10.1677/jme.0.0050103. [DOI] [PubMed] [Google Scholar]
- 11.Roth P, Stanley ER. Colony stimulating factor-1 expression is developmentally regulated in the mouse. J Leuk Biol. 1996;59:817–23. doi: 10.1002/jlb.59.6.817. [DOI] [PubMed] [Google Scholar]
- 12.Roth P, Stanley ER. The biology of CSF-1 and its receptor. Curr Topics Microbiol Immunol. 1992;181:141–67. doi: 10.1007/978-3-642-77377-8_5. [DOI] [PubMed] [Google Scholar]
- 13.Sherr CJ, Rettenmier CW, Sacca R, Roussel MF, Look AT, Stanley ER. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985;41:665–76. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- 14.Hume DA, Yue X, Ross IL, Favot P, Lichanska A, Ostrowski MC. Regulation of CSF-1 receptor expression. Mol Reprod Dev. 1997;46:46–52. doi: 10.1002/(SICI)1098-2795(199701)46:1<46::AID-MRD8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 15.Arceci RJ, Pampfer S, Pollard JW. Expression of CSF-1/c-fms and SF/c-kit mRNA during preimplantation mouse development. Dev Biol. 1992;151:1–8. doi: 10.1016/0012-1606(92)90207-w. [DOI] [PubMed] [Google Scholar]
- 16.Athanassakis-Vassiliadis I, Papamatheakis J, Vassiliadis S. Specific CSF-1 binding on murine placental trophoblasts and macrophages serves as a link to placental growth. J Recept Res. 1993;13:739–51. doi: 10.3109/10799899309073690. [DOI] [PubMed] [Google Scholar]
- 17.Athanassakis I, Bleackley RC, Paetkau V, Guilbert I, Barr RJ, Wegmann TG. The immunostimulatory effect of T cells and T cell lymphokines on murine fetal derived placental cells. J Immunol. 1987;138:37–44. [PubMed] [Google Scholar]
- 18.Armstrong DT, Chaouat G. Effects of lymphokines and immune complex on murine placental cell growth in vitro. Biol Reprod. 1989;40:466–74. doi: 10.1095/biolreprod40.3.466. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Lloret MI, Morrish DW, Wegmann TG, Honore L, Turner AR, Guilbert LJ. Demonstration of functional cytokine–placental interactions: CSF-1 and GM-CSF stimulate human cytotrophoblast differentiation and peptide hormone secretion. Exp Cell Res. 1994;214:46–54. doi: 10.1006/excr.1994.1232. [DOI] [PubMed] [Google Scholar]
- 20.Rappolee DA, Werb Z. The role of growth factors in mammalian pregastrulation development. Adv Dev Biol. 1994;3:41–71. [Google Scholar]
- 21.Pollard JW, Hunt JS, Wiktor-Jedrzejczak W, Stanley ER. A pregnancy defect in the osteopetrotic (op/op) mouse demonstrates the requirement for CSF-1 in female fertility. Dev Biol. 1991;148:273–83. doi: 10.1016/0012-1606(91)90336-2. [DOI] [PubMed] [Google Scholar]
- 22.Pollard JW, Stanley ER. Pleiotropic roles for CSF-1 in development defined by the mouse mutation osteopetrotic (op) Adv Dev Biochem. 1996;4:153–7. [Google Scholar]
- 23.Chaouat G, Menu E, Bonneton C, Kinsky R. Immunological manipulation in animal pregnancy and models of pregnancy failure. Curr Opin Immunol. 1989;1:1153–6. doi: 10.1016/0952-7915(89)90008-3. [DOI] [PubMed] [Google Scholar]
- 24.Toder V, Shepshelovich J, Carp H, Altaratz H, Strassburger D. Cytokine function in immunopotentiated females. In: Chaouat G, Mowbray JF, editors. Cellular and molecular biology of the maternal–fetal relationship. Paris: INSERM/John Libbey Eurotext; 1991. pp. 131–9. [Google Scholar]
- 25.Carp HJA, Toder V, Torchinsky A, Portuguese S, Lipitz S, Gazit E, Mashiach S and the Recurrent Miscarriage Immunotherapy Trialists Group. Allogenic leukocyte immunization after five or more miscarriages. Hum Reprod. 1997;12:250–5. doi: 10.1093/humrep/12.2.250. [DOI] [PubMed] [Google Scholar]
- 26.Arck PC, Merali FS, Stanisz AM, Stead RH, Chaouat G, Manuel J, Clark DA. Stress-induced murine abortion associated with substance P-dependent alteration in cytokines in maternal uterine decidua. Biol Reprod. 1995;53:814–9. doi: 10.1095/biolreprod53.4.814. [DOI] [PubMed] [Google Scholar]
- 27.Chaouat G, Menu E, Smedt D, et al. The emerging role of IL-10 in pregnancy. Am J Reprod Immunol. 1996;35:325–9. doi: 10.1111/j.1600-0897.1996.tb00488.x. [DOI] [PubMed] [Google Scholar]
- 28.Gorivodsky M, Zemlyak I, Orenstein H, Savion S, Fein A, Torchinsky A, Toder V. Tumor necrosis factor alpha mRNA and protein expression in the uteroplacental unit of mice with pregnancy loss. J Immunol. 1998;160:4280–8. [PubMed] [Google Scholar]
- 29.Chaouat G, Kiger N, Wegmann TG. Vaccination against spontaneous abortion in mice. J Reprod Immunol. 1983;5:389–92. doi: 10.1016/0165-0378(83)90248-6. [DOI] [PubMed] [Google Scholar]
- 30.Torchinsky A, Fein A, Carp HJA, Toder V. MHC-associated immunopotentiation affects the embryo response to teratogens. Clin Exp Immunol. 1994;98:513–9. doi: 10.1111/j.1365-2249.1994.tb05521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torchinsky A, Fein A, Toder V. Modulation of mouse sensitivity to cyclophosphamide-induced embryopathy by nonspecific intrauterine immunopotentiation. Toxicol Methods. 1995;5:131–41. [Google Scholar]
- 32.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 33.Toder V, Strassburger D, Carp H, Irlin I. Mouse model for the treatment of immune pregnancy loss. Am J Reprod Immunol. 1991;26:42–46. doi: 10.1111/j.1600-0897.1991.tb00701.x. [DOI] [PubMed] [Google Scholar]
- 34.Baines MG, Duglos AJ, deFougerolles AR, Gendron RL. Immunological prevention of spontaneous early embryo resorption is mediated by non-specific immunostimulation. Am J Reprod Immunol. 1996;35:34–38. doi: 10.1111/j.1600-0897.1996.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 35.Katano K, Matsumoto Y, Ogasawara M, Aoyama T, Ozaki Y, Kajiura S, Aoki K. Low serum M-CSF levels are associated with unexplained recurrent abortion. Am J Reprod Immunol. 1997;38:1–5. doi: 10.1111/j.1600-0897.1997.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 36.Wood GW, Hausmann E, Choudhuri R. Relative role of CSF-1, MCP-1/JE, and RANTES in macrophage recruitment during successful pregnancy. Mol Reprod Dev. 1997;46:62–70. doi: 10.1002/(SICI)1098-2795(199701)46:1<62::AID-MRD10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 37.Toder V, Strassburger D, Irlin Y, Carp H, Pecht M, Trainin N. Nonspecific immunopotentiators and pregnancy loss: complete Freund adjuvant reverses high fetal resorption rate in CBA/J × DBA/2 mouse combination. Am J Reprod Immunol. 1990;24:63–66. doi: 10.1111/j.1600-0897.1990.tb01040.x. [DOI] [PubMed] [Google Scholar]
- 38.Wegmann TG, Lin H, Guilbert L, Mossmann TR. Bidirectional cytokine interactions in the maternal–fetal relationship: is a successful pregnancy a TH2 phenomenon ? Immunol Today. 1993;14:353–6. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 39.Raghupathy R, Tangri S. Immunodystrophism, T cells, cytokines and pregnancy failure. Am J Reprod Immunol. 1996;35:291–6. doi: 10.1111/j.1600-0897.1996.tb00484.x. [DOI] [PubMed] [Google Scholar]
- 40.Gorivodsky M, Torchinsky A, Zemliak I, Savion S, Fein A, Toder V. TGFβ2 mRNA expression and pregnancy failure in mice. Am J Reprod Immunol. 1999 in press. [PubMed] [Google Scholar]
- 41.Robertson SA, Seamark RF, Guilbert LJ, Wegmann TG. The role of cytokines in gestation. Crit Rev Immunol. 1994;14:239–92. doi: 10.1615/critrevimmunol.v14.i3-4.30. [DOI] [PubMed] [Google Scholar]
- 42.Pollard JW, Bartocci A, Arceci R, Orlofsky A, Ladner MB, Stanley ER. Apparent role of the macrophage growth factor CSF-1 in placental development. Nature. 1987;330:484–6. doi: 10.1038/330484a0. [DOI] [PubMed] [Google Scholar]
- 43.Roussel MF. Regulation of cell cycle entry and G1 progression by CSF-1. Mol Reprod Dev. 1997;46:11–18. doi: 10.1002/(SICI)1098-2795(199701)46:1<11::AID-MRD3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 44.Vassiliadis S, Athanassakis I. Two novel colony-stimulating factor 1 (CSF-1) properties: it post-transcriptionally inhibits interferon-specific induction of class II antigens and reduces the risk of fetal abortion. Cytokine. 1994;6:295–9. doi: 10.1016/1043-4666(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 45.Oghiso Y, Yamada Y, Ando K, Ishihara H, Shibata Y. Differential induction of prostaglandin E2-dependent and -independent immune suppressor cells by tumor-derived GM-CSF and M-CSF. J Leuk Biol. 1993;53:86–92. doi: 10.1002/jlb.53.1.86. [DOI] [PubMed] [Google Scholar]
- 46.Daya S, Rosenthal KL, Clark DA. Immunosuppressor factor(s) produced by decidua-associated suppressor cells: a proposed mechanism for fetal allograft survival. Am J Obstet Gynecol. 1987;156:344–50. doi: 10.1016/0002-9378(87)90281-x. [DOI] [PubMed] [Google Scholar]
- 47.Tartakovsky B. CSF-1 induces resorption of embryos in mice. Immunol Letters. 19891990;23:65–70. doi: 10.1016/0165-2478(89)90157-0. [DOI] [PubMed] [Google Scholar]
- 48.Tartakovsky B, Ben-Yair E. Cytokines modulate proliferation development and pregnancy. Dev Biol. 1991;146:345–52. doi: 10.1016/0012-1606(91)90236-v. [DOI] [PubMed] [Google Scholar]
- 49.Chaouat G, Menu E, Clark DA, Dy M, Minkowski M, Wegmann TG. Control of fetal survival in CBA × DBA/2 mice by lymphokine therapy. J Reprod Fertil. 1990;89:447–58. doi: 10.1530/jrf.0.0890447. [DOI] [PubMed] [Google Scholar]
- 50.Aboagye-Mathiesen G, Zdravkovic M, Toth FD, Ebbesen P. Effects of human trophoblast-induced interferons on the expression of proto-oncogenes c-fms/CSF-1R, EGF-R and c-erbB2 in invasive and non-invasive trophoblast. Placenta. 1997;18:155–61. doi: 10.1016/s0143-4004(97)90087-4. [DOI] [PubMed] [Google Scholar]
- 51.Jacobsen SE, Ruscetti FW, Dubois CM, Keller JR. Tumor necrosis factor α directly and indirectly regulates hematopoietic progenitor cell proliferation: role of colony-stimulating factor receptor modulation. J Exp Medicine. 1992;175:1759–72. doi: 10.1084/jem.175.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]