Abstract
We have previously shown that natural killer (NK) cells play a role in protection against leishmaniasis. Furthermore, we have shown that NK cells in mononuclear cells derived from unexposed donors are induced to proliferate in vitro in response to leishmanial antigens. Since interleukin (IL)-12, a strong inducer of NK cells, acts on the early events in NK cells and T-cells, and is considered as an adjuvant for use in a potential antileishmaniasis antigen, we wished to investigate how this cytokine influences the in vitro Leishmania induced proliferative and cytokine response in healthy donors. We demonstrate that in an innate response to Leishmania antigen involving NK cells, a critical level of IL-12 is required to induce interferon (IFN)-γ secretion below which, IL-10 is released in amounts which apparently inhibit IFN-γ secretion and cellular proliferation. However, at higher IL-12 levels, there is simultaneous secretion of IFN-γ and IL-10 as well as proliferation of cells. In a similar vein, exogenous IL-10 in turn inhibited IFN-γ secretion as well as proliferation when used at low/medium concentrations, but at high concentrations this effect was abolished and replaced by the simultaneous detection of IFN-γ, IL-10 and proliferation. The contribution of NK cells in cross regulation of these two very important immuneregulatory cytokines and the effect of exogenous IL-12 in a Leishmania driven response are discussed.
Keywords: natural killer cells, cytokines, IL-10, IL-12, Leishmania, regulation
INTRODUCTION
Cytokines are being considered for use in immune-modulation and therapy against a number of human disorders including infectious diseases. Studies aimed at elucidating the mechanisms of action of many of these cytokines have generally used genetically well defined inbred mouse strains [1–5]. The potential use of interleukin 12 (IL-12) as an adjuvant in a vaccine against leishmaniasis has been elegantly demonstrated using highly susceptible BALB/c mice [6–8]. IL-12 given in conjunction with leishmanial antigens [9] or DNA [10] prevented the generation of TH2 responses in normally susceptible mice, favouring instead Th1 cell generation. Thus the macrophage activating cytokine interferon-gamma (IFN-γ) was rapidly generated at high levels when the mice were subsequently challenged with infectious doses of Leishmania major. It has also been shown that in some mouse strains, the early unacquired, innate immune responses to Leishmania infection are regulated by natural killer (NK) cells [11], a cell type controlled, amongst others by IL-12 [12]. IL-12 is a pro-inflammatory cytokine that induces T-cells and NK cells to secrete IFN-γ. Furthermore, phagocytic cells activated by IFN-γ are enhanced for production of cytokines including IL-12. Apart from its antileishmanial activity, IFN-γ has, in turn, an important role in Th1 cell development as well as a feed back effect on IL-12 production by macrophages [13,14]. However, the effects of IL-12 on the immune response seem to also depend on the activation state of T-cells, being shown to be able to exacerbate an established Th2 response [15]. These apparently paradoxical findings illustrate that the immune-regulatory function of IL-12 is complex. In addition to CD4+ T-cells, IL-12 induces priming for high IFN-γ production in CD8+ T-cells [16]. The down-regulatory cytokine IL-10 is intricately involved in this regulation through its ability to suppress accessory cell function including the production of IL-12 [17].
We have previously shown that there exists an innate capacity of peripheral blood mononuclear cells from Leishmania-unexposed blood donors to respond by NK cell proliferation and IFN-γ production when stimulated in vitro with Leishmania antigen [18] and suggested that such capacity for reactivity to the antigens of a pathogen to which the host has not been exposed could have consequences for the outcome of subsequent infection. We investigate how IL-12 influences this apparently innate response and show that the response can be influenced by exogenous IL-12 in a dose-dependent manner and that it is sensitive to the down-regulatory effects of IL-10, a cytokine produced by a number of cell types including Th2 cells, macrophages [19] and more recently shown to be produced by NK cells [20]. Most strikingly, we noted that in this response, involving NK, a critical level of IL-12 is required to induce IFN-γ secretion. Below this critical IL-12 concentration, IL-10 production is induced in amounts which apparently inhibit the ability of the cells to secrete IFN-γ and to proliferate. However, at higher IL-12 levels there is simultaneous secretion of IFN-γ, IL-10 and proliferation of cells. In a similar vein, exogenous IL-10 inhibited IFN-γ secretion as well as proliferation when used at low/medium concentrations, but at high concentrations this effect was abolished and replaced by the simultaneous detection of IFN-γ, IL-10 and proliferation. The potential implications for the incorporation of adjuvants such as IL-12 in vaccines [9,21] for human leishmaniasis are discussed.
MATERIALS AND METHODS
Blood donors
Peripheral blood mononuclear cells (PBMC) were obtained from defibrinated venous blood or buffy coats from Swedish blood donors. All blood donors were healthy with no present or past history of infection with Leishmania.
Preparation of mononuclear cells
Mononuclear cells (MNC) were isolated from venous blood or buffy coats using Ficoll gradient (Pharmacia, Uppsala, Sweden) separation as previously described [22]. Cells were resuspended in complete medium containing 10% heat inactivated normal human AB serum (Bloodcentralen, Sabbartsbergs Hospital, Stockholm, Sweden), 2 mml-glutamine, 100 U penicillin and 100 μg/ml streptomycin (Gibco, Irvine, UK), and were counted and a portion kept to be used as whole MNC while the rest was depleted of or enriched for NK cells.
Enrichment and depletion of NK cells from MNC
The remaining MNC were depleted of NK cells using magnetic beads following treatment of cells with mouse anti-CD56 antibodies (Becton Dickinson Europe, Erembodegem, Belgium) as previously described [23]. Briefly MNC were resuspended at 40 × 106 cells/tube, 0.08 μg/1 × 106 MNC of mouse anti-CD56 monoclonal antibody added and incubated on ice for 15 min. After two washes in RPMI (Gibco) to remove excess antibody, the cells were resuspended in 2% foetal calf serum in PBS and their viability assessed. Sheep antimouse IgG-coated immunomagnetic Dynabeads-M450 (Dynal AS, Oslo, Norway) were added at 7.5 × 105 Dynabeads/106 viable MNC. After incubation at 4°C, with gentle rotation, for 30 min, CD56+ cells (bound to the magnetic Dynal beads) were removed from the other cells magnetically, using the magnetic particle concentrator (Dynal AS). Both cell preparations were kept, the positively selected containing CD56+ cells (NK enriched) and the negatively selected population depleted of CD56+ cells (NK depleted). To separate NK cells from the magnetic beads to which they were bound, the NK enriched suspension was incubated overnight at 37°C in 5 ml complete medium. Released magnetic beads were removed as above. The viability of the cells was rechecked before use and found to be high in all cases.
Antigens used for cell stimulation assays
Freeze thawed promastigotes of Leishmania aethiopica isolated from four patients (two with localized cutaneous leishmaniasis, and two with diffuse cutaneous leishmaniasis) were mixed just prior to antigen preparation, as previously described [24] and used at a final concentration of 1.25 × 106 parasites per ml. Phytoheamagglutinin (PHA, Murex Diagnostic, Chatillon, France) were used at a final concentration of 12.5 μg/ml.
Stimulation of cell preparations
Responsiveness of the whole, NK-depleted and NK enriched cells to L. aethiopica antigen stimulation, were tested in triplicate cultures as previously described [24]. Cellular responsiveness to the T cell mitogen PHA were also assessed as controls. Both the whole MNC and NK-depleted preparations were distributed in 96-well tissue culture plates at 2 × 105 cells/well, while the NK enriched cells were distributed at 1 × 105 cells/well. Cultures were incubated in 5% CO2 in air at 37°C. Seventy-two hours after incubation supernatants were removed and stored at − 70°C for subsequent assessment of cytokine secretion. Cultures for proliferation assessment were harvested 6 days after stimulation (for antigen stimulation) or 3 days (for PHA stimulation) following 18 h pulse with 1 μCi/well of tritiated thymidine (Amersham, Bucks, UK). Proliferative responses were assessed by measurement of counts per minute (CPM) of the incorporated tritiated thymidine in a β-scintillation counter (Betaplate, Wallac, Turku, Finland). Results are expressed as raw CPM or stimulation indices (SI = CPM stimulated cultures/CPM unstimulated cultures).
The effect of recombinant IL-10 and IL-12 on the normal response to L. aethiopica
Recombinant human IL-10 (specific activity of 107 U/mg) which was a kind gift from Dynax (Palo Alto, CA, USA). Recombinant human IL-12 (specific activity of 5.26 × 106 U/mg) (AMS Biotechnologies, Stockholm, Sweden) were individually added to L. aethiopica stimulated cultures in the presence or absence of IL-2 (25 U/ml) (Genzyme Diagnostics, Cambridge, MA, USA) at the start of culture.
Measurement of cytokines in supernatants
Commercial enzyme-linked immunosorbent assays (ELISA) were used to assess the release of IFN-γ (MabTech, Stockholm, Sweden), and IL-10 (AMS Biotechnologies) into supernatants following stimulation. The ELISAs used have the same sensitivity for natural and recombinant cytokines.
Statistical analysis
Means were compared by Student's t-test.
RESULTS
Proliferative responses to L. aethiopica antigen
The proliferative response of unfractionated mononuclear cells (MNC) from healthy individuals to L. aethiopica has been evaluated over time and strong proliferative responses of up to 50000 CPM have been noted in some individuals. Such high levels of reactivity are not frequently achieved when cells of patients with L. aethiopica infection are stimulated [25]. A whole range of responses are observed in the cells of the blood donors and it is evident that not all donor cells proliferate markedly to L. aethiopica stimulation (Fig. 1). Nonetheless, blood donors can be divided into responders and non responders to L. aethiopica stimulation and in this communication an arbitrary definition of responder of CPM = 5000 or SI = 5.0 is used. Reproducible responses were obtained when the same individuals were retested on more than one occasion (data not shown).
Fig. 1.
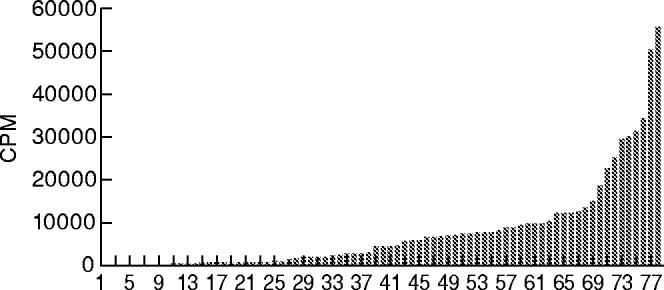
Proliferative response (counts per minute, CPM) of unfractionated peripheral blood mononuclear cells (PBMC) from Normal Swedish blood donors to L. aethiopica promastigote antigens. Each number and each histogram represents one individual donor.
The effect of NK cell depletion on the in vitro proliferative responses in healthy Swedish donors
In previous studies, we noted using fluorescence-activated cell sorter analysis that NK cells were the main proliferating phenotype when cells from healthy unexposed donors were stimulated with L. aethiopica antigen. To further evaluate these findings, MNC from eight of the healthy blood donors were depleted of NK-cells using the indirect technique of immunomagnetic isolation of CD56+ cells. The resultant purity (i.e. absence of CD3–CD56+ cells) ranged from 97.9% to 99.7%. Cells were recounted and stimulated as for the MNC. The effect of depletion varied in responders and in nonresponders. Depletion of NK cells from the cells of responders resulted in a marked reduction of the proliferative response to L. aethiopica compared with undepleted cultures in three of four donors (Fig. 2). There was little effect or sometimes enhanced proliferation of NK-cell depletion in cells of nonresponding donors. Mock depletion using antimouse IgG coated beads had no effect on the proliferative potential in the selected samples tested this way (data not shown).
Fig. 2.
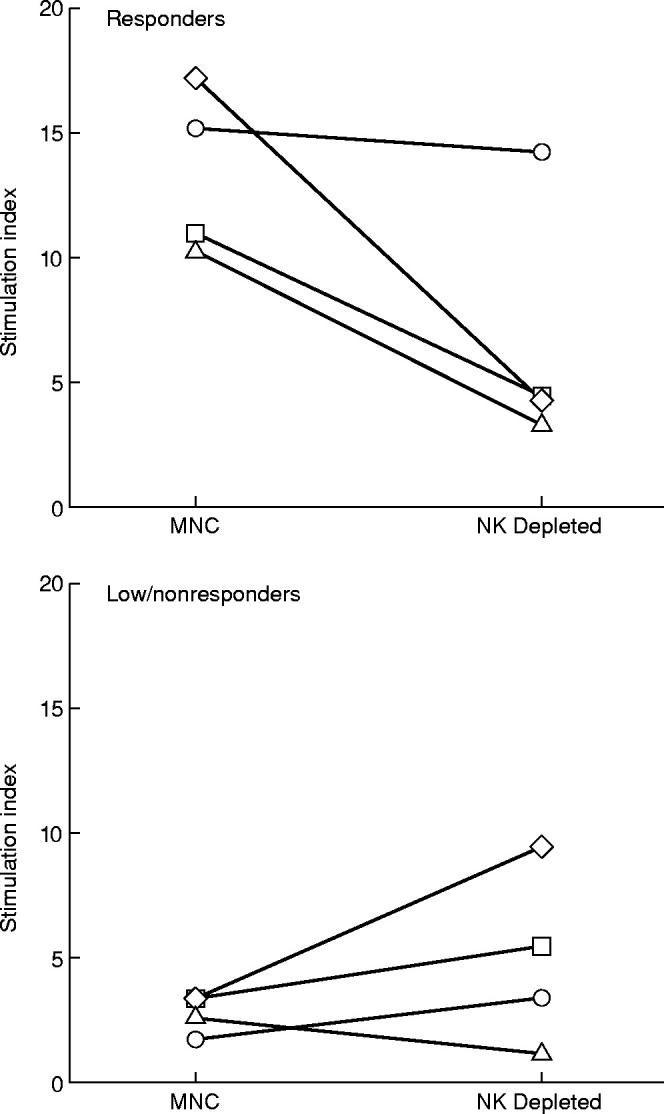
The effect of depletion of natural killer (NK) cells from MNC on the L. aethiopica induced proliferation in responder and nonresponder donors. Each line represents the stimulation indices of cultures from the same individual before and after NK cell depletion.
Response of purified NK cells to L. aethiopica stimulation
Due to the relatively low level of NK cells obtained, the NK enriched cultures were tested only at one concentration, 1 × 105/well. There was no proliferation to L. aethiopica by purified NK cells from any of the eight donors (data not shown).
The differential effect of varying doses of exogenous rIL-12 on the L. aethiopica induced proliferative and cytokine response in healthy Swedish blood donors
The supernatants of L. aethiopica stimulated cultures were harvested 72 h after stimulation and tested for levels of IFN-γ and IL-10 in another set of eight donors (Fig. 3). The mean L. aethiopica induced stimulation index (± s.d.) was 13.6 ± 8.5, but contrary to expectation, supernatants from these cultures contained little IFN-γ (109.5 ± 78.0 pg/ml). Addition of 10 U of rIL-12 to L. aethiopica significantly enhanced the secretion of IFN-γ into the supernatants (304 ± 57.2 pg/ml; P < 0.01), but the proliferation was abrogated. Further increase of rIL-12 to 50 U was reflected in variable but measurable IL-10 (5.1 ± 10.2 U/ml), decreased IFN-γ (261.0 ± 29.0 pg/ml) and no proliferation. This trend continued at 100 U of rIL-12, inducing increased IL-10 (31.1 ± 19.8 U/ml), decreased IFN-γ (197.0 ± 69.1 pg/ml) and no proliferation. Addition of 500 U of rIL-12 resulted in a shift that was expressed as proliferation to levels as high as cultures stimulated with L. aethiopica alone (SI = 10.3 ± 4.5), a significant enhancement of IFN-γ secretion (11514 ± 4368 pg/ml; P < 0.01) and no further increase in IL-10 levels.
Fig. 3.
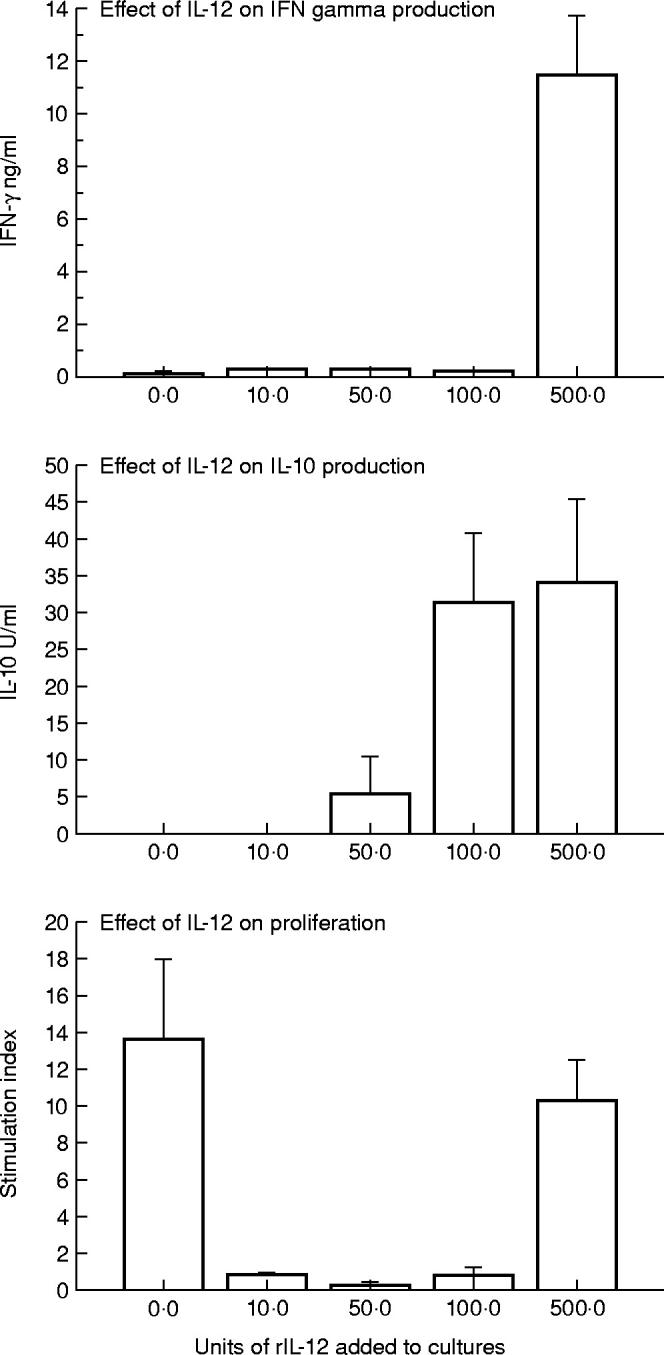
The effect of addition of various doses of exogenous rIL-12 on L. aethiopica response in peripheral blood mononuclear cells (PBMC). The results are expressed as mean ± s.d. of eight donors tested.
The differential effect of varying doses of exogenous rIL-10 on the L. aethiopica induced proliferative and cytokine response in healthy Swedish blood donors
PBMC from 10 Swedish blood donors were tested with or without varying concentrations of added rIL-10. Up to 100 U/ml rIL-10 inhibited the L. aethiopica induced proliferation in a dose dependent manner in all those tested and addition of 10 U of exogenous rIL-10 was essentially able to abrogate the L. aethiopica induced response (Fig. 4). In this group of individuals, variable but measurable IL-10 was induced in cultures stimulated with L. aethiopica antigen alone, 8.1 ± 18.0 (mean ± s.d.). Addition of 50 U of exogenous rIL-10 to the L. aethiopica antigen resulted in a mean recovery of a similar amount as added (47.5 ± 23.2). No enhanced recovery of IL-10 in the supernatant was achieved by the addition of higher concentrations of rIL-10 (100–500 U). As expected, there was a significant decrease (P < 0.05) in IFN-γ in the culture supernatants when rIL-10 of between 10 and 100 U were added together with L. aethiopica. Unexpectedly, however, 500 U rIL-10 showed a tendency to an increase in IFN-γ levels although this difference was not statistically significant. At this high concentration of added IL-10, cell proliferation as measured by SI which was inhibited at lower rIL-10 concentrations also showed a tendency towards enhancement, although this difference was just below the level of significance (100 units versus 500 units; P = 0.0511).
Fig. 4.
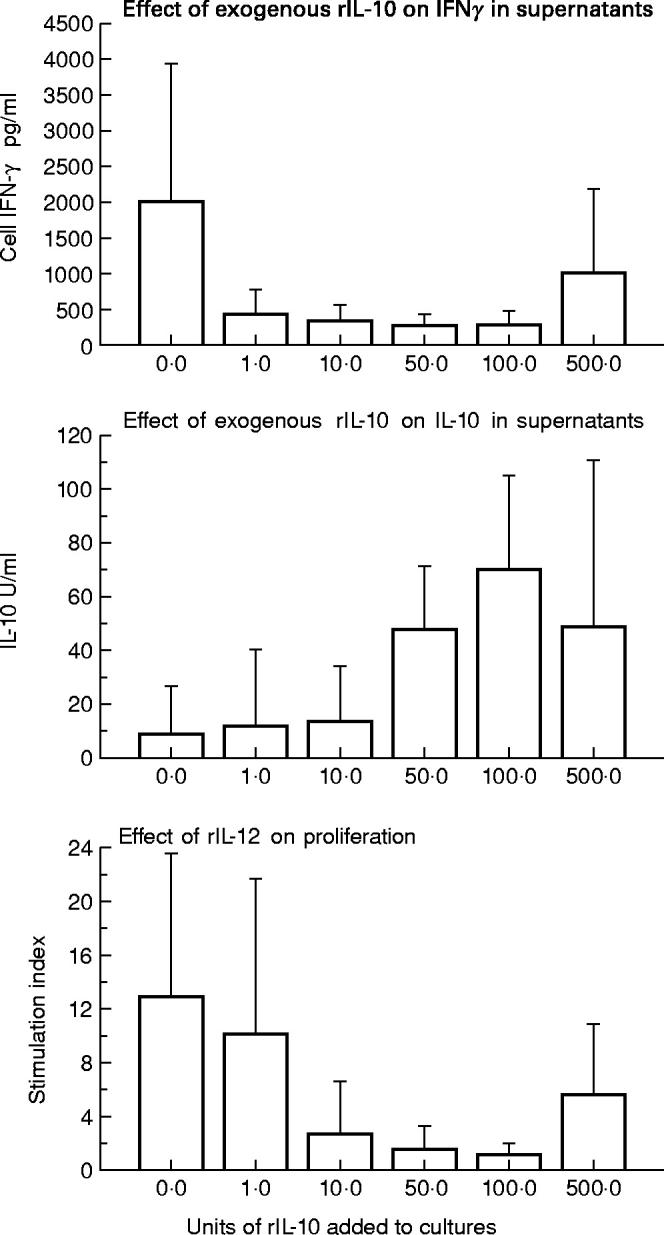
The effect of addition of various doses of exogenous rIL-10 on L. aethiopica response in peripheral blood mononuclear cells (PBMC). The results are expressed as mean ± s.d. of 10 donors tested.
DISCUSSION
Mononuclear cells from normal non-Leishmania exposed donors may respond to leishmanial antigens with strong IFN-γ production and proliferation in vitro. This response has previously been shown to include NK cell activation and features of innate immune responses [18,26], although some T-cell responses were also noted in these responses. The importance of NK cells in this infection has recently been illustrated in an endemic situation where a potential role of NK cells in protection against human L. aethiopica induced disease was demonstrated [27]. We confirm these findings and show that strong in vitro responses to leishmanial antigens are reduced when NK cells are depleted from PBMC of unexposed donors. Purified NK cells, prepared as described, however, did not proliferate to L. aethiopica at the concentrations tested. This might suggest that other cell types or the presence of cytokines such as IL-2, are required for this response to occur. The specific role of NK cells in the mixed PBMC response is not clear at this point since NK cells have multiple effector functions, including the capacity for antigen presentation. In this context, some populations of NK cells have been shown to present both conventional antigens and superantigens to T-cells [28]. Monocytes, which are induced by microbial products to secrete the NK cell stimulating cytokine, IL-12, are probably crucial for the observed activation. The possible differential capacity of monocytes from responder and nonresponder donors to induce cytokines in response to Leishmania antigen would need to be tested. IL-12 is a potent inducer of NK cells to produce IFN-γ and to proliferate [29]. In contrast, IL-10, produced by a number of cells including monocytes and Th2 cells, is reported to have opposite effects to IL-12. In particular, IL-10 has been shown to down-regulate macrophage function [30,31] and induce negative effects on IFN-γ production by T-cells through direct inhibition of IL-12 production by monocyte-macrophages [17]. The potential sources of IL-10 in the system studied here are multiple, including T-cells (CD4+ or CD8+) [19], antigen presenting cells [32] and NK cells themselves [20]. The finding that depletion of NK cells resulted in reduced proliferation to L. aethiopica, in the responder donors, coupled with the observation that addition of IL-12 only had an appreciable effect in the responders (data not shown) suggests a differential capacity of Leishmania stimulated NK cells to respond to IL-12.
We are aware that the nature of the Leishmania antigen used here could suggest that the responses measured are multiple responses to cross reactive epitopes. However, previous data showing the induction of NK cells by this antigen preparation [18], as well as the reduction of the response by NK cell depletion led us to concentrate on this cell type in the mononuclear cell mixture. This does not exclude some T-cell involvement in the proliferation observed as previously reported [26]. The slight to quite substantial enhanced proliferation after NK cell depletion in nonresponder cells might suggest that in this group of donors, NK cells have a suppressive effect on such T-cell proliferation.
The present study set out to evaluate the in vitro effect of rIL-12 (a candidate component in an antileishmaniasis vaccine) and IL-10 to influence the antileishmanial response in unexposed healthy donors, the individuals to which a potential vaccine will be given. The data presented here demonstrates that at low to moderate concentrations, cells responded to IL-12 by, as expected, measurable IFN-γ secretion but less expectedly, also by inhibited proliferation. This inhibited proliferation coincided with increased levels of secreted IL-10 in the supernatant. At high IL-12 levels (500 U) very highly elevated amounts of IFN-γ were induced which was apparently enough to over-ride the IL-10 effect in the supernatant, allowing for the L. aethiopica driven proliferation to occur. Exogenous rIL-10 in turn, at concentrations of ≥ 100 U were detected in lower amounts than added, even though 500 U was within the limits of detection of the ELISA assay. Possible interpretation for this lowered level of detection is difficult. Control experiments of incubation of varying levels of rIL-10 over a period of time, in medium alone without cells, showed that the added IL-10 was short lived and not recoverable even after 4 h incubation at 37°C (data not shown). Thus, what is being measured in the cultures is not just the added IL-10. One possibile explanation could be that at this high level, IL-10 is utilized or made unavailable for measurement in the supernatants, thus a plateau of measurable IL-10 was reached. The high level of 500 U of exogenous IL-10 with lowered level of detection coincided with a tendency towards enhanced IFN-γ induction and proliferation. Whether high levels of IFN-γ contributed to the reduced levels of IL-10 in the supernatant and enhanced proliferation is presently unclear. However, recent data have shown that NK cells express IL-10 receptor and that IL-10 enhances NK cell production of IFN-γ [33]. Further work would be needed to elucidate whether NK cell induced IFN-γ was responsible for the enhanced IFN-γ production when high concentrations of IL-10 were added to the L. aethiopica stimulated cultures, an explanation which would be consistent with our findings.
The results suggest that at a given concentration, which may differ in different individuals, IL-10 may be a negative regulator of IL-12, reducing the potential damaging effects of high levels of IL-12 leading in turn to modulated levels of IFN-γ and cellular proliferation. Very high concentrations of IL-10, in turn, may be regulated through feed back or by as yet unknown molecule(s). NK cells may be involved in utilizing the IL-10 to prevent excess accumulation in the cultures and resulting in cell proliferation and IFN-γ secretion as described [33]. The ability of IL-12 to induce IL-10 production has previously been reported when IL-12 was added during the restimulation of resting memory cells from allergic patients [34], in human T-cell clones [35], of both CD4+ and CD8+ phenotype [36]. The study here describes IL-12 induced IL-10 in antigen (Leishmania) stimulated mononuclear cell cultures from donors not previously exposed to leishmanial antigens. In this mixed cell culture, a contribution to the effects observed could come from NK cells, memory T-cells responding to cross-reacting epitopes present in the leishmanial antigen or other cells types capable of IL-10 secretion. The exact cell types involved have not been addressed in this study; however, findings in this mixed culture situation may be relevant if the phenomenon is present in vivo. This would be important in the context of giving IL-12 (as a potential adjuvant in an antileishmanial vaccine) to an individual with an innate high capacity to produce this cytokine in response to leishmanial antigens. At least two scenarios are possible. First, administration of IL-12 may not enhance the capacity of cells from such an individual to produce stronger IFN-γ response (antileishmanial), but rather down-regulate this potential through the induction of IL-10. Second, the elevated IL-10 may not inhibit the effects of IL-12 but via NK cells could enhance the IFN-γ secretion capacity of NK cells. Even if the dose of IL-12 that would be in a potential antileishmanial vaccine is not high enough to switch on an IL-10 response through a feedback action, the capacity of IL-12 to induce IL-10 secretion must be appreciated in an appropriate dose for a vaccine formulation.
Acknowledgments
This study was supported by funds from The Swedish Medical Research Council (MFR) and the Swedish International Development Agency (SIDA/SAREC).
REFERENCES
- 1.Lehn M, Weiser WY, Engelhorn S, et al. IL-4 inhibits H2O2 production and antileishmanial capacity of human cultured monocytes mediated by IFN-gamma. J Immunol. 1989;143:3020–4. [PubMed] [Google Scholar]
- 2.Sadick MD, Heinzel FP, Holaday BJ, et al. Cure of murine Leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990;17:115–27. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott P, Caspar P, Sher A. Protection against Leishmania major in BALB/c mice by adoptive transfer of a T cell clone recognizing a low molecular weight antigen released by promastigotes. J Immunol. 1990;144:1075–9. [PubMed] [Google Scholar]
- 4.Bogdan C, Schroppel K, Lohoff M, et al. Tumor necrosis factor-α in combination with interferon gamma but not with interleukin 4 activated murine macrophages for elimination of Leishmania major amastigotes. Eur J Immunol. 1990;20:1131–5. doi: 10.1002/eji.1830200528. [DOI] [PubMed] [Google Scholar]
- 5.Hatzigeorgiou DE, He S, Sobel J, et al. IL-6 down-modulates the cytokine-enhanced antileishmanial activity in human macrophages. J Immunol. 1993;151:3682–92. [PubMed] [Google Scholar]
- 6.Heinzel EP, Schoenhhaut DS, Rerko RM, et al. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993;177:1505–9. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sypek JP, Chung CL, Mayor SE, et al. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797–802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scharton-Kersten T, Afonso LC, Wysocka M, et al. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J Immunol. 1995;154:5320–30. [PubMed] [Google Scholar]
- 9.Afonso LC, Scharton TM, Vieira LQ, et al. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235–7. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 10.Gurnathan S, Sacks DL, Brown DR, et al. Vaccination with DNA encoding the immunodominent LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J Immunol. 1997;186:1137–47. doi: 10.1084/jem.186.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scharton T, Scott P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–77. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trinchieri G, Wysocka M, D'Andrea A, et al. Natural killer cell stimulatory factor (NKSF) or interleukin-12 is a key regulator of immune response and inflammation. Prog Growth Factor Res. 1992;4:355–68. doi: 10.1016/0955-2235(92)90016-b. [DOI] [PubMed] [Google Scholar]
- 13.Kubin M, Chow JM, Trinchieri G. Differential regulation of interleukin-12 (IL-12), tumor necrosis factor alpha, and IL-1 beta production in human myeloid leukemia cell lines and peripheral blood mononuclear cells. Blood. 1994;83:1847–55. [PubMed] [Google Scholar]
- 14.Wenner C, Guler M, Macatonia S, et al. Roles of IFN-gamma and IFN-alpha in IL-12-induced T helper cell-1 development. J Immunol. 1996;156:1442–7. [PubMed] [Google Scholar]
- 15.Finkelman F, Madden K, Cheever A, et al. Effects of interleukin 12 on immune responses and host protection in mice infected with intestinal nematode parasites. J Exp Med. 1994;179:1563–72. doi: 10.1084/jem.179.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paganin C, Frank IGT. Priming for high interferon-γ production induced by interleukin-12 in both CD4+ and CD8+ T cell clones from HIV-infected patients. J Clin Invest. 1994;96:1677–82. doi: 10.1172/JCI118209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Andrea A, Aste AM, Valiante NM, et al. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–8. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akuffo H, Maasho K, Howe R. Natural and acquired resistance to Leishmania: cellular activation of Leishmania aethiopica of mononuclear cells from unexposed individuals is through the stimulation of NK cells. Clin Exp Immunol. 1993;94:516–21. doi: 10.1111/j.1365-2249.1993.tb08227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Yssel HWMR, Roncarolo MG, et al. IL-10 is produced by subsets of human CD4+ T cell clones and peripheral blood T cells. J Immunol. 1992;149:2378–84. [PubMed] [Google Scholar]
- 20.Mehrotra PT, Donnelly RP, Wong S, et al. Production of IL-10 by human natural killer cells stimulated with IL-2 and/or IL-12. J Immunol. 1998;160:2637–44. [PubMed] [Google Scholar]
- 21.Scott P. IL-12: initiation cytokine for cell-mediated immunity. Science. 1993;260:496–7. doi: 10.1126/science.8097337. [DOI] [PubMed] [Google Scholar]
- 22.Bøyum A. Separation of lymphocytes from blood and bone marrow. Scand J Clin Lab Invest. 1969;21(Suppl.):77–89. [Google Scholar]
- 23.Naume B, Nonstad U, Steinkjer B, et al. Immunomagnetic isolation of NK and LAK cells. J Immunol Methods. 1991;136:1–9. doi: 10.1016/0022-1759(91)90242-8. [DOI] [PubMed] [Google Scholar]
- 24.Maasho K, Akuffo HO. Cells from healthy non-exposed individuals produce cytokines to selected fractions of Leishmania promastigotes. Scand J Immunol. 1992;36:179–84. doi: 10.1111/j.1365-3083.1992.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 25.Akuffo HO, Fehniger TE, Britton S. Differential recognition of Leishmania aethiopica antigens by lymphocytes from patients with local and diffuse cutaneous leishmaniasis. J Immunol. 1988;141:2461–6. [PubMed] [Google Scholar]
- 26.Akuffo HO, Britton SFF. Contribution of non-Leishmania-specific immunity to resistance to Leishmania infection in humans. Clin Exp Immunol. 1992;87:58–64. doi: 10.1111/j.1365-2249.1992.tb06413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maasho K, Sanchez F, Schurr E, et al. Indication of the protective role of natural killer cells in human cutaneous leishmaniasis in an area of endemicity. Infect Immun. 1998;66:2698–704. doi: 10.1128/iai.66.6.2698-2704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D'Orazio JA, Stein-Streilein J. Human natural killer (NK) cells present staphylococcal enterotoxin B (SEB) to T-lymphocytes. Clin Exp Immunol. 1996;104:366–73. doi: 10.1046/j.1365-2249.1996.08698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trinchieri G, Kubin M, Bellone G, et al. Cytokine cross-talk between phagocytic cells and lymphocytes: relevance for differentiation/activation of phagocytic cells and regulation of adaptive immunity. J Cell Biochem. 1993;53:301–8. doi: 10.1002/jcb.240530406. [DOI] [PubMed] [Google Scholar]
- 30.de Waal Malefyt R, Abrams J, Bennett B, et al. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiorentino DF, Zlotnik A, Vieira P, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–51. [PubMed] [Google Scholar]
- 32.de Spits H. Functional characterization of human IL-10. Int Arch Allergy Immunol. 1992;99:8–15. doi: 10.1159/000236329. [DOI] [PubMed] [Google Scholar]
- 33.Carson WE, Lindeman MJ, Baiocchi R, et al. The functional characterization of interleukin-10 receptor expression on human natural killer cells. Blood. 1995;85:3577–85. [PubMed] [Google Scholar]
- 34.Marshall J, Secrist H, DeKruyff R, et al. IL-12 inhibits the production of IL-4 and IL-10 in allergen-specific human CD4+ T lymphocytes. J Immunol. 1995;155:111–7. [PubMed] [Google Scholar]
- 35.Jeannin P, Delneste Y, Seveso M, et al. IL-12 synergizes with IL-12 and other stimuli in inducing IL-10 production by human T cells. J Immunol. 1996;156:3159–65. [PubMed] [Google Scholar]
- 36.Gerosa F, Paganin C, Peritt D, et al. Interleukin-12 primes human. CD, 4 and CD8 T cell clones for high production of both interferon-γ and interleukin-10. J Exp Med. 1996;183:2559–69. doi: 10.1084/jem.183.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]


