Abstract
Mannan-binding lectin (MBL) is an acute phase protein which activates the classical complement pathway at the level of C4 and C2 via two novel serine proteases homologous to C1r and C1s. We recently reported that haemolysis via this lectin pathway requires alternative pathway amplification. The present experiments sought to establish the basis for this requirement, and hence focused on the activity and regulation of the C3 convertases. Complement activation was normalized between the lectin and classical pathways such that identical amounts of bound C4 and of haemolytically active C4,2 sites were present on the indicator cells. Under these conditions, there was markedly less haemolysis, associated with markedly less C3 and C5 deposited, via the lectin pathway than via the classical pathway, particularly when alternative pathway recruitment was blocked by depletion of factor D. Lectin pathway activation was associated with enhanced binding in the presence of MBL of complement control proteins C4bp and factor H to C4b and C3b, respectively, with decreased stability of the C3-converting enzyme C4b,2a attributable to C4bp. Immunodepletion of C4bp and/or factor H increased lectin pathway haemolysis and allowed lysis to occur in absence of the alternative pathway. Thus, the lectin pathway of humans is particularly susceptible to the regulatory effects of C4bp and factor H, due at least in part to MBL enhancement of C4bp binding to C4b and factor H binding to C3b.
Keywords: mannan-binding lectin, complement, RCA, innate immunity, inflammation
INTRODUCTION
Complement can be activated by antibody via the classical pathway, and independent of antibody via the classical, alternative and recently discovered lectin pathways [1–6]. Central to this system are the C3 and C4 proteins which, when activated to C4b and C3b, covalently attach to nearby targets and lead to formation of the C3 convertase enzymes [4]. The classical pathway convertase is formed by association of C2a with C4b and the alternative pathway convertase by association of Bb with C3b. The C3 convertases are critical to activation of the rest of the complement cascade and formation of the macromolecular attack complex and cytolysis [2]. A family of plasma and membrane regulatory proteins, the ‘regulators of complement activation’ (RCA), has evolved to help control this process [1,7] at this critical C3 convertase step. These include the plasma RCA proteins C4bp (C4-binding protein), which controls the C3 convertase of the classical pathway, and factor H, which controls the C3 convertase of the alternative pathway.
Although both antibody and mannan-binding lectin (MBL) activate the classical pathway to form the C3-convertase, they do so through different serine proteases, C1r and C1s in the classical pathway [7] and MASP-1 and MASP-2 in the lectin pathway [8,9] and with different reaction characteristics. Mannan-coated erythrocytes sensitized with MBL lyse upon incubation in human serum-Mg-ethyleneglycol tetraacetic acid (EGTA) [10], indicating that MBL reacts with the MASP enzymes and initiates haemolysis via the lectin pathway in the absence of calcium, in striking contrast to an absolute calcium requirement for the association of C1q with C1r and C1s and classical pathway haemolysis. This calcium-independent MBL-initiated haemolysis led to the observation that even though as a C-type lectin MBL requires calcium for binding to mannan, it is retained by mannan-coated E in the absence of calcium [10]. Furthermore, in contrast to antibody, MBL requires alternative pathway amplification in order to initiate cytolysis via the complement system in normal human serum [10]. A recent investigation demonstrating that C-reactive protein inhibits complement-dependent haemolysis via the lectin pathway by its ability to inhibit the alternative pathway indicated that this was due, at least in part, to enhancing the inhibitory activity of factor H on C3b [11,12], calling attention to the RCA proteins in control of lectin pathway activity. In the present study, we report that regulation by C4bp and factor H are important in the inability of the lectin pathway to initiate haemolysis in the absence of the alternative pathway in human serum, in keeping with an expanded role for the RCA proteins in nonimmune complement activation.
MATERIALS AND METHODS
Buffers
Gelatin-veronal-buffered saline consisting of 5 mm veronal (pH 7.4), 0.145 m NaCl and 0.1% gelatin (GVB); GVB containing 10 mm CaCl2 and 0.5 mm MgCl2 (GVB++); a mixture of equal parts of GVB++ and 5% glucose++ to make the ionic strength equal to 0.075 m NaCl (GGVB++); GVB containing 0.5 mm MgCl2, 10 mm EGTA and glucose to make the ionic strength equal to 0.075 m NaCl (Mg-EGTA); and GVB containing 10 mm ethylenediamine tetraacetic acid (EDTA-GVB), were prepared as previously described [10,12].
Reagents
Saccharomyces cerevisiae mannan (M) was purchased from Sigma Chemical Company (St Louis, MO, USA). Monoclonal anti-MBL antibody was purchased from Statens Serum Institut, Copenhagen, Denmark. Rabbit anti-sheep erythrocyte (E) haemolysin containing both IgG and IgM was obtained from Colorado Serum Co. (Denver, CO, USA). Anti-human C4, C3, C5, C4bp, properdin and factor H were purchased from Calbiochem (La Jolla, CA, USA). Streptavidin conjugated with R-phycoerythrin was purchased from Dako (Gostrup, Denmark), for use in flow cytometry.
Agammaglobulinaemic, complement deficient and complement-depleted sera
Agammaglobulinaemic human serum (AHS), and human sera deficient in C8 (C8D) and in C2 (C2D) absorbed twice with E and M, with MBL levels of 3.11, 1.78 and 3.17 μg/ml, respectively, prior to and ≤ 0.1 μg/ml following absorption by passage through a small mannan-Sepharose column, were used to avoid potential effects of natural anti-E or anti-mannan antibodies and to avoid loss of indicator cells because of haemolysis; previous experiments had showed comparable lectin pathway haemolytic activity in NHS and AHS [10]. C3-depleted human serum (MBL level, 2.54 μg/ml) was purchased from Calbiochem, and C4-deficient guinea pig serum was kindly provided by Dr Michael M. Frank (Durham, NC, USA). Serum depleted in C1q and factor D was prepared by column chromatography on BioRex 70 (BioRad, Hercules, CA) [10,13]. Factor H- and C4bp-depleted human serum was prepared by immunoaffinity chromatography as previously described [14]. Briefly, 2 ml of human serum was made 10 mm with EDTA and passed through an anti-H- or anti-C4bp-Sepharose 4B immunoabsorbent column (10 ml) which had been equilibrated with EDTA-VBS. The effluent was concentrated to the original serum volume, and had factor H, C4bp and MBL levels of 2, < 1 and 2.0 μg/ml, respectively (starting levels of H, C4bp and MBL were 1000, 200 and 3.8 μg/ml, respectively).
Purified human complement components
C2, C3, C4, C8 and D were purchased from Calbiochem. C4bp was purchased from Sigma Chemical Company. Factor H was prepared as previously described [11].
Enzyme-linked immunosorbent assay for C4bp and factor H
C4bp and factor H concentrations were assayed by sandwich enzyme-linked immunosorbent assay methodology [12].
Mannan-binding lectin
Human MBL was prepared by sequential affinity column chromatography by a minor modification of the method of Kawasaki et al. [15], as previously described [10].
Preparation of antibody-sensitized and mannan-coated erythrocytes
Antibody-sensitized sheep erythrocytes (EA) and E coated with mannan (E-M) and sensitized with MBL (E-M-MBL) were prepared as previously described [10,16–19].
Preparation of indicator cells bearing C4, C3 and C5
Indicator cells bearing C4, C3 and C5 (EAC4,3,5; E-M-MBL-C4,3,5) were prepared as previously described [10,18]. Briefly, EA and E-M-MBL were washed, resuspended to 108 cells/ml in GVB++ and Mg-EGTA, respectively, and prewarmed to 30°C. An equal volume of C8D serum (1:6.25–1:400) was added (30 min, 30°C), and the mixture was washed, incubated for 1 h to decay off C2 and resuspended to the original volume in GVB++. The cells showing the highest level of C4 binding (96 mean channel fluorescence intensity (MCF) in Fig. 1) were used as ‘high C4 binding cells’, and cells showing C4 binding of 22 MCF were used as ‘low C4 binding cells’; the tmax of the high and low C4-binding cells were identical for cells formed via the lectin and the classical pathways, respectively, indicating that C4 binding accurately reflected the formation of haemolytically active C4 sites.
Fig. 1.
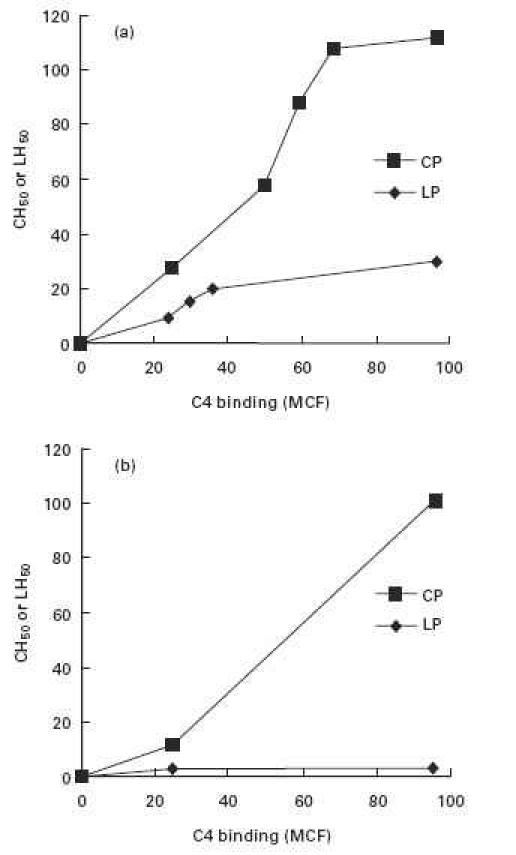
C-dependent haemolysis (expressed as 50% haemolytic units) and C4 binding (expressed as mean channel fluorescence, MCF) via the classical (CP) and lectin (LP) pathways in a constant amount (1:10) of agammaglobulinemic human serum (AHS), using sheep erythrocytes sensitized with increasing amounts of antibody and mannan plus MBL, respectively; (a) unmodified AHS and (b) AHS depleted of factor D to exclude alternative pathway amplification. Much less haemolysis was observed via the lectin pathway at each level of C4 binding, and no lysis at all was observed in the absence of alternative pathway amplification.
Haemolytic assays
The 50% haemolytic titres for classical, alternative and lectin pathway activity (CH50, AH50 and LH50, respectively) were determined as previously described [10,18,19]. For these and all other results presented, a minimum of three replicate experiments was performed.
C4 consumption assay
C4 consumption via the two pathways was assayed as previously described [18]. Briefly, EA (108/ml in GGVB++) and E-M-MBL (108/ml in Mg-EGTA) were incubated with an equal volume of C8D serum for 15 min at 37°C. After centrifugation, serial dilutions of the supernatant were assayed for residual C4 by incubation with C4-deficient guinea pig serum and EA.
Assay of the decay rate of the classical and lectin pathway C3 convertases
The effect of C4bp on the intrinsic decay rate of the C3 convertases of both pathways was carried out kinetically as previously described [20]. Briefly, EAC1,4 and E-M-MBL-C4 were prepared by incubating EA (108/ml in GGVB++) and E-M-MBL (108/ml in Mg-EGTA), respectively, with an equal volume of 1:10 C2D serum for 15 min at 37°C. The cells were washed, incubated (5 min, 30°C) with purified human C2 (2.5 μg/108 cells), and resuspended at 30°C in GGVB++ and Mg-EGTA, respectively, to determine the decay rates of the C3 convertases of the two pathways; 100 μl samples were withdrawn at various time intervals and added to C2D serum (1:10) in Mg-EGTA to measure haemolysis in the usual way.
Flow cytometry assay of MBL, C4, C3, C5, C4bp, factor H and properdin on sheep E
The indicator cells (100 μl; 108 cells/ml) were mixed with 100 μl of 1:100 biotinylated anti-human MBL, C4, C3, C5, H, C4bp and properdin (P), respectively, and incubated at 4°C for 1 h. After washing three times, 100 μl 1:10 R-phycoerythrin-conjugated streptavidin solution was added and incubated 1 h at 4°C. The cells again were washed and resuspended to 1 ml in GVB++ and assayed for the above proteins using an Ortho Cytoron flow cytometer (Ortho Diagnostic Systems, Raritan, NJ, USA) to quantify orange fluorescence. The fluorescence of cells is presented (with background staining subtracted) as the MCF of 104 cells as measured using logarithmic amplification, except as noted. In this configuration, a 22-channel increase represents an approximate doubling of fluorescence [21].
Statistical analysis
All experiments were performed in duplicate a minimum of three times. The standard errors of the mean are shown in Figs 2,3 and 6.
Fig. 2.
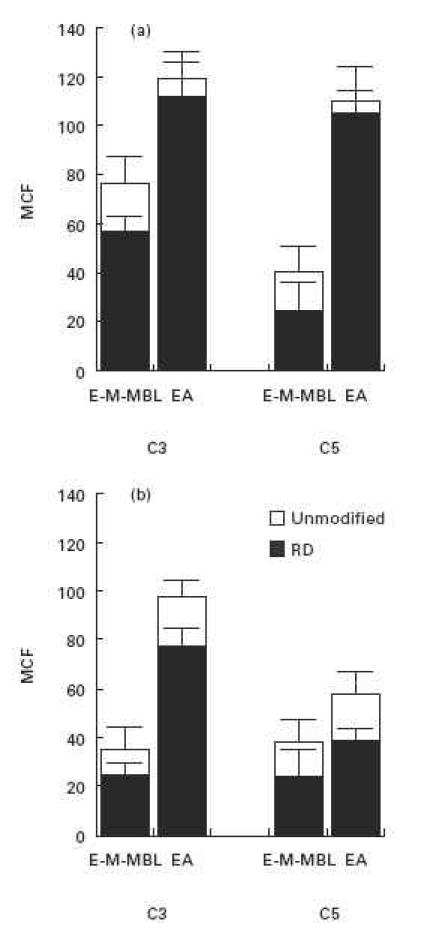
C3 and C5 binding via the lectin (E-M-MBL) and classical (EA) pathways, respectively, in a constant amount of C8D serum using cells sensitized with varying amounts of mannan and antibody in order to yield comparably high (a) and low (b) binding of C4; the darkened bars refere to C8D serum which was depleted of factor D in order to exclude alternative pathway amplification. Much less C3 and C5 were bound via the lectin pathway at both high and low levels of C4 deposition; inhibition of alternative pathway amplification reduced the level of C3 and C5 binding via the lectin pathway, as well as via the classical pathway at the lower level of C4 deposition. Standard errors of the mean are indicated by error bars.
Fig. 3.
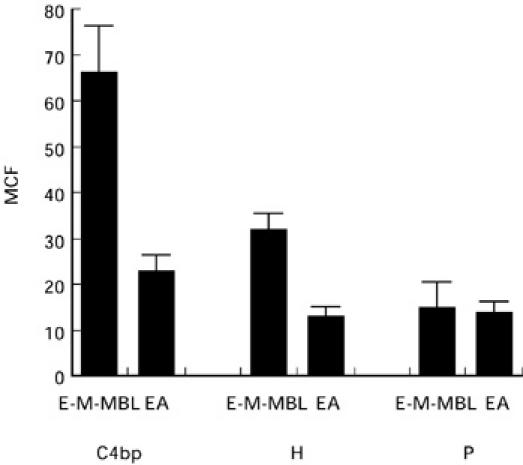
Binding of the complement regulatory proteins C4bp, factor H and properdin (P) to E-M-MBL via the lectin pathway and EA via the classical pathway, using indicator cells prepared with comparable amounts of C4 bound; regulatory protein binding is expressed as mean channel fluorescence (MCF). Much greater amounts of C4bp and factor H, but not properdin, were bound during lectin pathway activation. Standard errors of the mean are indicated by error bars.
Fig. 6.
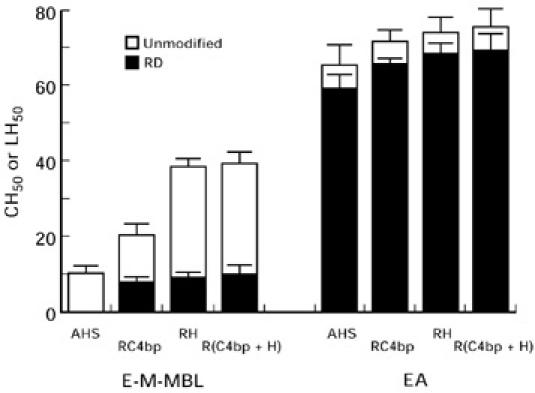
Lysis of E-M-MBL via the lectin pathway and EA via the classical pathway in agammaglobulinemic human serum (AHS), AHS depleted of C4bp (RC4bp), AHS depleted of factor H (RH) and AHS depleted of both C4bp and factor H designated as R(C4bp + H), using otherwise unmodified sera (open bars) and sera which first had been depleted of factor D (RD, solid bars) to exclude alternative pathway amplification. Lysis via the lectin pathway was much greater with an intact alternative pathway, but nonetheless could occur in absence of the alternative pathway in serum depleted of either C4bp or factor H. Standard errors of the mean are indicated by error bars.
RESULTS
C4 binding and haemolysis via the lectin pathway
We first compared haemolysis at increasing levels of C4 bound, from low (22 MCF) to high (96 MCF) C4 amounts (Fig. 1). Lysis via the lectin pathway was significantly less than lysis via the classical pathway at every level of C4b binding (Fig. 1a). Lysis did not occur at all via the lectin pathway in the absence of alternative pathway amplification (Fig. 1b), even when amounts of C4 sufficient for maximal antibody-initiated haemolysis were bound. Thus, C4 binding (96 MCF) sufficient to yield a CH50 titre of 115 U/ml via the classical pathway led to a LH50 of only 22 U/ml via the lectin pathway and no lectin pathway lysis at all when alternative recruitment was prevented by depletion of factor D.
C3b and C5b binding and haemolysis via the lectin pathway
The degrees of C3b and C5b bound were determined at both high and low levels of C4b deposition (Fig. 2). Significantly less C3b and C5b were bound via the lectin than via the classical pathway, under both conditions (P < 0.005). We next determined the degree of C3b and C5b bound in serum depleted of factor D (RD). There was significant reduction of C3b and C5b binding via the lectin pathway at both high and low levels of deposited C4b (P = 0.001 and 0.019 for C3 binding, and 0.05 and 0.06 for C5 binding, respectively), and also at the low level of C4b deposited via the classical pathway (P < 0.001); normal levels were restored upon addition of purified factor D. However, there was no reduction in classical pathway C3b and C5b deposition at the high level of bound C4b (P = 0.57 and 0.50). These results are consistent with the haemolytic studies shown in Fig. 1, and emphasize the importance of alternative pathway amplification to C3b and C5b binding as well as haemolysis via the lectin pathway. They also provide evidence that this amplification is significant for antibody-initiated classical pathway haemolysis when C4 binding is limited.
Binding of C4bp, factor H and properdin during lectin pathway activation
To further characterize the reduced C3b and C5b deposition observed during complement activation via the lectin pathway, we examined the binding of three complement regulatory proteins (C4bp, factor H and P) that control the assembly and/or stability of the C3 convertases. Complement receptor 1 (CR1) and membrane cofactor protein (MCP) were not evaluated because they are not present on the sheep E membrane [22–25], and decay accelerating factor was not evaluated because the action of this cell membrane RCA is limited to autologous complement components [26]. As shown in Fig. 3, much larger amounts of both C4bp and factor H bound to E-M-MBL than to EA at equivalent levels of membrane-bound C4b (P < 0.002). No significant difference of properdin binding was observed to the two indicator cells (P = 0.85). C4bp and H binding to EA were comparable to negative control indicator cells or chromic chloride-treated E, E-M, or when purified C4bp or factor H was added to E-M-MBL, demonstrating the specificity of the reaction. Similarly, no significant C4bp binding was observed in the absence of C4b, nor was there significant binding of factor H in absence of C3b, indicating that C4bp and factor H were binding to C4b and C3b, respectively, on the cell surface. Thus, there was much greater binding of C4bp and factor H during lectin pathway than during classical pathway activation.
Stability of the lectin pathway-induced C3 convertase in the presence and absence of C4bp
Since C4bp binding was increased during lectin pathway activation, the stability of the C3 convertase formed by this pathway was determined in the presence and absence of C4bp. As shown in Fig. 4, the intrinsic decay rates of the C3 convertases formed by the two pathways were identical in the absence of C4bp, with the t1/2 = 5.25 min for each pathway. However, the C3 convertase of the lectin pathway was much less stable in the presence of C4bp (t1/2 = 1.25 min) than was the C3 convertase of the classical pathway (t1/2 = 3.5 min).
Fig. 4.
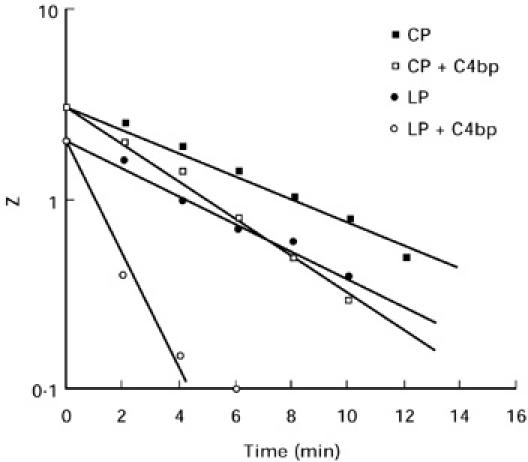
Stability of the C4b,2a convertases formed via the classical pathway (CP) and lectin pathway (LP), respectively, in the presence and absence of C4bp. The intrinsic decay rates were identical in the absence of C4bp (t1/2 = 5.25 min), but the convertase formed via the lectin pathway decayed much more rapidly in the presence of C4bp than did the classical pathway convertase (t1/2 = 1.25 min compared to 3.5 min). Lysis is presented as haemolytic sites per cell (Z) for these calculations.
Enhancement of lectin pathway-mediated haemolysis by depletion of C4bp and factor H
To test the functional significance of the greater susceptibility of the lectin pathway to C4bp inhibition, we compared lectin pathway haemolytic activity in C4bp-depleted (RC4bp) and unmodified AHS. Enhancement of lysis (from 8 to 17 LH50 U/ml) was observed in RC4bp in Mg-EGTA (Fig. 5a). Enhancement of lysis decreased as increasing amounts of C4bp were added to the depleted serum, with optimal restoration seen with 5 μg C4bp/100 μl serum (Fig. 5b), a level well below the normal C4bp serum concentration of 250 μg/ml. Next, we investigated the effect of factor H depletion on lectin pathway-mediated haemolysis. As shown in Fig. 5c, lysis of E-M-MBL was significantly enhanced in factor H-depleted AHS (RH), compared to AHS in Mg-EGTA (from 8 to 44 LH50 U/ml). The enhancement of lysis decreased as the amounts of factor H were increased, with complete restoration of inhibition obtained at 50 μg H/100 μl serum (Fig. 5d), a level approximately equal to the normal factor H serum concentration of 500 μg/ml. These experiments point to an important role for C4bp and factor H in regulation of haemolysis via the lectin pathway.
Fig. 5.
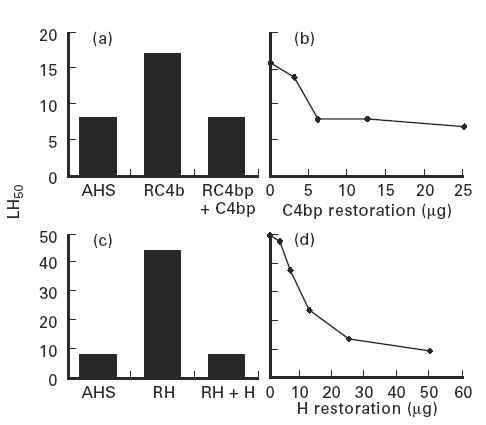
Lectin pathway activity expressed as percentage haemolysis of E-M-MBL in agammaglobulinemic human serum (AHS), AHS depleted of C4bp (RC4bp) and RC4bp reconstituted with purified C4bp (a); the dose response curve for C4bp restoration is shown in (b) and expressed as 50% lectin pathway haemolytic units (LH50). Comparable experiments using serum depleted of factor H (RH) are shown in (c,d). Hemolytic activity was greatly enhanced in the absence of C4bp and, to an even greater extent, in the absence of factor H.
Alternative pathway requirement for lectin pathway-mediated haemolysis in serum depleted of C4bp and factor H
As shown in Fig. 6 (left panel), lysis remained reduced in absence of alternative pathway amplification in human serum depleted of C4bp or factor H, or both, but there no longer was an absolute requirement for alternative pathway amplification in order for lectin pathway lysis to occur. Thus, the enhancement from 8 to 17 LH50 U/ml upon depletion of C4bp was reduced to 6 LH50 U/ml when factor D was depleted as well; the enhancement from 8 to 44 LH50 U/ml upon depletion of factor H was reduced to 8 LH50 U/ml when factor D was depleted as well; and the enhancement from 8 to 36 LH50 U/ml upon depletion of both C4bp and factor H was reduced to 9 LH50 U/ml when factor D was depleted as well. However, even the reduced levels of lysis observed in absence of alternative pathway amplification in the inhibitor-depleted sera contrasted with the complete absence of lysis in AHS depleted of factor D (LH50 of 8 U/ml prior to depletion of factor D). C3b and C5b binding to E-M-MBL was enhanced in serum depleted of C4bp or factor H, or both, at both high and low levels of bound C4b. No significant enhancement of classical pathway-mediated lytic activity was observed in C4bp- or factor H-depleted human serum (65 to 71 and 74 CH50 U/ml, respectively; P = 0.2 and 0.1, respectively), nor was the alternative pathway required for classical pathway haemolysis (Fig. 6, right panel). Thus, although lectin pathway lysis was increased in the absence of C4bp and/or factor H, alternative pathway amplification still was required for maximal lysis; classical pathway lysis was not significantly influenced by depletion of these regulatory proteins.
DISCUSSION
The present experiment demonstrated, in three ways, that the lectin pathway is particularly susceptible to control by both C4bp and factor H. First, binding of both C4bp and factor H to E-M-MBL during C activation via the lectin pathway was significantly greater than to EA during C activation via the classical pathway when these were incubated in human serum under conditions of equivalent C4 binding. Normalization at the C4 level was needed to make this comparison, since in the absence of purified MASP reagents equilibration of activation could not be established at an earlier step in the reaction sequence, with normalization established based on both C4 binding and haemolytically active C4b2a sites. Second, the C3 convertase formed via the lectin pathway decayed at a faster rate in the presence of C4bp than the convertase formed via the classical pathway. Third, lectin pathway-mediated lysis was markedly increased in sera depleted of C4bp and factor H, to a much greater extent than was antibody-initiated lysis via the classical pathway; indeed, the alternative pathway was no longer absolutely required for lectin pathway-induced haemolysis in either C4bp- or factor H-depleted serum unless these sera were reconstituted with the missing C4bp or factor H. Taken together, these experiments point to a special role for C4bp and factor H in the regulation of haemolytic activity via the lectin pathway (Fig. 7).
Fig. 7.
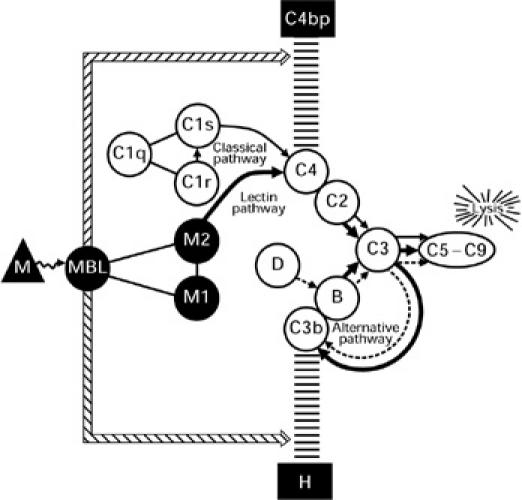
Diagrammatic sketch of complement activation and regulation during haemolysis initiated by mannan-binding lectin (MBL) and the MASP enzymes (designated M1 and M2) via the lectin pathway (solid thick line) after reacting with mannan (M)-coated sheep erythrocytes. The scaffolded lines represent the inhibitory effects of complement regulatory proteins C4bp and factor H on C4b and C3b, respectively. The enhanced binding of both regulatory proteins during MBL-initiated complement activation is indicated by the thin double line, although the mechanism by which this occurs is not yet clear. The classical and alternative pathways are indicated by thin solid and dotted lines, respectively; arrows in the complement pathways indicate enzymatic cleavages.
It is not yet clear why the binding and activity of C4bp and factor H are so greatly increased during lectin pathway activation. Perhaps the presence of MBL enhances the binding of C4bp to C4b and factor H to C3b, comparable to the manner in which sialic acid, heparin and CRP increase the binding of factor H to C3b [11,26,27], as diagramatically represented in Fig. 7. Alternatively, as recently evaluated [27a], the increased binding of factor H to E-M-MBL might simply reflect complement activation at a surface from which immunoglobulin is absent and thus unable to covalently bind and protect nascent C3b from attachment of factor H and subsequent degradation by I (an enzyme which cleaves and inactivates C3b) [28,29]. C4b also forms covalent bonds with IgG and IgM [30,31], but immunoglobulin seems unable to protect nascent C4b from interaction with C4bp [27a]. It also is possible that other proteins, such as serum amyloid P (SAP) [32] or CRP [10,12], influence the reactivity of C4bp with C4b and/or factor H with C3b. Whatever the underlying mechanism, these experiments indicate that C4bp and factor H are of major importance in regulation of lectin pathway activity.
The enhanced haemolysis observed in sera depleted of C4 bp and factor H, in contrast to immunoglobulin-initiated activation of the classical pathway, still required alternative pathway amplification for maximum haemolysis to occur. Thus, a greater susceptibility to the activity of these control proteins cannot entirely explain the insufficiency of haemolysis via the lectin pathway in absence of alternative pathway amplification. The basis for this requirement, which may reside at the level of activation or control of the MASP enzymes, is not yet clear. The E-M-MBL provided an adequate stimulus for lectin pathway activation, since more lysis was observed in guinea-pig serum via the lectin pathway than via the classical pathway in identical assays with the same indicator cells used in the present report [33]. Lesser lectin pathway lysis which required alternative pathway amplification also was observed when E sensitized with endotoxic lipopolysaccharide from the Ra mutant of Salmonella minnesota (E-RaLPS) were used as the indicator cell to compare lectin pathway activation by MBL with classical pathway activation by rabbit anti-RaLPS in assays normalized for C4 binding [34]. Nonetheless, there clearly is a preferential susceptibility of lectin pathway activation to the activity of C4bp and factor H. Perhaps multiple forms of immunoglobulin-independent C activation are preferentially regulated by these RCA control proteins and require alternative pathway amplification in order to initiate maximum haemolysis, with this requirement accentuated when the degree of C activation is limited. Even immunoglobulin-initiated classical pathway activation may require alternative pathway amplification for maximal haemolysis when C4b and C3b deposition is limited, as suggested in the present report. Hemolysis via the lectin pathway may depend upon a balance between the strength of classical pathway activation and regulation of the classical and alternative pathway C3 convertases by C4bp and factor H. The biological significance of the lectin pathway is yet to be clarified. Its early appearance in phylogenetic development [35], and the association of MBL deficiency with increased susceptibility to certain infectious processes [36–38], indicate its importance in host defense. Since MBL levels increase early during infection and inflammation, perhaps MBL leads to C activation and C-dependent opsonization and cytolysis prior to antibody formation or acts cooperatively with small amounts of antibody. Elucidation of the factors that influence MBL interactions with the complement system should yield further insight into its role in inflammation and host defense.
Acknowledgments
This paper was presented in part to the XVII Annual International Complement Workshop in Rhodes, 11–16 October 1998 [39]. Supported by a Thai Royal Government Scholarship to C.S., and is presented by Y.Z. in partial fulfillment of the requirements for the PhD from Rush University; H.G. holds the Thomas J. Coogan Sr Chair in Immunology established by Marjorie Lindheimer Everett.
REFERENCES
- 1.Liszewski MK, Farries TC, Lublin DM, Rooney IA, Atkinson JP. Control of the complement system. Adv Immunol. 1996;61:201–83. doi: 10.1016/s0065-2776(08)60868-8. [DOI] [PubMed] [Google Scholar]
- 2.Volanakis JE, Fearon TD. Molecular biology of the complement system. In: Koopman WJ, editor. Arthritis and allied conditions: a textbook of rheumatology. 13. Philadelphia, PA: Williams & Wilkins Press; 1997. [Google Scholar]
- 3.Gewurz H, Ying SC, Jiang H, Lint TF. Nonimmune activation of the classical complement pathway. Behring Inst Mitt. 1993;93:138–47. [PubMed] [Google Scholar]
- 4.Law SK, Dodds AW. The internal thioester and the covalent binding properties of the complement proteins. C3 and C4. Protein Sci. 1997;6:263–74. doi: 10.1002/pro.5560060201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hourcade D, Holers VM, Atkinson JP. The regulators of complement activation (RCA) gene cluster. Adv Immunol. 1989;45:381–416. doi: 10.1016/s0065-2776(08)60697-5. [DOI] [PubMed] [Google Scholar]
- 6.Holmskov U, Malhotra R, Sim RB, Jensenius JC. Collectins: collagenous C-type lectins of the innate immune defense system. Immunol Today. 1994;15:67–74. doi: 10.1016/0167-5699(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 7.Arlaud GJ, Thielens NM, Illy C. Assembly of the C1 complex. Behring Inst Mitt. 1993;93:189–95. [PubMed] [Google Scholar]
- 8.Matsushita M, Endo Y, Fujita T. MASP1 (MBL-associated serine protease 1) Immunobiology. 1998;199:340–7. doi: 10.1016/S0171-2985(98)80038-7. [DOI] [PubMed] [Google Scholar]
- 9.Vorup-Jensen T, Jensenius JC, Thiel S. MASP-2, the C3 convertase generating protease of the MBLectin complement activating pathway. Immunobiology. 1998;199:348–57. doi: 10.1016/S0171-2985(98)80039-9. [DOI] [PubMed] [Google Scholar]
- 10.Suankratay C, Zhang XH, Zhang Y, Lint TF, Gewurz H. Requirement for the alternative pathway as well as C4 and C2 in complement-dependent hemolysis via the lectin pathway. J Immunol. 1998;160:3006–13. [PubMed] [Google Scholar]
- 11.Mold C, Kingzette M, Gewurz H. C-reactive protein inhibits pneumococcal activation of the alternative pathway by increasing the interaction between factor H and C3b. J Immunol. 1984;133:882–5. [PubMed] [Google Scholar]
- 12.Suankratay C, Mold C, Zhang Y, Potempa LA, Lint TF, Gewurz H. Complement regulation in innate immunity and the acute-phase response: inhibition of mannan-binding lectin-initiated complement cytolysis by C-reactive protein (CRP) Clin Exp Immunol. 1998;113:353–9. doi: 10.1046/j.1365-2249.1998.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Praz F, Barreira MC, Lesavre P. A one-step procedure for preparation of classical pathway (C1q) and alternative pathway (factor D) depleted human serum. J Immunol Methods. 1982;50:227–31. doi: 10.1016/0022-1759(82)90229-0. [DOI] [PubMed] [Google Scholar]
- 14.Sim RB, Day AJ, Moffatt BE, Fontaine M. Complement factor I and cofactors in control of complement system convertase enzymes. Methods Enzymol. 1993;223:13–35. doi: 10.1016/0076-6879(93)23035-l. [DOI] [PubMed] [Google Scholar]
- 15.Kawasaki N, Kawasaki T, Yamashina I. Isolation and characterization of a mannan-binding protein from human serum. J Biochem (Tokyo) 1983;94:937–47. doi: 10.1093/oxfordjournals.jbchem.a134437. [DOI] [PubMed] [Google Scholar]
- 16.Perucca PJ, Faulk WP, Fudenberg HH. Passive immune lysis with chromic chloride-treated erythrocytes. J Immunol. 1969;102:812–20. [PubMed] [Google Scholar]
- 17.Ikeda K, Sannoh T, Kawasaki N, Kawasaki T, Yamashina I. Serum lectin with known structure activates complement through the classical pathway. J Biol Chem. 1987;262:7451–4. [PubMed] [Google Scholar]
- 18.Harrison RA, Lachmann PJ. Complement technology. In: Weir DM, editor. Handbook of experimental immunology. Palo Alto, CA: Blackwell Scientific Publications; 1986. [Google Scholar]
- 19.Gewurz AT, Lint TF, Imherr SM, Garber SS, Gewurz H. Detection and analysis of inborn and acquired complement abnormalities. Clin Immunol Immunopathol. 1982;23:297–311. doi: 10.1016/0090-1229(82)90116-7. [DOI] [PubMed] [Google Scholar]
- 20.Gigli I, Fujita T, Nussenzweig V. Modulation of the classical pathway C3 convertase by plasma proteins C4 binding protein and C3b inactivator. Proc Natl Acad Sci USA. 1979;76:6596–600. doi: 10.1073/pnas.76.12.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spear GT, Takefman DM, Sullivan BL, Landay AL, Jennings MB, Carlson JR. Anti-cellular antibodies in sera from vaccinated macaques can induce complement-mediated virolysis of human immunodeficiency virus and simian immunodeficiency virus. Virology. 1993;195:475–80. doi: 10.1006/viro.1993.1398. [DOI] [PubMed] [Google Scholar]
- 22.Wilson JG, Ratnoff WD, Schur PH, Fearon DT. Decreased expression of the C3b/C4b receptor (CR1) and the C3d receptor (CR2) on B lymphocytes and of CR1 on neutrophils of patients with systemic lupus erythematosus. Arthritis Rheum. 1986;29:739–47. doi: 10.1002/art.1780290606. [DOI] [PubMed] [Google Scholar]
- 23.Ross GD, Medof ME. Membrane complement receptors specific for bound fragments of C3. Adv Immunol. 1985;37:217–67. doi: 10.1016/s0065-2776(08)60341-7. [DOI] [PubMed] [Google Scholar]
- 24.Seya T, Turner JR, Atkinson JP. Purification and characterization of a membrane protein (gp45–70) that is a cofactor for cleavage of C3b and C4b. J Exp Med. 1986;163:837–55. doi: 10.1084/jem.163.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cole JL, Housley GA, Jr, Dykman TR, MacDermott RP, Atkinson JP. Identification of an additional class of C3-binding membrane proteins of human peripheral blood leukocytes and cell lines. Proc Natl Acad Sci USA. 1985;82:859–63. doi: 10.1073/pnas.82.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lublin DM, Atkinson JP. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu Rev Immunol. 1989;7:35–58. doi: 10.1146/annurev.iy.07.040189.000343. [DOI] [PubMed] [Google Scholar]
- 27.Kazatchkine MD, Fearon DT, Silbert JE, Austen KF, Suankratay C, Zhang Y, Jones TF, Lint TF, Giewury H. Enhancement of lectin pathway haemolysis by immunoglobulins. Clin Exp Immunol. 1999;117:435–41. doi: 10.1046/j.1365-2249.1999.00996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medof ME, Lam T, Prince GM, Mold C. Requirement for human red blood cells in inactivation of C3b in immune complexes and enhancement of binding to spleen cells. J Immunol. 1983;130:1336–40. [PubMed] [Google Scholar]
- 29.Fries LF, Gaither TA, Hammer CH, Frank MM. C3b covalently bound to IgG demonstrates a reduced rate of inactivation by factors H and I. J Exp Med. 1984;160:1640–55. doi: 10.1084/jem.160.6.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kishore N, Shah D, Skanes VM, Levine RP. The fluid-phase binding of human C4 and its genetic variants, C4A3 and C4B1, to immunoglobulins. Mol Immunol. 1988;25:811–9. doi: 10.1016/0161-5890(88)90117-4. [DOI] [PubMed] [Google Scholar]
- 31.Jarvis JN, Lockman JC, Levine RP. IgM rheumatoid factor and the inhibition of covalent binding of C4b to IgG in immune complexes. Clin Exp Rheumatol. 1993;11:135–41. [PubMed] [Google Scholar]
- 32.Garcia de Frutos P, Dahlback B. Interaction between serum amyloid P component and C4b-binding protein associated with inhibition of factor I-mediated C4b degradation. J Immunol. 1994;152:2430–7. [PubMed] [Google Scholar]
- 33.Zhang Y, Suankratay C, Zhang X-H, Jones DR, Lint TF, Gewurz H. Calcium independent hemolysis via the lectin pathway of complement activation in the guinea pig and other species. Immunology. 1999 doi: 10.1046/j.1365-2567.1999.00810.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Suankratay C, Lint TF, Gewurz H. Lysis via the lectin pathway of complement activation: minireview and lectin pathway enhancement of endotoxin-initiated haemolysis. Immunopharmacology. 1999;42:81–90. doi: 10.1016/s0162-3109(99)00029-6. [DOI] [PubMed] [Google Scholar]
- 35.Ji X, Azumi K, Sasaki M, Nonaka M. Ancient origin of the complement lectin pathway revealed by molecular cloning of mannan binding protein-associated serine protease from a urochordate, the Japanese ascidian, Halocynthia roretzi. Proc Natl Acad Sci USA. 1997;94:6340–5. doi: 10.1073/pnas.94.12.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Epstein J, Eichbaum Q, Sheriff S, Ezekowitz RA. The collectins in innate immunity. Curr Opin Immunol. 1996;8:29–35. doi: 10.1016/s0952-7915(96)80101-4. [DOI] [PubMed] [Google Scholar]
- 37.Garred P, Madsen HO, Balslev U, et al. Susceptibility to HIV infection and progression of AIDS in relation to variant alleles of mannose-binding lectin. Lancet. 1997;349:236–40. doi: 10.1016/S0140-6736(96)08440-1. [DOI] [PubMed] [Google Scholar]
- 38.Sumiya M, Super M, Tabona P, et al. Molecular basis of opsonic defect in immunodeficient children. Lancet. 1991;337:1569–70. doi: 10.1016/0140-6736(91)93263-9. [DOI] [PubMed] [Google Scholar]
- 39.Suankratay C, Mold C, Zhang Y, Jones DR, Lint TF, Gewurz H. Complement-dependent hemolysis in human serum via the lectin pathway: regulation by C4bp, factor H and immunoglobulins (abstract) Mol Immunol. 1998;35:390. [Google Scholar]


