Abstract
The aim of this study was to evaluate the prevalence of IgG, IgA and IgM anti-β2-GPI antibodies in anti-phospholipid syndrome (APS), and to establish the clinical significance of IgA type antibodies compared with the other isotypes. Anti-β2-GPI antibodies were measured in the sera of 70 patients by solid-phase enzyme immunoassay in γ-irradiated polystyrene plates coated with human purified β2-GPI. Thirty-three out of the 70 patients were classified as having APS: three of them had primary, and 30 had secondary APS related to systemic lupus erythematosus (SLE). The remaining 37 patients had SLE without APS. Anti-β2-GPI antibodies of IgG, IgA and IgM isotypes were present in 84.8%, 59.3% and 51.5% of patients with APS. Both the frequency and the level of each isotype were significantly higher in patients with APS. This association was very strong for IgA (P = 0.0004 for the antibody frequency and P < 0.0001 for the antibody level), as well as for IgG type antibodies (P < 0.0001 and P < 0.0001), whereas it was weaker for IgM (P = 0.01 and P = 0.04). A strong relationship was demonstrated between increased IgA anti-β2-GPI antibody levels and a history of venous thrombosis, thrombocytopenia, heart valve disease, livedo reticularis and epilepsy. IgG anti-β2-GPI antibodies were associated with the presence of lupus anticoagulant (LA) in addition to the main features of APS. However, antibodies of IgM isotype were related only to thrombocytopenia and heart valve disease. We recommend the evaluation of anti-β2-GPI antibodies of IgA isotype in addition to IgG in patients with clinical suspicion of APS.
Keywords: anti-β2-glycoprotein I antibodies, IgA, IgG, IgM, anti-phospholipid syndrome
INTRODUCTION
Anti-phospholipid (aPL) antibodies—anti-cardiolipin (aCL) antibodies and lupus anticoagulant (LA)—are closely associated with connective tissue disorders such as systemic lupus erythematosus (SLE) [1]. Patients with aPL antibodies are at risk of thromboembolic manifestations, intra-uterine fetal loss and thrombocytopenia, representing a condition termed as anti-cardiolipin or anti-phospholipid syndrome (APS) [2]. APS has been classified as primary, without evidence of any underlying disease, and secondary, associated mainly with SLE. Anti-cardiolipin antibodies can be detected by solid-phase immunoassay using cardiolipin as antigen, but binding of aCL antibodies depends on the presence of β2-GPI [3]. It has been shown that aCL antibodies recognize a modified β2-GPI structure induced by the interaction of the glycoprotein with anionic phospholipids or negatively charged solid surfaces (β2-GPI-dependent aCL antibodies) [4,5]. In contrast, aCL antibodies found in patients with infection bind to native cardiolipin (β2-GPI-independent aCL antibodies) [6,7]. Recent studies have demonstrated that phospholipid-free β2-GPI can be used to detect aPL antibodies [8,9]. Moreover, the association of anti-β2-GPI antibodies with the manifestations of APS is stronger than that of aCL antibodies [10,11].
Predominantly IgG and IgM aCL antibodies have been studied in patients with primary and secondary APS, and there are only few reports on the frequency of IgA antibodies. Association between IgA aCL antibodies and APS has been suggested already [12–14], but this relationship could not be confirmed by others [15,16]. Moreover, IgA aCL have been found also in other conditions, such as Henoch–Schönlein purpura [17], or HTLV-1-associated tropical spastic paraparesis [18]. Even less data are available about IgA anti-β2-GPI antibodies, since investigations on the frequency and significance of anti-β2-GPI antibodies also referred to IgG and rarely to IgM isotypes [9,11,19,20].
In addition to thromboembolic manifestations, intra-uterine fetal loss and thrombocytopenia, other symptoms have also been included as part of the APS, such as skin involvement (livedo reticularis, and skin ulcers) [21], neurologic defects [22], or heart valve disease [23]. Here we report the frequency of IgG, IgA and IgM type anti-β2-GPI antibodies in patients with primary and secondary APS, and the association of the various anti-β2-GPI isotypes with the different manifestations of the APS: venous and arterial thrombosis, thrombocytopenia, intra-uterine fetal loss, livedo reticularis, epilepsy and heart valve disease.
PATIENTS AND METHODS
Patient population
Samples from 70 patients were selected for the study from sera sent to the laboratory for routine aPL antibody determination (aCL and aβ2-GPI antibodies), with the diagnosis of known or suspected APS. The patient population included 57 women and 10 men with SLE, and three women with primary APS. SLE patients with a history of thromboembolic manifestations, spontaneous fetal loss or thrombocytopenia (< 150 G/l) along with the presence of LA or IgG type aCL antibodies were classified as having secondary APS. Thirty out of the 67 SLE patients fulfilled these criteria. Serum samples from these patients were stored at −70°C until assaying for IgG, IgA and IgM anti-β2-GPI and aCL antibodies. Medical records of the study subjects were retrospectively reviewed, and clinical symptoms and laboratory parameters such as previous arterial and venous thrombosis, thrombocytopenia (< 150 G/l), intra-uterine fetal loss, livedo reticularis or skin ulcers, epilepsy, heart valve disease and the presence of LA were registered. Deep venous thromboses were diagnosed by ultrasonography, and occurred in the lower limb in the majority of cases (n = 12). One patient had deep vein thrombosis in the upper limb, and one other patient in the vena cava inferior. Arterial occlusions included stroke (n = 9) and multiple cerebral infarctions (n = 5) as confirmed by magnetic resonance imaging; transient ischaemic attack, based on clinical findings (n = 3); and myocardial infarction with typical electrocardiographic features and elevated creatinine kinase MB fraction (n = 2). Fetal loss was defined as two or more spontaneous abortions (before week 20 of gestation), or one or more intra-uterine fetal deaths (after week 20 of gestation). Eleven women fulfilled these criteria, and some of them had a history of both spontaneous abortion and intra-uterine fetal death. Twelve patients had a history of thrombocytopenia (< 150 G/l). Eleven of them also had other clinical features of the APS: five patients had venous thrombosis, four had arterial thrombosis, while three had a history of spontaneous fetal loss in addition to thrombocytopenia. Moreover, two patients had skin involvement, two had cardiac valve disease and one had epilepsy. There was only one patient with thrombocytopenia as the only clinical feature related to the APS. Valve abnormalities—as confirmed by echocardiography—involved the aortic valve in two patients and the mitral valve in 11 other patients. For the diagnosis of APS aPL antibodies were detected on at least two different occasions, but aPL antibody positivity is known to persist for years in some of these patients. In the present study four patients were identified with aCL and anti-β2-GPI antibodies, but no clinical symptoms of APS, and two other patients with aCL and anti-β2-GPI antibodies, and symptoms related to the APS but not fulfilling the diagnostic criteria (one patient had epilepsy and avascular bone necrosis and one had livedo reticularis in addition to pulmonary hypertension).
Measurement of antibodies to β2-GPI
Antibodies to β2-GPI were determined by solid-phase enzyme immunoassay. Wells of γ-irradiated polystyrene plates (Greiner Labortechnik, Frickenhausen, Germany) were coated with 0.5 μg of human β2-GPI/well (Crystal Chemicals, Chicago, IL) in PBS pH 7.4 overnight. The plates were subsequently washed in PBS containing 0.05% Tween 20 (PBS–T) before blocking by 1% essentially fatty acid-free bovine serum albumin (Sigma Chemical Co., St Louis, MO) in PBS (1% BSA–PBS). Samples diluted 1:100 by 1% BSA–PBS were added to the wells in duplicates and incubated for 1 h at room temperature. Antibodies of different isotypes were detected by peroxidase-conjugated anti-human IgG, IgA, or IgM (Dako A/S, Glostrup, Denmark) and by o-phenylenediamine–H2O2 reagent. Absorbances were measured by Labsystems Multiskan MS ELISA reader at 492 nm. The IgG anti-β2-GPI ELISA was previously standardized by international reference sera [24], and the results were expressed as standard IgG anti-β2-GPI units (SGU). The cut-off value (14.6 SGU/ml) was determined as the mean antibody level + 3 s.d. of 50 healthy controls. Quantities of IgA and IgM anti-β2-GPI antibodies were presented as optical density (OD492 nm), and were considered positive when absorbances exceeded the mean + 3 s.d. values of 50 healthy blood bank donors.
Measurement of aCL antibodies
IgG, IgA and IgM type antibodies to CL were determined by commercial aCL enzyme immunoassay (Cogent Diagnostics, Penicuik, UK). Positive results were classified as low positive, moderate positive and high positive according to the protocol of the manufacturer.
Detection of lupus anticoagulant
Activated partial thromboplastin time (APTT) was applied as screening test and the presence of LA was confirmed by thromboplastin dilution test or diluted Russel's viper venom test.
Statistical analysis
The Mann–Whitney U-test was used to evaluate the association between the antibody levels and various signs of APS. The relationship between the frequency of anti-β2-GPI or aCL antibodies and the manifestations of the APS was assessed by Fisher's exact test. Sensitivity and specificity of the different aCL and anti-β2-GPI isotypes were calculated for APS. Spearman's correlation coefficients were used to demonstrate the correlation between the various antibodies. P < 0.05 was considered statistically significant.
RESULTS
Levels and frequency of anti-β2-GPI antibodies of IgG, IgA and IgM isotypes
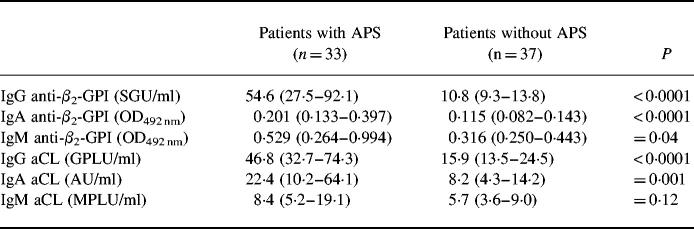
Two patients (one with APS and one without APS) with selective IgA deficiency (IgA < 0.01 g/l) were omitted from the analysis of the levels and frequencies of IgA antibodies. We found that the amounts of anti-β2-GPI antibodies of all isotypes were significantly higher in patients with APS than in those without APS (P < 0.0001 for IgG and IgA, and P = 0.04 for IgM). The levels of IgG and IgA, but not of IgM type aCL antibodies were also increased in patients with APS (P < 0.0001 for IgG, P = 0.001 for IgA and P = 0.12 for IgM) (Table 1).
Table 1.
Anti-β2-GPI and anti-cardiolipin antibody levels, in patients with or without anti-phospholipid syndrome (APS)
Antibody levels are presented as medians and 25th to 75th percentiles (in parentheses). Statistical significance of the differences between patients with and without APS was assessed by the Mann–Whitney U-test.
OD492 nm, optical density at 492 nm; SGU, standard IgG anti-β2-GPI units; GPLU, IgG phospholipid unit; MPLU, IgM phospholipid unit.
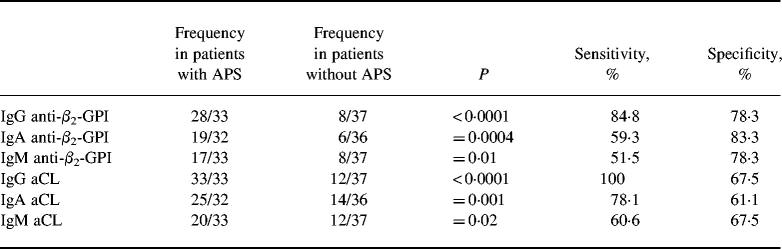
Thirty-six patients had IgG anti-β2-GPI antibodies, while 25/25 patients were positive for IgA and IgM anti-β2-GPI. The occurrence of anti-β2-GPI antibodies, as well as of aCL antibodies of each isotype, were significantly more frequent in patients with APS (Table 2). The positivity for IgM type antibodies (both aCL and anti-β2-GPI antibodies) represented only a weak association with the APS, while the relationship with IgA type antibodies was very strong, similar to that of IgG (Table 2). The control subjects were all negative for both IgA and IgM anti-β2-GPI antibodies.
Table 2.
Relationship between the occurrence of IgG, IgA and IgM anti-β2-GPI and anti-cardiolipin (aCL) antibodies and anti-phospholipid syndrome (APS)
Relationship between the presence of anti-phospholipid (aPL) antibodies and APS was assessed by Fisher's exact test. Sensitivity and specificity values of each antibody were calculated for APS.
Relationships between IgG, IgA and IgM anti-β2-GPI antibodies and the symptoms of APS
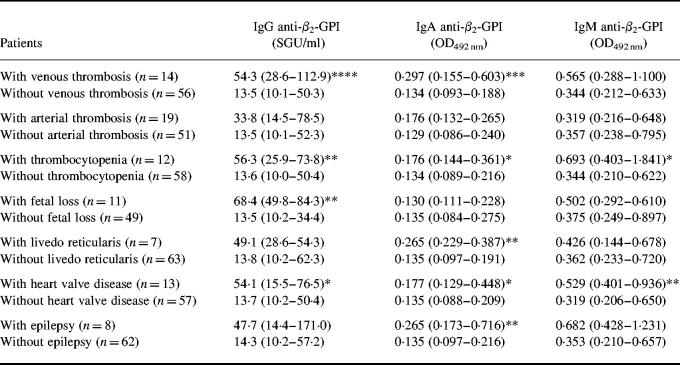
The association of anti-β2-GPI antibodies of all isotypes with arterial or venous thrombosis, thrombocytopenia, spontaneous fetal loss, livedo reticularis, epilepsy, heart valve disease and the presence of LA were calculated (Table 3). IgA anti-β2-GPI antibody values were significantly increased in patients with venous thrombosis (P = 0.007) and thrombocytopenia (P = 0.02), and also in patients with livedo reticularis (P = 0.01), heart valve disease (P = 0.02) and epilepsy (P = 0.01). Moreover, nearly significant elevation was detected in patients with arterial thrombosis (P = 0.06). IgG anti-β2-GPI antibody levels were significantly higher in patients with previous venous thrombosis (P = 0.003), thrombocytopenia (P = 0.01), intra-uterine fetal loss (P = 0.01) and heart valve disease (P = 0.03). Elevated IgG antibody levels were measured also in patients with arterial thrombosis, close to significance (P = 0.06). Increased amounts of IgM anti-β2-GPI antibodies were related only to thrombocytopenia (P = 0.04) and heart valve disease (P = 0.01).
Table 3.
The levels of IgG, IgA and IgM anti-β2-GPI antibodies in patients with or without the symptoms of anti-phospholipid syndrome (APS)
Levels of anti-β2-GPI antibodies are presented as medians and 25th to 75th percentiles (in parentheses). Statistical significance of the differences between patients with and without the characteristic features of APS was assessed by the Mann–Whitney U-test.
*P < 0.05; **P = 0.01; ***P = 0.007; ****P = 0.003.
Anti-cardiolipin antibody levels of IgA isotype were significantly higher in patients with previous venous thrombosis (P = 0.03), livedo reticularis (P = 0.04) and epilepsy (P = 0.01). Elevated IgG aCL antibody values were measured in patients with venous thrombosis (P = 0.0009), arterial thrombosis (P = 0.02) and spontaneous fetal loss (P = 0.03), while increased IgM aCL antibody levels did not associate with any of these symptoms.
The presence of LA correlated only with the occurrence of IgG type anti-β2-GPI (P = 0.006) and aCL antibodies (P = 0.01), but not with IgA and IgM type antibodies.
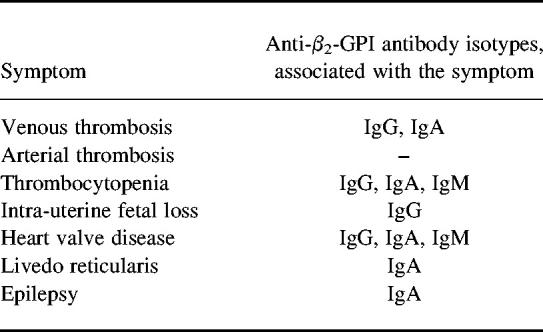
Subgroups of patients with APS based on the association of the different symptoms with various isotype distributions of anti-β2-GPI antibodies
The simultaneous measurement of various isotypes of aPL antibodies can result in formation of subgroups among patients with APS. Table 4 shows that some manifestations of the APS were related to a single isotype of anti-β2-GPI antibodies: intra-uterine fetal loss to IgG, epilepsy and livedo reticularis to IgA isotype. Venous thrombosis associated with both IgG and IgA type anti-β2-GPI antibodies, while thrombocytopenia and heart valve disease associated with all three isotypes.
Table 4.
Association of the symptoms of anti-phospholipid syndrome (APS) with the different anti-β2-GPI antibody isotypes
Correlation between the various types of aPL antibodies
The correlation between anti-β2-GPI and aCL antibodies was very strong for all three isotypes (IgG, r = 0.77; IgA, r = 0.80; IgM, r = 0.90; P < 0.0001). The quantity of IgG anti-β2-GPI antibodies significantly correlated with the amount of IgA (r = 0.67, P < 0.0001), as well as with the level of IgM anti-β2-GPI antibodies (r = 0.45, P < 0.0001). However, no correlation was found between IgA and IgM isotypes (r = 0.14, P = 0.23). The presence of IgG anti-β2-GPI antibodies was often associated with the presence of IgA or IgM antibodies (eight and nine cases, respectively). All three isotypes could be detected in the sera of 12 patients, while in some cases only a single type of anti-β2-GPI antibody was present: IgG in seven, IgA in four, and IgM in three patients. All subjects with single IgG anti-β2-GPI antibody had one or more clinical features of APS. Among patients with single IgA anti-β2-GPI antibody, one had no signs of APS, while three of them had two features: arterial thrombosis + livedo reticularis, arterial thrombosis + epilepsy, and arterial thrombosis + heart valve disease. Only one of the three patients with a single IgM anti-β2-GPI antibody had a history of thrombocytopenia.
DISCUSSION
The association between thrombosis and anti-β2-GPI antibodies is well known [9,25], and these antibodies are thought to have a direct pathogenic role in the development of this symptom [26,27]. This strong association refers to anti-β2-GPI antibodies of IgG isotype. The importance of antibodies of IgM isotype is, however, controversial [19,20]. Few data are available on IgA anti-β2-GPI antibodies, which were shown to associate with APS by only one study [28], and with thrombosis by another one [29]. Moreover, in spite of increasing information on the role of anti-β2-GPI antibodies in thrombosis, the relationship of these antibodies with the other characteristic symptoms of APS remains to be investigated.
The aim of this study was to evaluate the prevalence of anti-β2-GPI antibodies of IgG, IgA and IgM isotype in APS, and to establish the clinical significance of the IgA type antibodies compared with the other isotypes, concerning the different manifestations of APS.
In our study IgA anti-β2-GPI antibodies were detected in 59.3% of patients with APS, representing the highest specificity (83.3%) (Table 2). We could confirm the recently described association between IgA anti-β2-GPI antibodies and previous thrombosis. Moreover, we found strong correlation between increased IgA anti-β2-GPI antibody levels and thrombocytopenia, livedo reticularis, heart valve disease and epilepsy. Interestingly, in patients with livedo reticularis and epilepsy, only the IgA anti-β2-GPI antibody level was significantly elevated. IgG anti-β2-GPI antibodies were present in 84.8% of patients with APS, and their amount was significantly increased in patients with venous thrombosis, thrombocytopenia, intra-uterine fetal loss and heart valve disease. IgM anti-β2-GPI antibodies occurred in 51.5% of patients with APS. While some previous studies reported that these antibodies were associated with thrombosis, thrombocytopenia [19] and fetal loss [20,30], we could confirm the relationship only with thrombocytopenia, but found a correlation with heart valve disease.
In most cases, anti-β2-GPI antibodies of IgA isotype were detected simultaneously with the IgG type antibodies. On the other hand, there were four patients with single IgA type antibody, and three of them had a history of symptoms characteristic of APS. It is well known that aPL antibody levels fluctuate with the activity of disease. We could detect these antibodies at a certain period of the illness, but we could not exclude the possibility that these patients had antibodies of IgG and/or IgM type in another period of their disease. We presume that the dominant immune response against β2-GPI is IgG type, which can be associated with antibodies of IgA and/or IgM types. During the course of the disease the quantity of IgG antibodies may decrease, which may or may not be accompanied by a decrease in the quantity of the other isotypes. Further prospective long-term studies are needed to elucidate the kinetics of the autoantibody response against β2-GPI. Our results, however, clearly show that the measurement of IgA type anti-β2-GPI antibodies is more informative than that of IgM.
Fewer symptoms of APS were related to aCL antibodies of IgG and IgA isotypes than to anti-β2-GPI antibodies, whereas IgM aCL antibodies did not associate with any symptoms. The specificity of aCL antibodies of all isotypes for APS was lower than that of anti-β2-GPI antibodies (Table 2).
To our knowledge this is the first report on the association of anti-β2-GPI antibodies (IgG, IgA, and IgM type) with heart valve disease, as well as on the association of IgA anti-β2-GPI antibodies with the history of thrombocytopenia, livedo reticularis and epilepsy. Different anti-β2-GPI antibody isotypes seem to associate with different clinical features of APS, forming distinct subgroups of patients. IgG anti-β2-GPI antibodies represent the highest sensitivity for APS. However, the relationship with IgA anti-β2-GPI antibodies is also very strong, including some unique associations. Despite its similar prevalence to that of IgA anti-β2-GPI, the correlation between IgM anti-β2-GPI antibodies and APS was found to be weak. Our results suggest that measurement of IgA type antibodies can give new information for the clinician about patients with clinical suspicion of APS. Furthermore. the simultaneous measurement of various isotypes of aPL antibodies can result in creating subgroups among patients with APS, representing special clinical entities from the aspects of diagnosis and therapy.
REFERENCES
- 1.Harris EN, Gharavi AE, Hughes GR. Anti-phospholipid antibodies. Clin Rheum Dis. 1985;11:591–609. [PubMed] [Google Scholar]
- 2.Hughes GR, Harris EN, Gharavi AE. The anticardiolipin syndrome. J Rheumatol. 1986;13:486–9. [PubMed] [Google Scholar]
- 3.Galli M, Comfurius P, Maassen C, et al. Anticardiolipin antibodies (ACA) directed not to cardiolipin but to a plasma protein cofactor. Lancet. 1990;335:1544–7. doi: 10.1016/0140-6736(90)91374-j. [DOI] [PubMed] [Google Scholar]
- 4.Pengo V, Biasiolo A, Fior MG. Autoimmune antiphospholipid antibodies are directed against a cryptic epitope expressed when β2-glycoprotein I is bound to a suitable surface. Thromb Haemost. 1995;73:29–34. [PubMed] [Google Scholar]
- 5.Maatsura E, Igarashi Y, Yasuda T, et al. Anticardiolipin antibodies recognize β2-glycoprotein I structure altered by interacting with an oxygen modified solid phase surface. J Exp Med. 1994;179:457–62. doi: 10.1084/jem.179.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunt JE, McNeil HP, Morgan GJ, et al. A phospholipid–β2-glycoprotein I complex is an antigen for anticardiolipin antibodies occurring in autoimmune disease but not with infection. Lupus. 1992;1:83–90. doi: 10.1177/096120339200100204. [DOI] [PubMed] [Google Scholar]
- 7.McNally T, Purdy G, Mackie IJ, et al. The use of an anti-β2-glycoprotein-I assay for discrimination between anticardiolipin antibodies associated with infection and increased risk of thrombosis. Br J Haernatol. 1995;91:471–3. doi: 10.1111/j.1365-2141.1995.tb05324.x. [DOI] [PubMed] [Google Scholar]
- 8.Arvieux J, Roussel B, Jacob MC, et al. Measurement of antiphospholipid antibodies by ELISA using β2-glycoprotein I as an antigen. J Immunol Methods. 1991;143:223–9. doi: 10.1016/0022-1759(91)90047-j. [DOI] [PubMed] [Google Scholar]
- 9.Viard JP, Amoura Z, Bach JF. Association of anti-β2-glycoprotein I antibodies with lupus-type circulating anticoagulant and thrombosis in systemic lupus erythematosus. Am J Med. 1992;93:181–6. doi: 10.1016/0002-9343(92)90049-h. [DOI] [PubMed] [Google Scholar]
- 10.Cabral AR, Cabiedes J, Alarcon-Segovia D. Clinical manifestations of the antiphospholipid syndrome in patients with systemic lupus erythematosus associate more strongly with anti-β2-glycoprotein-I than with antiphospholipid antibodies. J Rheumatol. 1995;22:1899–906. [PubMed] [Google Scholar]
- 11.Roubey RAS, Maldonado MA, Byrd SN. Comparison of an enzyme-linked immunosorbent assay for antibodies to β2-glycoprotein I and a conventional anticardiolipin immunoassay. Arthritis Rheum. 1996;36:1606–7. doi: 10.1002/art.1780390922. [DOI] [PubMed] [Google Scholar]
- 12.Gharavi AE, Harris EN, Asherson RA, Hughes GR. Anticardiolipin antibodies: isotype distribution and phospholipid specificity. Ann Rheum Dis. 1987;46:1–6. doi: 10.1136/ard.46.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalunian KC, Peter JB, Midlekauff HR, Sayre J, Ando DG, Mangotich M, Hahn BH. Clinical significance of a single test for anti-cardiolipin antibodies in patients with systemic lupus erythematosus. Am J Med. 1988;85:602–8. doi: 10.1016/s0002-9343(88)80229-8. [DOI] [PubMed] [Google Scholar]
- 14.Lopez LR, Santos ME, Espinoza LR, La-Rosa FG. Clinical significance of immunoglobulin A versus immunoglobulins G and M anti-cardiolipin antibodies in patients with systemic lupus erythematosus. Correlation with thrombosis, thrombocytopenia, and recurrent abortion. Am J Clin Pathol. 1992;98:449–54. doi: 10.1093/ajcp/98.4.449. [DOI] [PubMed] [Google Scholar]
- 15.Loizou S, Cifiner C, Weetman AP, Walport MJ. Immunoglobulin class and IgG subclass distribution of anticardiolipin antibodies in patients with systemic lupus erythematosus and associated disorders. Clin Exp Imunol. 1992;90:434–9. doi: 10.1111/j.1365-2249.1992.tb05864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escalante A, Brey RL, Mitchell BD, Jr, Dreiner U. Accuracy of anticardiolipin antibodies in identifying a history of thrombosis among patients with systemic lupus erythematosus. Am J Med. 1995;98:559–65. doi: 10.1016/s0002-9343(99)80014-x. [DOI] [PubMed] [Google Scholar]
- 17.Burden AD, Gibson IW, Rodger RS, Tillman DM. IgA anticardiolipin antibodies associated with Henoch–Schönlein purpura. J Am Acad Dermatol. 1994;31:857–60. doi: 10.1016/s0190-9622(94)70246-2. [DOI] [PubMed] [Google Scholar]
- 18.Wilson WA, Morgan-Ost C, Barton EN, Smikle M, Hanchard B, Blattner WA, Doggett S, Gharavi AE. IgA antiphospholipid antibodies in HTLV-1-associated tropical spastic paraparesis. Lupus. 1995;4:138–41. doi: 10.1177/096120339500400210. [DOI] [PubMed] [Google Scholar]
- 19.Swadzba J, De-Clerck LS, Stevens WJ, Bridts CH, van-Cotthem KA, Musial J, Jankowski M, Szczeklik A. Anticardiolipin, anti-β2-glycoprotein I, antiprothrombin antibodies and lupus anticoagulant in patients with systemic lupus erythematosus with a history of thrombosis. J Rheumatol. 1997;24:1710–5. [PubMed] [Google Scholar]
- 20.Forastiero RR, Martinuzzo ME, Cerrato GS, Kordich LC, Carreras LO. Relationship of anti-β2-glycoprotein I and anti-prothrombin antibodies to thrombosis and pregnancy loss in patients with antiphospholipid antibodies. Thromb Haemost. 1997;78:1008–14. [PubMed] [Google Scholar]
- 21.Hughes GR. Connective tissue diseases and skin. Clin Exp Dermatol. 1984;9:535–44. doi: 10.1111/j.1365-2230.1984.tb00856.x. [DOI] [PubMed] [Google Scholar]
- 22.Herranz MT, Rivier G, Khamashta MA, Blaser KU, Hughes GR. Association between antiphospholipid antibodies and epilepsy in patients with systemic lupus erythematosus. Arthritis Rheum. 1994;37:568–71. doi: 10.1002/art.1780370418. [DOI] [PubMed] [Google Scholar]
- 23.Khamashta MA, Cervera R, Asherson RA, et al. Association of antibodies against phospholipids with heart valve disease in systemic lupus erythematosus. Lancet. 1990;335:1541–4. doi: 10.1016/0140-6736(90)91373-i. [DOI] [PubMed] [Google Scholar]
- 24.Erickson EN, Najmey SS, Keil LB, et al. Reference calibrators for IgG antibodies to β2-glycoprotein I: preparation, properties, and availability to investigators. Clin Chem. 1996;42:1116–7. [PubMed] [Google Scholar]
- 25.Tsutsumi A, Matsuura E, Ichikawa K, Fujisaku A, Mukai M, Kobayashi S, Koike T. Antibodies to β2-glycoprotein I and clinical manifestations in patients with systemic lupus erythematosus. Arthritis Rheum. 1996;39:1466–74. doi: 10.1002/art.1780390905. [DOI] [PubMed] [Google Scholar]
- 26.Arnout J. The pathogenesis of the antiphospholipid syndrome: a hypothesis based on parallelism with heparin-induced thrombocytopenia. Thromb Haemost. 1996;75:536–41. [PubMed] [Google Scholar]
- 27.Blank M, Faden D, Tincani A, et al. Immunization with anticardiolipin cofactor (β2-glycoprotein I) induces experimental antiphospholipid syndrome in naive mice. J Autoimmun. 1994;7:441–55. doi: 10.1006/jaut.1994.1032. [DOI] [PubMed] [Google Scholar]
- 28.Fanopoulus D, Teodorescu MR, Varga J, Teodorescu M. High frequency of abnormal levels of IgA anti-β2-glycoprotein I antibodies in patients with systemic lupus erythematosus: relationship with antiphospholipid syndrome. J Rheumatol. 1998;25:675–80. [PubMed] [Google Scholar]
- 29.Tsutsumi A, Matsuura E, Ichikawa K, Fujisaku A, Mukai M, Koike T. IgA class anti-β2-glycoprotein I in patients with systemic lupus erythematosus. J Rheumatol. 1998;25:74–78. [PubMed] [Google Scholar]
- 30.Falcon CR, Martinuzzo ME, Forastiero RR, Cerrato GS, Carreras LO. Pregnancy loss and autoantibodies against phospholipid-binding proteins. Obstet Gynecol. 1997;89:975–80. doi: 10.1016/s0029-7844(97)00115-4. [DOI] [PubMed] [Google Scholar]