Abstract
We calculated the percentage of Th1, Th2, Th0 cells and the Th1:Th2 cell ratio of peripheral blood from normal pregnant subjects and preeclampsia patients using flow cytometry which can analyse both the surface marker, CD4, and intracellular cytokines, interleukin (IL)-4 and interferon (IFN)-γ. In normal pregnancy, the percentage of Th1 cells was significantly lower in the third trimester, and the ratios of Th1:Th2 were significantly lower in the second and third trimester than in nonpregnant subjects. In contrast, the percentage of Th1 cells and the ratios of Th1:Th2 in preeclampsia were significantly higher than in normal third trimester pregnant subjects. The percentage of Th2 cells in preeclampsia was significantly lower than in third trimester of normal pregnancy. Additionally, peripheral blood mononuclear cells from these subjects and patients were cultured with phytohemagglutinin stimulation, and IL-4 and IFN-γ concentrations were determined in the supernatant by enzymed linked immunosorbent assays. The percentage of Th1 and Th2, and the ratios of Th1:Th2 were correlated with cytokine (IFN-γ and IL-4) secretion level. These results demonstrated that Th2 cells were predominant in the second and third trimesters of normal pregnancy, but Th1 cells predominated in preeclamptic patients.
Keywords: Th1, Th2, pregnancy, preeclampsia, flow cytometry
INTRODUCTION
T cells may be classified as Th1 cells, which synthesize interleukin (IL)-2, interferon (IFN)-γ, and tumour necrosis factor (TNF)-β, and induce cellular immunity, or Th2 cells, which synthesize IL-4, IL-5, IL-6 and IL-10, and induce antibody production [1]. Wegman et al. [2] suggested that in pregnant women, cytokines produced by Th2 cells predominate over those produced by Th1 cells, resulting in the maintenance of pregnancy. This hypothesis has been tested by consecutive experiments using mice [3–7]. There are different reports indicating that, in normal pregnant women, production of Th2 cell-derived cytokines by peripheral blood mononuclear cells is increased and that of Th1 cell-derived cytokines decreased [8]. On the other hand, there are various reports demonstrating that in normal pregnant women both circulating IFN-γ-producing (Th1) cells and IL-4-producing (Th2) cells are increased [9], while Russell et al. [10] reported that the synthesis of Th1 derived cytokine, IL-2, was reduced, but the synthesis of the Th2 derived cytokine, IL-10, did not change in normal pregnancy. Ekerfelt et al. [11] reported that in the second and third trimester of pregnancy, significantly large numbers of circulating IL-4 secreting cells (Th2) were induced by paternal leucocytes as compared to unrelated leucocytes in a one way mixed lymphocyte reaction (MLR), but the numbers of circulating IFN-γ secreting cells (Th1) were unchanged. Thus, there is little consensus among the findings in peripheral blood Th1 and Th2 cells in normal pregnancy.
Previous reports have maintained greater amounts of plasma levels of IL-2 (a Th1 type cytokine) [12,13] and IL-12 which stimulate the production of Th1 type cytokine; IFN-γ [14,15], but other studies have yielded opposing findings, such as elevation in IL-4 or IL-6 (Th2 type cytokines) [16–18] and lack of an increase in serum IL-12 in patients with preeclampsia [19]. Recently, we demonstrated the increased secretion of Th1 cell-derived cytokine, IL-2 and IFN-γ and the decreased secretion of Th2 cell derived cytokine, IL-4 in preeclamptic patients [20].
Before the present study, the definitions of the Th1:Th2 cell dichotomy in humans came from studies of cell culture supernatants of complex mixtures of lymphocytes [8,10,21], frequency analysis by ELISPOT assays [9,11], and studies using T cell clones [22]. The production of cytokines by such cells may be studied on the protein level by measuring the bulk production of cytokine production. Th1 cell-derived IFN-γ is also synthesized by natural killer (NK) cells [23,24], and IL-6 and IL-10 are also synthesized by B cells and monocytes, as well as by Th2 cells [1,25]. Based on these considerations, it must be presumed impossible to obtain an accurate evaluation of increases or decreases in the levels of Th1 and Th2 cell numbers in the peripheral blood of pregnant women using ELISPOT assay. T cell clones are long-term in vitro propagated lines, so studies in fresh ex vivo T cells may reveal differences in cytokine pattern. To study fresh, ex vivo cytokine production at the single cell level, we performed recently developed technique of flow cytometry for intracellular cytokines [26,27]. This method has the capacity for rapid analysis of large numbers of cells which are required for making statistically significant measurements. Thus, in the present study using flow cytometry, we were able to investigate changes in the numbers of Th0, Th1 and Th2 cells, as well as in the Th1:Th2 cell ratio in the peripheral blood of normal pregnant women and women with preeclampsia.
MATERIALS AND METHODS
Subjects
Peripheral blood mononuclear cells were collected from 29 nonpregnant women, 26 normal pregnant women in the first trimester (gestational age at sampling: 9.7 ± 2.3 weeks, mean ± s.d.), 31 in the second trimester (gestational age at sampling: 24.7 ± 3.4 weeks, mean ± s.d.) and 30 in the third trimester (gestational age at sampling: 35.7 ± 3.3 weeks, mean ± s.d.), 26 postpartum women 4 weeks after delivery, and 11 women with preeclampsia (gestational age at sampling: 34.1 ± 3.6 weeks, mean ± s.d.). Normal pregnancy was defined as one in which the women remained normotensive and nonproteinuric, delivered at between 37 and 42 weeks and in which the pregnancy was not complicated by foetal growth retardation or maternal problems. All subjects of postpartum group were breast feeding. Preeclampsia was diagnosed when the diastolic blood pressure was greater than 90 mmHg on two or more consecutive occasions greater than 4 h apart and when there was more than 300 mg of protein in a 24-h urine collection or 2+ proteinuria detected on reagent strip on two occasions more than 4 h apart. Preeclampsia was characterized as severe because of blood pressure ≥ 160/110 mmHg in all patients. Preeclamptic patients were matched individually with normal pregnant women in the third trimester of similar age, and gestational age at sampling. None had histories or showed signs of autoimmune diseases, as judged by clinical examination.
Staining for intracellular cytokines and cell surface antigens for flow cytometric analysis
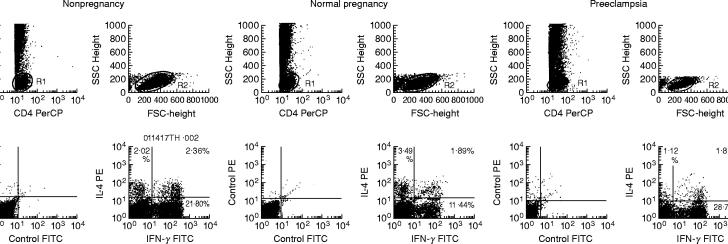
Peripheral whole blood diluted with 1:2 RPMI-1640 was stimulated for 4 h with phorbol 12-myristate 13-acetate (PMA) (50 ng/ml) and the calcium ionophore A23187 (250 ng/ml) in the presence of 10 μm brefeldin A, which blocks intracellular transport processes resulting in the accumulation of cytokine proteins in the Golgi complex. The Fc receptors on the mononuclear cells were pre-blocked with an excess of purified murine immunoglobulin (Ig)G. These mononuclear cells were stained with Peridinin chlorophyll protein (PerCP)-conjugated monoclonal antibody specific for the cell surface antigen CD4 (Becton Dickinson, Mountain View, CA, USA) and washed twice with phosphate buffered saline (PBS). After adding FACSTM Lysing Solution (Becton Dickinson) for 5 min to lyse the erythrocytes, cells were centrifuged at 500 g for 5 min, and the supernatant was removed. FACSTM Permeabilizing Solution (Becton Dickinson) was added for 10 min at room temperature to make cell permeable. The cells were washed twice with PBS. These permeabilized cells were stained with fluorescein-isothiocyanate (FITC) labelled anti-human IFN-γ monoclonal antibody (Becton Dickinson), and PE-labelled anti-human IL-4 monoclonal antibody (Becton Dickinson). Fluorochrome-conjugated, isotype-muched IgG1 and IgG2a were used as controls for detecting nonspecific binding. After two washes with PBS, flow cytometry was performed using a FACScan (Becton Dickinson). Fifty thousand cells were aquired in the list mode and analysed with CELL Quest software (Becton Dickinson). Analysis gates were set for CD4+ cells (Fig. 1, upper left). CD4+ mononuclear cells are composed of T-lymphocytes and monocytes. CD4+ T lymphocytes were identified by characteristic side scatter parameters (Fig. 1, R1). CD4+ T lymphocytes were further identified by characteristic forward and side scatter parameters (Fig. 1, R2). Correlated PE (IL-4) and FITC (IFN-γ) fluorescence were displayed in the panel (Fig. 1, lower right). The data used fluorochrome-conjugated, isotype-muched IgG1 and IgG2a and are also shown in Fig. 1(lower left). CD4+IFN-γ+IL-4− cells as Th1 cells, CD4+IFN-γ−IL-4+ cells as Th2 cells and CD4+IFN-γ+IL-4+ cells as Th0 cells were examined. Cell viability was assessed by assaying for the presence of the activation marker CD69 [28] on the CD4+ T cells using flow cytometry. When less than 90% of the CD4+ T cells expressed CD69 antigen, samples were excluded from the study. Thus, more than 90% of the CD4+ T cells expressed CD69 antigen in this study.
Fig. 1.
Percentage of Th1, Th2, and Th0 cells in nonpregnant women, normal pregnant women, and preeclamptic patients. Peripheral blood mononuclear cells were stained with Peridinin chlorophyll protein (PerCP)-conjugated CD4 monoclonal antibody, fluorescein-isothiocyanate (FITC)-conjugated anti IFN-γ monoclonal antibody and PE-conjugated anti-interleukin (IL)-4 monoclonal antibody, as described in Materials and Methods. A gate was set on the CD4+ lymphocytes by characteristic forward and side scatter parameters (R1 and R2). The FITC (anti-interferon (IFN)-γ) and PE (anti-IL-4) fluorescence data were analysed. Isotype muched fluorochrome-conjugated IgG1 and IgG2a were used as control. The horizontal bar shows FITC fluorescence and the vertical bar shows PE fluorescence. Fluorescence intensity is determined on a logarithmic scale.
IFN-γ and IL-4 production by PHA-stimulated peripheral blood mononuclear cells
Heparinized peripheral blood samples were obtained from 10 nonpregnant women, 19 normal pregnant women, 11 postpartum women and 12 preeclamptic patients. Mononuclear cells were isolated by Ficoll–Hypaque (Amersham Pharmacia Biotech AB, Uppsala, Sweden) gradient centrifugation. Peripheral blood mononuclear cells suspended at 1 × 106/ml in RPMI-1640 medium supplemented with 10% foetal calf serum were stimulated for 1 day with 5 μg/ml of PHA (Difco Labs, Defroid, MI, USA) at 37°C in a moist atmosphere with 5% CO2. Supernatants were harvested and IFN-γ and IL-4 concentrations were evaluated with commercially available enzyme linked immunosorbent assays (R&D Systems, Minneapolis, MN, USA).
Statistical analysis
The data were analysed by anova, Fisher's protected least significant difference, and Fisher's Z-transformation test. P < 0.05 was considered statistically significant.
RESULTS
Percentage of Th1, Th2, and Th0 cells and the Th1:Th2 cell ratio in the peripheral blood CD4+ T cells of nonpregnant women, normal pregnant women and postpartum women
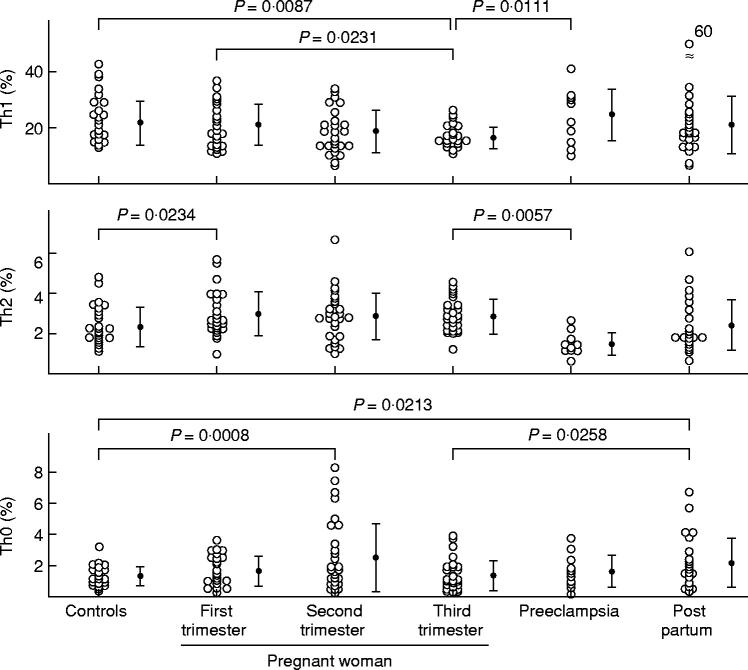
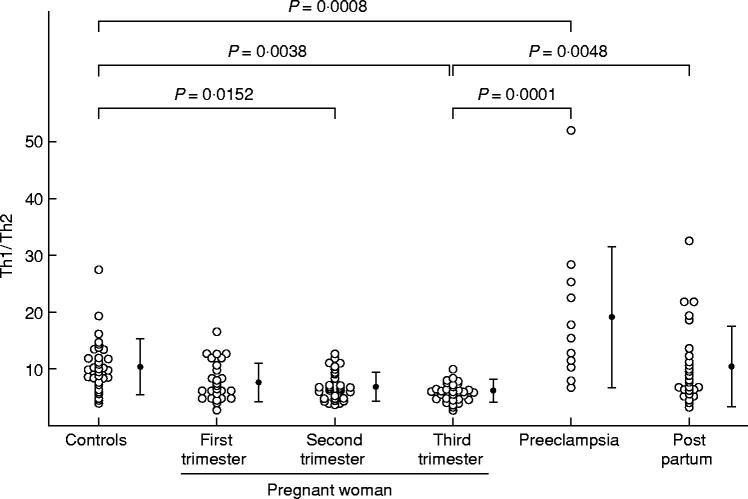
The percentage of Th1 cells was significantly decreased in normal pregnant women in third trimester than in nonpregnant women (Figs 1 and 2). The percentage of Th2 cells was significantly higher in normal pregnant women in first trimester than in nonpregnant women (Fig. 2). The percentages of Th2 cells in second and third trimester pregnant subjects were rather high but not significantly higher than in nonpregnant subjects (Fig. 2). The percentages of Th0 cells in second trimester pregnant subjects and postpartum women were significantly higher than in nonpregnant women (Fig. 2). The ratios of Th1:Th2 in second and third trimester pregnant subjects were significantly lower than in nonpregnant women. The Th1:Th2 ratios returned to the same levels as in nonpregnant women 4 weeks after labour (Fig. 3).
Fig. 2.
Percentage of Th1, Th2 and Th0 cells, and Th1:Th2 cell ratio in nonpregnant women, normal pregnant women, postpartum women, and preeclamptic patients. The data were analysed by anova and Fisher's protected least significant difference.
Fig. 3.
Th1:Th2 cell ratio in nonpregnant women, normal pregnant women, postpartum women, and preeclamptic patients. The data were analysed by anova and Fisher's protected least significant difference.
Percentage of Th1, Th2, and Th0 cells and the Th1:Th2 cell ratio in the peripheral blood CD4+ T cells of preeclamptic patients
In preeclamptic patients, the percentage of Th1 cells and the Th1:Th2 cell ratio were significantly higher than in third trimester pregnant women (Figs 1–3). On the other hand, the percentage of Th2 cells in preeclamptic patients was significantly lower than in normal late pregnant women (Fig. 2).
Correlation between percentage of Th1 and concentration of IFN-γ, percentage of Th2 and concentration of IL-4, and the ratios of Th1:Th2 and the concentration of IFN-γ and IL-4
A significant, positive correlation was observed between percentage of Th1 cells and IFN-γ concentration in culture supernatant (r = 0.628, P < 0.0001). Similarly, significant correlations were noted between the percentage of Th2 cells and IL-4 concentration in culture supernatant (r = 0.616, P < 0.0001), and between the ratios of Th1:Th2 and IFN-γ concentration/IL-4 concentration in culture supernatant (r = 0.796, P < 0.0001) (Fig. 4).
Fig. 4.

Correlation between percentage of Th1 cells and interferon (IFN)-γ concentrations in culture supernatant, percentage of Th2 cells and interleukin (IL)-4 concentrations, and the ratios of Th1:Th2 and the ratios of IFN-γ concentration/IL-4 concentration in nonpregnant women, normal pregnant women, postpartum women, and preeclamptic patients. The data were analysed by Fisher's Z-transformation test.
DISCUSSION
The results of the present study using flow cytometry elucidated the numbers of peripheral blood Th1 and Th2 cells and the Th1:Th2 ratio in both normal pregnant women and those with preeclampsia, and demonstrated that, in normal pregnancy, Th1 cell levels were decreased in the third trimester, Th2 cell levels were increased in the first trimester, and, in relation to Th1:Th2 ratios, Th2 cells were more predominant in the mid- and third trimesters compared with non-pregnant women. Furthermore, the present study also demonstrated that peripheral blood Th1:Th2 ratios returned to the same levels as in non-pregnant women 4 weeks after labour. The percentage of Th1 and Th2, and the ratios of Th1:Th2 were correlated with cytokine (IFN-γ and IL-4) secretion levels in this study. These results are in agreement with a report that the pattern of cytokines secreted by spleen cells from pregnant mice indicated a predominance of Th2 cells [3,4,6]. The present study also suggests that the predominance of Th2 cell-derived cytokines observed in the peripheral blood mononuclear cells of pregnant women [8,10,11], can be explained by changes in the Th1:Th2 cell ratio in the peripheral blood. Ekerfelt et al. [11] performed a one-way MLR analysis using paternal leucocytes as stimulators, and identified both IL-4 and IFN-γ synthesizing cells using the ELISPOT technique. The results showed no difference in the level of IFNγ-producing cells throughout the overall gestation period, but revealed increased levels of IL-4 positive cells during the mid- and third trimesters. In contrast, the results of the present study demonstrated reduced numbers of IFN-γ-positive cells in the third trimester in pregnant women, with increased numbers of IL-4-positive cells in the first trimester. Thus, our findings differ from those of Ekerfelt et al. [11], although the Th1:Th2 ratios observed in the two studies are in agreement. Ekerfelt et al. [11] showed a selective bias in normal pregnancy toward Th2 selectively for paternal allo-antigen. It might be useful to know how many Th1, Th2 and Th0 cells were induced by paternal lymphocytes by utilizing the flow cytometry. Wegman et al. [2] hypothesized that in the mid- and third trimesters of pregnancy, Th2 cells were predominant, allowing maintenance of the pregnancy. Nevertheless, in the first trimester of pregnancy, the Th1:Th2 cell ratio did not changed in this study. Piccini et al. [22] also reported that there was no significant difference in the production of IL-4, IFN-γ, IL-10, TNF-β and leukaemia inhibitory factor (LIF) by T cell clones generated from peripheral blood by women with unexplained recurrent abortions and normal women. This raises the question of why the foetus and placenta are unaffected by maternal lymphocytes. One possible reasons for this is that Th2 cells may be more predominant in the maternal immune system at the decidua than in the peripheral blood during the first trimester of pregnancy. Piccinni et al. [22] demonstrated a decreased production of LIF, IL-4 and IL-10 by decidual T cell clones of women with unexplained recurrent abortions in comparison with that of women with normal gestation. They speculated that the defective production of LIF and/or Th2 cytokines in the decidua may contribute to the development of unexplained recurrent abortions. Recently, we also reported that Th2 cells predominated in early pregnancy decidua, while no significant differences were found in the percentages of Th1, Th2 and Th0 cells in the peripheral blood T cells of nonpregnant women and women in early pregnancy [29]. These indicate that Th2 type cytokines secreted by T cells in the uterus might be necessary for maintenance of normal pregnancy during the first trimester of pregnancy. The microenviroment of the uterus or hormonal enviroment in pregnant states might affect the peripheral blood Th2:Th1 balance during mid- and third trimesters of pregnancy. Progesterone may be responsible for a swich from Th1 to Th2 at the feto–maternal interface [30]. It remains to be clarified how and where Th1 and Th2 cells differentiate from Th0 cells in pregnant subjects.
In normal pregnancy the maternal immune system is favourably regulated, and there is no foetal rejection, but in preeclampsia the immunological regulatory mechanism appears to be disrupted. It is also known that in sera from women suffering preeclampsia, high levels of Th1 cell-derived cytokines, IL-2, and IL-12 which induces the differentiation of Th1 cells from Th0 cells are observed [12,13]. Although blood levels of IL-4 and IL-6 have similarly been reported to increase in preeclampsia [16–18], this observation has not been further investigated. The present study is the first to show that in the peripheral blood of women experiencing preeclampsia, Th1 cell counts are clearly increased, and the number of Th2 cells decreased, and that the Th1:Th2 ratio results in a clear predominance of Th1 cells. We have been reported that more IL-2 and IFN-γ were produced by unstimulate and PHA-stimulated cultured peripheral blood mononuclear cells from preeclampsia patients than by those from normal pregnant women, and concentration of the Th2 type cytokine IL-4 in supernatants from cultures of PHA-stimulated peripheral blood mononuclear cells was lower in preeclamptic patients than in normal pregnant group [20]. This suggests that increases in the Th1 cell count in preeclampsia cause, increased levels of IL-2 and IFN-γ in the culture supernatants, and decreases in the Th2 cell count in preeclampsia cause decreased level of IL-4 in the culture supernatants. It is also known that IL-12 induces the differentiation of Th1 cells from Th0 or Th2 cells [31], and that in preeclampsia the concentration of IL-12 in the sera increases [14,15] although another study reported the lack of an increase in serum IL-12 in patients with preeclampsia [19]. This leads us to suggest that IL-12 causes the predominance of Th1 cells in preeclampsia. Independently, it has been reported that there are relatively few trophoblasts in the myometrium in preeclampsia [32,33]. We suggested that in preeclampsia, the predominance of Th1 cells results in maternal NK cells and/or T cells attacking the trophoblast [34,35]. In addition, we also observed positive correlations between mean blood pressure and concentrations of the Th1 type cytokines IL-2 and IFN-γ [20]. Further analyses are needed to elucidate the mechanisms by which hypertension and proteinuria that occur in preeclampsia are induced by a predominance of Th1 type cytokine.
REFERENCES
- 1.Mosmann TR, Sad S. The expanding universe of T cell subsets — Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 2.Wegmann TG, Lin H, Guilbert L, Mosmann TH. Bidirectional cytokine interactions in the maternal–fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–6. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 3.Lin H, Mosmann TR, Guilbert L, Tuntipopitat S, Wegmann TG. Synthesis of T helper 2-type cytokines at the maternal–fetal interface. J Immunol. 1993;151:4562–73. [PubMed] [Google Scholar]
- 4.Delassus S, Countinho GS, Saucier D, Darche S, Kourilky P. Differential cytokine expression in maternal blood and placenta during murine gestation. J Immunol. 1994;152:2411–20. [PubMed] [Google Scholar]
- 5.Chaouat G, Assal-Meliani A, Martal J, et al. IL-10 prevents naturally occuring fetal loss in the CBA (DBA/2) mating combination and local defect in IL-10 production in this abortion–pron combination is occured by in vivo injection of IFNtau. J Immunol. 1995;154:4261–8. [PubMed] [Google Scholar]
- 6.Krishnan L, Guilbert LJ, Russell AS, Wegmann TG, Mosmann TR, Belosevic M. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigen specific IFNγ responses and increased production of T helper 2 cytokines. J Immunol. 1996;156:644–52. [PubMed] [Google Scholar]
- 7.Krishnan L, Guilbert LJ, Wegmann TG, Belosevic M, Mosmann TR. T helper 1 response against Leishmania major in pregnant C57BL/6 mice increases implantation failure and fetal resorptions. Correlation with increased IFN-γ and TNF and reduced IL-10 production by placental cells. J Immunol. 1996;156:653–62. [PubMed] [Google Scholar]
- 8.Marzi M, Vigano A, Trabattoni D, et al. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol. 1996;106:127–33. doi: 10.1046/j.1365-2249.1996.d01-809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthiesen L, Ekerfelt C, Berg G, Ernerudh J. Increased numbers of circulating interferon-gamma and interleukin-4-secreting cells during normal pregnancy. Am J Reprod Immunol. 1998;39:362–7. doi: 10.1111/j.1600-0897.1998.tb00370.x. [DOI] [PubMed] [Google Scholar]
- 10.Russell AS, Johnston C, Crew C, Maksymowych WP. Evidence for reduced Th1 function in normal pregnancy: a hypothesis for the remission of rheumatoid arthritis. J Rheumatol. 1997;24:1045–50. [PubMed] [Google Scholar]
- 11.Ekerfelt C, Matthiesen L, Berg G, Ernerudh J. Paternal leukocytes selectively increase secretion of IL-4 in peripheral blood during normal pregnancies: demonstrated by a novel one-way MLC measuring cytokine secretion. Am J Reprod Immunol. 1997;38:320–6. doi: 10.1111/j.1600-0897.1997.tb00307.x. [DOI] [PubMed] [Google Scholar]
- 12.Sunder-Plassmann G, Derffer K, Wagner L, et al. Increased serum activity of interleukin-2 in patients with preeclampsia. J Autoimmun. 1989;2:203–5. doi: 10.1016/0896-8411(89)90156-x. [DOI] [PubMed] [Google Scholar]
- 13.Hamai Y, Fujii T, Yamashita T, et al. Evidence for an elevation in serum interleukin-2 and tumor necrosis factor-α levels before the clinical manifestations of preeclampsia. Am J Reprod Immunol. 1997;38:89–93. doi: 10.1111/j.1600-0897.1997.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 14.Dudley DJ, Hunter C, Mitchell MD, Varner MW, Gately M. Elevations of serum interleukin-12 concentrations in women with severe preeclampsia and HELLP syndrome. J Reprod Immunol. 1996;31:97–107. doi: 10.1016/0165-0378(96)00976-x. [DOI] [PubMed] [Google Scholar]
- 15.Daniel Y, Kupherminc MJ, Baram A, et al. Plasma interleukin-12 is elevated in patients with preeclampsia. Am J Reprod Immunol. 1998;39:376–80. doi: 10.1111/j.1600-0897.1998.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 16.Omu AE, Makhseed M, Al-Qattan F. The comparative value of interleukin-4 in sera of women with preeclampsia and cord sera. Nutrition. 1995;11:688–91. [PubMed] [Google Scholar]
- 17.Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CWG. Interleukin-6, tumor necrosis factor and soluble tumor necrosis factor receptors in women with preeclampsia. Br J Obstet Gynecol. 1995;102:20–5. doi: 10.1111/j.1471-0528.1995.tb09020.x. [DOI] [PubMed] [Google Scholar]
- 18.Greer IA, Lyall F, Perera T, Boswell F, Macara LM. Increased concentrations of cytokines interleukin-6 and interleukin-1 receptor antagonist in plasma of women with preeclampsia: a mechanism for endothelial dysfunction? Obstet Gynecol. 1994;84:937–40. [PubMed] [Google Scholar]
- 19.Sacks GP, Scott D, Tivnann H, Mire-Sluis T, Sargent IL, Redman CWG. Interleukin-12 and preeclampsia. J Reprod Immunol. 1997;34:155–8. doi: 10.1016/s0165-0378(97)00028-4. [DOI] [PubMed] [Google Scholar]
- 20.Saito S, Umekage H, Sakamoto Y, et al. Increased Th1-type immunity and decreased Th2-type immunity in patients with preeclampsia. Am J Reprod Immunol. 1999;41:297–306. doi: 10.1111/j.1600-0897.1999.tb00442.x. [DOI] [PubMed] [Google Scholar]
- 21.Hill JA, Polgar K, Anderson DJ. T-helper 1 type immunity to trophoblast in women with recurrent spontaneous abortion. JAMA. 1995;273:1933–6. [PubMed] [Google Scholar]
- 22.Piccinni M-P, Beloni L, Livi C, Maggi E, Scarselli G, Romagnani S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nature Med. 1998;4:1020–4. doi: 10.1038/2006. [DOI] [PubMed] [Google Scholar]
- 23.Cuturi MC, Anegon I, Sherman F, et al. Production of hematopoietic colony-stimulating factors by human natural killer cells. J Exp Med. 1989;169:569–83. doi: 10.1084/jem.169.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biassoni R, Ferrini S, Prigione I, Pelak VS, Sekaly RP, Long EO. Activated CD3− CD16+ natural killer cells express a subset of the lymphokine genes induced in activated αβ+ and γδ+ T cells. Scand J Immunol. 1991;33:247–52. doi: 10.1111/j.1365-3083.1991.tb01769.x. [DOI] [PubMed] [Google Scholar]
- 25.Kishimoto T, Hirano T. Molecular regulation of B lymphocyte response. Annu Rev Immunol. 1998;6:485–512. doi: 10.1146/annurev.iy.06.040188.002413. [DOI] [PubMed] [Google Scholar]
- 26.Prussin C, Metcalfe D. Detection of intracytoplasmic cytokine using flow cytometry nad directly conjugated anti-cytokine antibodies. J Immunol Method. 1995;188:117–28. doi: 10.1016/0022-1759(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 27.Ferrik DA, Schrenzel MD, Mulvania T, Hsieh B, Ferlin WG, Lepper H. Differential production of interferon-γ and interleukin-4 in response to Th1- and Th2- stimulating pathogens by γδ T cells in vivo. Nature. 1995;373:255–7. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 28.Testi R, Phillips JH, Lanier LL. Leu-23 induction as an early marker of functional CD3/T cell antigen receptor triggering: requirement for receptor cross-linking, prolonged elevation of intracellular [Ca+] and activation PKC. J Immunol. 1989;142:1854–60. [PubMed] [Google Scholar]
- 29.Saito S, Tsukaguchi N, Hasegawa T, Michimata T, Tsuda H, Narita N. Distribution of Th1, Th2 and Th0 and the Th1/Th2 cell ratios in human peripheral and endometrial T cells. Am J Reprod Immunol. 1999 doi: 10.1111/j.1600-0897.1999.tb00097.x. in press. [DOI] [PubMed] [Google Scholar]
- 30.Piccini MP, Guidizi MG, Biagiotti R, et al. Progesterone favors the development of human T helper cells producing Th-2 type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol. 1995;155:128–33. [PubMed] [Google Scholar]
- 31.Manetti R, Gerosa F, Giudizi MG, Biagiotti R, Romagnani S, Trinchieri G. Interleukin 12 induces stable priming for interferon gamma production during differentiation of human T helper 1 (Th1) cells and transient IFNγ production in established Th2 cell clones. J Exp Med. 1994;13:1273–83. doi: 10.1084/jem.179.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y, Damsky CH, Chiu K, Roberts JM, Fisher SJ. Preeclampsia is associated with abnormal expression of adhesior molecules by invasive cytotrophoblasts. J Clin Invest. 1993;91:950–60. doi: 10.1172/JCI116316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, Van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynecol. 1994;101:669–74. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 34.King A, Loke YW. Human trophoblast and JEG choriocarcinoma cells are sensitive to lysis by IL-2 stimulated decidual NK cells. Cell Immunol. 1990;129:435–41. doi: 10.1016/0008-8749(90)90219-h. [DOI] [PubMed] [Google Scholar]
- 35.Saito S, Morii T, Enomoto M, Sakakura S, Nishikawa K, Narita N, Ichijo M. The effect of interleukin 2 and transforming growth factor-β2 (TGFβ2) on the proliferation and natural killer activity of decidual CD16−CD56bright natural killer cells. Cell Immunol. 1993;152:605–13. doi: 10.1006/cimm.1993.1316. [DOI] [PubMed] [Google Scholar]