Abstract
Polymorphisms of the tumour necrosis factor (TNF) gene have been related to TNF production and outcome in a variety of inflammatory and malignant diseases. Proinflammatory cytokines and the inflammatory state appear to affect outcome in pancreatic cancer. Thus, the present study examined the TNFB and TNF-308 polymorphisms for their relationship to the inflammatory state and survival in pancreatic cancer. Sixty-four patients with advanced pancreatic cancer and 101 healthy subjects were genotyped for each polymorphism. Serum concentrations of the two TNF receptors and C-reactive protein (CRP) were measured in 45 of the cancer patients with no evidence of infection or jaundice, 1 month after surgical intervention. There was no difference in distribution of genotypes between the patient and control groups. There was no association between any genotype and concentrations of any of the measured inflammatory mediators. While those with an elevated CRP concentration had significantly poorer survival, there was no association between either TNF genotype and survival. This study found no association between TNF genotype and the inflammatory state or survival in advanced pancreatic cancer. Other cytokines may be more important than TNF in determining the inflammatory state and disease progress in pancreatic cancer.
Keywords: pancreatic cancer, inflammation, polymorphisms, tumour necrosis factor receptors, acute-phase protein response
INTRODUCTION
A number of polymorphisms within the gene coding for tumour necrosis factor (TNF) have been described and shown to influence outcome in a variety of conditions. Septic patients in intensive care homozygous for the TNFB2 allele of a polymorphism in the gene coding for TNF-β have been shown to have higher circulating concentrations of TNF-α and higher mortality [1]. Cells bearing the TNF2 allele of a G to A substitution polymorphism at −308 of the TNF-α promoter (TNF-308 A allele) exhibit increased transcription of the TNF gene [2] and have an increased risk of chronic bronchitis [3].
There has been increasing interest in the role of proinflammatory cytokines in malignant disease. In pancreatic cancer we have shown that the acute-phase response, activated by proinflammatory cytokines, is associated with poor survival [4]. The TNFB genotype has been examined in two groups of cancer patients. In a group of 102 patients with stage III and IV lung cancer, heterozygotes had reduced survival compared with the homozygotes [5]. Patients with oesophageal cancer homozygous for the TNFB1 allele have also been shown to have poor survival [6].
In the present study we aimed to assess the relationship of two polymorphisms of the TNF gene with survival and the inflammatory state in patients with advanced pancreatic cancer.
PATIENTS AND METHODS
Subjects
After informed consent, venous blood was collected for genotyping from 64 patients with a diagnosis of unresectable pancreatic cancer based on histological or unequivocal radiological or operative findings. Blood was also collected for genotyping from 101 healthy volunteers attending the Blood Transfusion Service Plasmapheresis Centre in Edinburgh.
Survival was noted from time of histological confirmation of pancreatic adenocarcinoma (80%) or of unequivocal radiological or operative findings in those for whom histological confirmation was not obtained.
Genotyping
DNA was extracted from 1-ml samples of EDTA anti-coagulated blood using a Puregene DNA isolation kit based on a simple salting out technique (Gentra Systems, NC).
TNFB Nco1 polymorphism
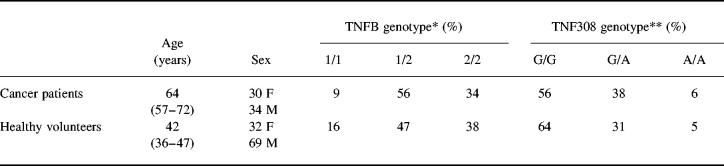
The polymerase chain reaction (PCR) was used to amplify a 368-bp fragment of the TNFβ genomic sequence using primers upstream 5′ CCGTGCTTCGTGCTTTGGACTA 3′ and downstream 5′ AGAGGGGTGCAT GCTTGGGTTC 3′ (Genosys, Pampisford, UK) [1]. The following PCR protocol was used: 95°C for 3 min, 45 cycles of 95°C for 30 s, 68°C for 40 s, 74°C for 48 s; and 74°C for 6 min using reagents supplied by Promega (Southampton, UK) on a Hybaid Omn-E thermal cycler (Teddington, UK). The PCR product was digested directly with 1 U of NcoI restriction enzyme (Promega, Madison, WI) at 37°C for 4 h. Restriction enzyme products were analysed on 1% NuSieve agarose (FMC Bioproducts, Rockland, ME) or 6% polyacrylamide gels (BioRad, Hemel Hempstead, UK). The cleaved product produced bands at 133 and 235 bp representing the allele TNFB1, while the uncleaved 368-bp product represented the allele TNFB2 (Fig. 1).
Fig. 1.
TNFB Nco1 polymorphism. The polymerase chain reaction (PCR) was used to amplify a 368-bp fragment of the of the TNFβ genomic sequence and the PCR product was digested directly with 1 U of NcoI restriction enzyme. Lane 1 shows a homozygous for the cleaved product produced bands at 133 and 235 bp (with incomplete digestion of the 368-bp band) representing the allele TNFB1, while the uncleaved 368-bp product in lane 3 represents the allele TNFB2. Lane 2 shows a heterozygote pattern.
TNF-308 G to A substitution
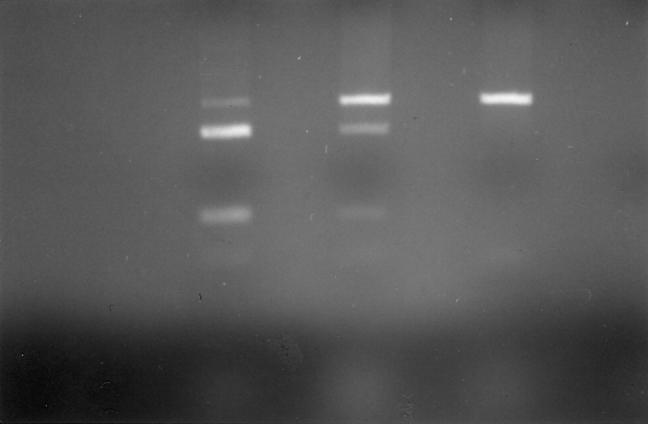
PCR was used to amplify a 107-bp fragment of the TNFβ genomic sequence using primers upstream 5′ AGGCAATAGGTTTTGAGGGCCAT 3′ and downstream 5′ TCCTCCCTGCTCCGATTCCG 3′ (Oswell) [7]. The following PCR protocol was used: 94°C for 3 min, 60°C for 1 min, 72°C for 1 min; 35 cycles of 94°C for 1 min, 60°C for 1 min, 72°C for 1 min; and 94°C for 1 min, 60°C for 1 min, 72°C for 5 min using reagents supplied by Promega (Southampton, UK) on a Hybaid Omn-E thermal cycler (Teddington, UK). The PCR product was digested directly with 1 U of NcoI restriction enzyme (Promega) at 37°C for 4 h. Restriction enzyme products were analysed on 9% polyacrylamide gels (BioRad). The cleaved product produced bands at 87 and 20 bp representing the TNF-308 A allele, while the uncleaved 107-bp product represented the TNF-308 G allele (Fig. 2).
Fig. 2.
TNF-308 G to A substitution. Polymerase chain reaction (PCR) was used to amplify a 107-bp fragment of the of the TNFβ genomic sequence which was digested with 1 U of NcoI. The cleaved product produced bands at 87 and 20 bp representing the TNF-308 A allele, while the uncleaved 107-bp product represented the TNF-308 G allele. Lanes 1–4, homozygote 87/20 TNF-308 A allele; lane 5, heterozygote 107, 87/20; lanes 6 and 7, homozygote 87/20; lane 8, homozygote 107 band TNF-308 G allele; lanes 9–11, homozygote 87/20 TNF-308 A allele.
Assessment of the inflammatory state
To assess the endogenous inflammatory state it was important to collect samples from cancer patients without evidence of infection or jaundice approximately 1 month after diagnosis, surgery or endobiliary stenting. It was possible to obtain venous blood from 45 such patients for the measurement of serum C-reactive protein (CRP) and soluble tumour necrosis factor receptor (TNF-R) concentrations.
Serum CRP concentrations were measured by an automated immunoturbidometric method (Abbott Labs, Maidenhead, UK). Limit of detection was 10 mg/l.
Serum concentrations of soluble TNF-RI and TNF-RII were measured by ELISA (Quantikine; R&D Systems, Abingdon, UK). Limits of detection were 0.156 ng/ml and 0.78 ng/ml, respectively.
Statistical analysis
Data are presented as median and interquartile range unless otherwise stated. Categorical variables were compared by the χ2 test. Relationships between genotype and the inflammatory state were assessed by the Kruskal–Wallis and Mann–Whitney U-test. Survival was analysed using the Kaplan–Meier technique and groups compared by the log rank test. P < 0.05 was taken to denote significance.
RESULTS
Characteristics of the subjects studied and TNF genotypes (Figs 1 and 2) are shown in Table 1. There was no significant difference between cancer patient and control groups in the distribution of either genotype.
Table 1.
Characteristics and tumour necrosis factor genotype of 64 patients with pancreatic cancer and 101 healthy subjects
Ages are median (interquartile range).
Comparison by χ2 test: *P = 0.35; **P = 0.89.
Serum concentrations of TNF-RI, TNF-RII and CRP presented by genotype are shown in Fig. 3. There were no significant differences in serum concentrations of either TNF-R or CRP between TNFB or TNF308 genotype. In addition, there was no significant difference between serum concentrations of TNF-RI, TNF-RII and CRP between those bearing allele 2 of either polymorphism and those with allele 1.
Fig. 3.
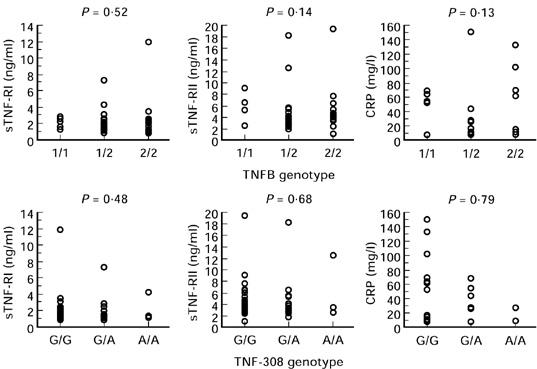
Serum concentrations of soluble tumour necrosis factor receptor (sTNF-R) I and II and C-reactive protein (CRP) from 45 patients with advanced pancreatic cancer with no evidence of infection or jaundice 4 weeks after surgery or bile duct stenting presented by TNFB and TNF-308 genotype. Statistical analysis by Kruskal–Wallis test.
Survival of patients with advanced pancreatic cancer stratified by the presence or absence of an elevated CRP concentration is shown in Fig. 4. Those with an elevated CRP concentration had a significantly shorter survival (median 137 (interquartile range 88–251) days versus 243 (152–320) days; P = 0.0073). Survival stratified by TNF genotype is shown in Figs 5 and 6. For the TNFB polymorphism, those with 1/1 genotype had a median survival of 235 (122–266) days, 1/2–202 (112–357) days and 2/2–256 (76–333) days. For the TNF-308 polymorphism, those with G/G genotype had a median survival of 202 (97–333) days, G/A–227 (136–294) days and A/A–82 (57–185) days. There was no significant relationship between either genotype and survival. In addition, the possession of the TNFB2 or TNF2 genotype made no difference to survival (P = 0.51 and 0.77, respectively).
Fig. 4.
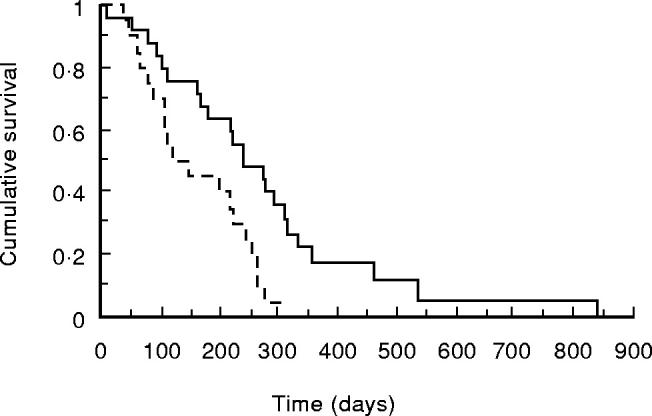
Kaplan–Meier survival curve of 45 patients with advanced pancreatic cancer with no evidence of infection or jaundice 4 weeks after surgery or bile duct stenting stratified for C-reactive protein (CRP). —-, CRP < 10 mg/l;–––––, CRP elevated. Comparison by log rank test, P = 0.0073.
Fig. 5.
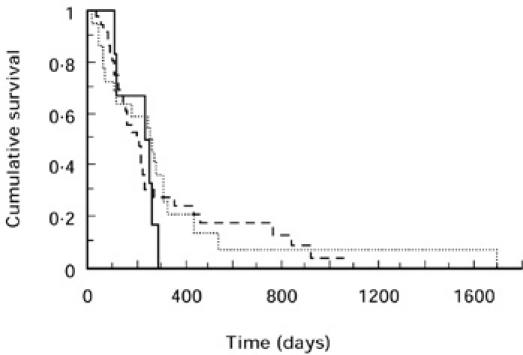
Kaplan–Meier survival curve of 64 patients with advanced pancreatic cancer for TNFB genotype. —-, 1/1; –––––, 1/2; ·····, 2/2. Comparison by log rank test, P = 0.78.
Fig. 6.
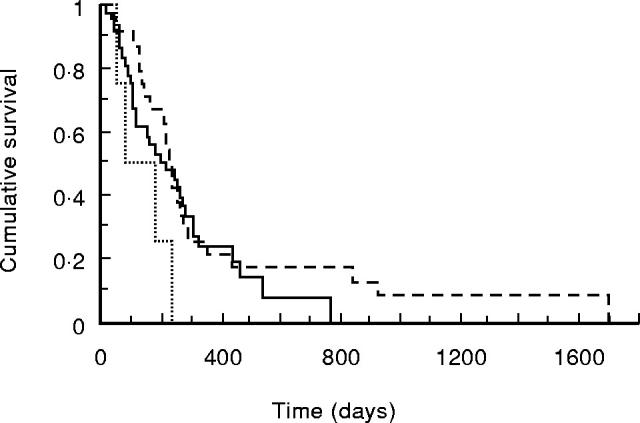
Kaplan–Meier survival curve of 64 patients with advanced pancreatic cancer for TNF-308 genotype. —-, G/G; –––––, G/A; ·····, A/A. Comparison by log rank test, P = 0.13.
DISCUSSION
Distribution of the TNF genotypes was similar between pancreatic cancer patients and controls. Frequencies of the genotypes were also similar to those described by other groups in healthy subjects and in patients with inflammatory and malignant diseases [1,5–8]. It has been suggested that the TNFB2 heterozygote is less common in patients with lung cancer than in the control population and may thus protect against this disease [5]. The present study provides no evidence to support this hypothesis in pancreatic cancer.
TNF is rarely detected in serum samples from patients with cancer [9]. However, the two TNF receptors are shed from cells in response to TNF release and therefore the serum concentration of soluble TNF receptors may provide a measure of TNF release [10]. Serum concentrations of the soluble TNF receptors have been shown to be progressively elevated with more advanced disease stage in cancer patients [11]. We have also shown that the serum concentrations of soluble TNF receptors correlate with the level of the acute-phase protein response in advanced pancreatic cancer [12]. However, in the present study we were unable to show any relationship between either TNF genotype and concentrations of the two TNF receptors. The administration of TNF to volunteers results in an acute-phase protein response [13,14]. Once again the present study showed no relationship between either TNF genotype and the acute-phase response as measured by serum CRP concentration. A relationship between the TNFB genotype and serum TNF concentration was found in septic patients [1] and between the TNF-308 genotype and TNF production by lymphoid cell lines [15]. However, others have found no relationship between TNF-308 genotype and TNF production by peripheral blood cells in culture [8]. It may be that serum concentrations of the TNF receptors and CRP are an inadequate measure of TNF production, or that other factors are more important in determining the inflammatory state in pancreatic cancer than the TNF genotype.
The present study demonstrates that advanced pancreatic cancer patients with an elevated serum CRP, despite having no evidence of infection or jaundice, have a significantly shorter survival than those without. We have previously described the importance of CRP in determining survival in pancreatic cancer [4]. However, despite the apparent importance of the inflammatory state, the present study found no association between survival and either TNF polymorphism. Those with the A/A genotype for the TNF-308 polymorphism had an almost significantly shorter survival than the other two groups (P = 0.054), but this genotype was seen in only 6% of patients, making it of little practical value. Previous studies of the TNFB polymorphism in cancer have suggested that two different genotypes, G/A and G/G, are associated with poor survival in lung and oesophageal cancer, respectively [5,6]. Although no measurement of the inflammatory state was made in the cancer patient groups, these genotypes have been associated with significantly lower TNF concentrations in septic patients [1]. This would appear to conflict with the observation that elevated proinflammatory cytokine levels are associated with a worse outcome in cancer [4,9]. It may be that other proinflammatory cytokines are of more importance in determining outcome in cancer patients, and that the cytokines of importance vary between different malignancies.
In conclusion, genotypes resulting from two polymorphisms of the TNF gene were not found to be associated with outcome or the inflammatory state in patients with advanced pancreatic cancer.
Acknowledgments
The authors wish to thank Jean Maingay and Kathryn Sangster for laboratory assistance, and the Blood Transfusion Service Plasmapheresis Centre, Edinburgh. We would also like to thank A. G. Wilson and F. S. di Giovine, University of Sheffield, for helpful discussions. This work was supported in part by Scotia Pharmaceuticals, by Ross Products Division of Abbott Laboratories and by the University of Edinburgh.
REFERENCES
- 1.Stüber F, Petersen M, Bokelmann F, Schade U. A genomic polymorphism within the tumor necrosis factor locus influences plasma tumor necrosis factor-α concentrations and outcome of patients with severe sepsis. Crit Care Med. 1996;24:381–4. doi: 10.1097/00003246-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor a promotor on transcriptional activation. Proc Natl Acad Sci USA. 1997;94:3195–9. doi: 10.1073/pnas.94.7.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haung S-L, Su C-H, Chang S-C. Tumor necrosis factor-α gene polymorphism in chronic bronchitis. Am J Respir Crit Care Med. 1997;156:1436–9. doi: 10.1164/ajrccm.156.5.9609138. [DOI] [PubMed] [Google Scholar]
- 4.Falconer JS, Fearon KCH, Ross JA, Elton R, Wigmore SJ, Garden OJ, Carter DC. Acute-phase protein response and survival duration of patients with pancreatic cancer. Cancer. 1995;75:2077–82. doi: 10.1002/1097-0142(19950415)75:8<2077::aid-cncr2820750808>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Shimura T, Hagihara M, Takebe K, Munkhbat B, Odaka T, Kato H, Nagamachi Y, Tsuji K. The study of tumor necrosis factor beta gene polymorphism in lung cancer patients. Cancer. 1994;73:1184–8. doi: 10.1002/1097-0142(19940215)73:4<1184::aid-cncr2820730410>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 6.O'Mahony L, Jackson J, Feighery C, Mealy K, Hennessy TPJ. Polymorphisms within the TNF region affect oesophageal cancer patient survival. Br J Surg. 1998;85:687. [Google Scholar]
- 7.Wilson AG, di Giovine FS, Blakemore AIF, Duff GW. Single base polymorphism in the human tumor necrosis factor alpha (TNFα) gene detectable by Nco I restriction of PCR product. Hum Mol Genet. 1992;1:353. doi: 10.1093/hmg/1.5.353. [DOI] [PubMed] [Google Scholar]
- 8.Westendorp RGJ, Langermans JAM, Huizinga TWJ, Elouali AH, Verweij CL, Boomsma DI, Vandenbrouke JP. Genetic influence on cytokine production and fatal meningococcal disease. Lancet. 1997;349:170–3. doi: 10.1016/s0140-6736(96)06413-6. [DOI] [PubMed] [Google Scholar]
- 9.Falconer JS, Fearon KCH, Plester CE, Ross JA, Carter DC. Cytokines, the acute-phase response, and resting energy expenditure in cachectic patients with pancreatic cancer. Ann Surg. 1994;219:325–31. doi: 10.1097/00000658-199404000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsson I, Gatanaga T, Gullberg U, Lantz M, Granger GA. Tumour necrosis factor binding proteins (soluble TNF receptor forms) with possible roles in inflammation and malignancy. Eur Cytokine Network. 1993;4:169–80. [PubMed] [Google Scholar]
- 11.Aderka D, Englemann H, Hornik V, Skornick Y, Levo Y, Wallach D, Kushtai G. Increased serum levels of soluble receptors for tumor necrosis factor in cancer patients. Cancer Res. 1991;51:5602–7. [PubMed] [Google Scholar]
- 12.Barber MD, Fearon KCH, Ross JA. Relationship of serum levels of interleukin-6, soluble interleukin-6 receptor and tumour necrosis factor receptors to the acute phase protein response in advanced pancreatic cancer. Clin Sci. 1999;96:83–87. [PubMed] [Google Scholar]
- 13.Selby P, Hobbs S, Viner C, et al. Tumour necrosis factor in man: clinical and biological observations. Br J Cancer. 1987;56:803–8. doi: 10.1038/bjc.1987.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warren RS, Starnes HF, Gabrilove JL, Oettgen HF, Brennan MF. The acute metabolic effects of tumor necrosis factor administration in humans. Arch Surg. 1987;122:1396–400. doi: 10.1001/archsurg.1987.01400240042007. [DOI] [PubMed] [Google Scholar]
- 15.Abraham LJ, French MAH, Dawkins RL. Polymorphic MHC ancestral haplotypes affect the activity of tumour necrosis factor-alpha. Clin Exp Immunol. 1993;92:14–18. doi: 10.1111/j.1365-2249.1993.tb05940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]