Abstract
We recently reported that indicator sheep erythrocytes (E) coated with mannan and sensitized with mannan-binding lectin (MBL) (E-M-MBL) are lysed by human serum in the absence of calcium via the lectin pathway of complement activation by a process which requires alternative pathway amplification and is associated with increased binding of and control by complement regulatory proteins C4 bp and factor H. In the present study, we investigated the effect of immunoglobulin (Ig) on this haemolysis. Co-sensitization of indicator E with anti-E haemolysin led to threefold enhancement of lectin pathway haemolysis in the absence of calcium, associated with increased binding of C3 and C5. Lysis was enhanced approximately twofold when E-M-MBL were chemically or immunologically coated with IgM or IgA, and fourfold when coated with IgG, prior to lysis in human serum-Mg-ethyleneglycol tetraacetic acid. The presence of haemolysin did not reduce the binding or inhibitory activity of C4 bp, and the enhancing activity of haemolysin was retained in serum depleted of C4 bp. By contrast, binding of factor H was greatly reduced in the presence of haemolysin, which had no enhancing effect in serum depleted of factor H. These experiments demonstrate the ability of IgG, IgM and IgA to enhance lectin pathway cytolysis, and that this enhancement occurs by neutralization of the inhibitory activity of factor H. Immunoglobulin enhancement of lectin pathway cytolysis represents another interaction between the innate and adaptive systems of immunity.
Keywords: acute phase reactants, complement, inflammation, inflammatory mediators, antibodies
INTRODUCTION
The complement system is a major effector of innate as well as adaptive immunity. Complement can be activated by many recognition molecules including antibody, mannan-binding lectin (MBL) and C-reactive protein (CRP), and represents a major bridge between the natural and acquired immune systems [1–3]. Complement cleavage products lead to antigen uptake via complement receptors expressed on B cells and follicular dendritic cells, humoral responses to T-cell dependent antigens and regulation of the repertoire of ‘natural’ IgM antibody and memory B cells [2–5]. Another example of complement bridging between the adaptive and innate immune systems is the ability of immunoglobulin, the major recognition molecule used by the classical pathway, to enhance alternative complement pathway activity [6–9].
A third complement pathway, the lectin pathway activated by MBL, has recently been described [10,11]. This pathway involves complement activation by MBL through two new serum serine proteases designated MASP-1 and MASP-2 [10,11], requires C4 and C2 for activation of C3 and the terminal components [12], and following calcium-dependent binding of MBL to mannan-coated erythrocytes leads to haemolysis in Mg-ethyleneglycol tetraacetic acid (EGTA) in the sera of multiple species [13–15]. Alternative pathway amplification also is required for lectin pathway haemolysis in human serum [13,16]. The inhibitory activities of C4 bp and factor H, which are augmented in the presence of MBL, regulate this haemolysis [16,17]. In the present paper, we report that immunoglobulin also has a significant regulating effect on lectin pathway cytolysis in human serum, by reversing the inhibitory activity of factor H. This synergism between immunoglobulin and MBL-initiated complement activation via the lectin pathway represents another bridge between the natural and adaptive immune systems.
MATERIALS AND METHODS
Buffers
Gelatin-veronal-buffered saline consisting of 5 mm veronal (pH 7.4), 0.145 m NaCl and 0.1% gelatin (GVB); GVB containing 10 mm CaCl2 and 0.5 mm MgCl2 (GVB++); a mixture of equal parts of GVB++ and 5% glucose++ to make the ionic strength equal to 0.075 m NaCl (GGVB++); GVB containing 0.5 mm MgCl2, 10 mm EGTA and glucose to make the ionic strength equal to 0.075 m NaCl (Mg-EGTA); and GVB containing 10 mm ethylenediamine tetraacetic acid (EDTA-GVB), were prepared as previously described [13].
Reagents
Saccharomyces cerevisiae mannan was purchased from Sigma Chemical Company (St Louis, MO, USA). Monoclonal anti-MBL antibody was purchased from Statens Serum Institut (Copenhagen, Denmark). Human IgM, IgG and IgA were purchased from Sigma Chemical Company. Rabbit anti-sheep haemolysin, containing both IgG and IgM, was purchased from Colorado Serum Company (Denver, CO); the erythrocyte determinants to which the antibody was directed were not identified. Purified rabbit anti-sheep erythrocyte IgM and IgG were purchased from Accurate Chemical and Scientific Corporation (Westbury, NY, USA). Anti-human C4, C3, C5, C4 bp, and factor H were purchased from Calbiochem (La Jolla, CA, USA) and biotinylated using EZ-LinkTM Sulfo-NHS-LC-Biotin obtained from Pierce (Rockford, IL, USA) according to the provider's instructions. Streptavidin conjugated with R-phycoerythrin was purchased from Dako (Gostrup, Denmark) for use in flow cytometry.
Agammaglobulinaemic, complement-deficient and complement-depleted sera
Agammaglobulinaemic human serum (AHS), and human sera deficient in C8 (C8D) and in C2 (C2D), respectively, were collected from patients with common variable immunodeficiency and previously identified complement deficient patients, and stored at − 70°C. AHS was used in all lytic experiments to avoid potential effects of natural anti-sheep erythrocytes (E) or anti-mannan (M) antibodies; previous experiments had showed that lectin pathway activity was comparable in normal human serum and AHS in the haemolytic assays used [13]. The AHS had < 2% normal haemolytic activity upon incubation with unsensitized sheep E and normal levels when antibody-sensitized E (EA) were used as the indicator cells. C8D serum absorbed × 2 with E and M was used in all complement binding experiments in order to avoid loss of indicator cells because of haemolysis, and for C4 consumption to permit comparison with C4 binding. Lectin pathway haemolytic activity in the C8D serum was reconstituted to the normal level upon addition of purified human C8. C3-depleted human serum was purchased from Calbiochem. The C-deficient and C-depleted sera had < 2% normal haemolytic activity, with normal levels restored upon addition of small amounts of the purified missing human component. Factor H- and C4 bp-depleted human serum was prepared by immunoaffinity chromatography as previously described [18]. Briefly, 2 ml of human serum was made 10 mm with EDTA and passed through an anti-H-or anti-C4 bp-Sepharose 4B immunoabsorbent column (10 ml) which had been equilibrated with EDTA-veronal-buffered saline (VBS). The effluent was concentrated to the original serum volume using a microconcentrator, and had factor H and C4 bp levels of 2 and < 1 μg/ml, respectively (starting levels of factor H and C4 bp were 1000 and 200 μg/ml, respectively).
Purified human complement components
C2, C3, C8 and D were purchased from Calbiochem. C4 bp was purchased from Sigma Chemical Company. Factor H was prepared as previously described [19].
Enzyme-linked immunosorbent assay for C4 bp and H
C4 bp and factor H [17] concentrations were assayed by sandwich enzyme-linked immunosorbent assay methodology. Briefly, microtitre plates were coated with anti-C4 bp or anti-H (5 μg/ml; 100 μl/well) in coating buffer (0.03 m Na2CO3, 0.02 m NaHCO3, pH 9.6) and incubated overnight at 4°C. The plates were washed three times with VBS (pH 7.4) containing 0.05% Tween 20 and blocked 2 h at room temperature by adding 200 μl VBS containing 1% BSA to each well. Samples and C4 bp/H standards were loaded in duplicate, incubated at room temperature for 1 h and washed as before. Biotinylated anti-C4 bp or anti-H diluted in VBS (100 μl, 2 μg/ml) was added to each well, followed by incubation at room temperature for 1 h. The plates were washed, streptavidin-horseradish peroxidase conjugate (1:1000 in VBS, 100 μl/well) was added, incubation was continued at room temperature for 1 h, and after additional washes, 100 μl substrate was added. Incubation was continued for 30 min at room temperature, the reaction was stopped by addition of 2 n HCl (100 μl/well), and the absorbance at 450 nm was read on a microplate reader.
Mannan-binding lectin
Human MBL was prepared by sequential affinity column chromatography by a minor modification of the method of Kawasaki et al. [20]. Briefly, human serum obtained by recalcification of pooled human citrated plasma of known MBL concentration was brought to 20 mm CaCl2 and allowed to clot for 1 h at 37°C and overnight at 4°C. After removal of the clot, the serum was dialysed extensively against ‘starting buffer’ containing 50 mm Tris HCl, 1 m NaCl, 20 mm CaCl2 and 0.05% (w/v) NaN3 (pH 7.8). After centrifugation at 10,000 g for 10 min, the supernatant was applied to a mannan-Sepharose 4B column (100 ml) equilibrated with starting buffer. The column was washed with starting buffer until protein was no longer detectable in the effluent, and the bound proteins were eluted with a buffer containing 50 mm Tris HCl, 1 m NaCl and 20 mm EDTA, pH 7.8. The eluate was brought to 50 mm CaCl2 and reapplied to a second, smaller (25 ml), otherwise identical mannan-Sepharose 4B column, and the bound proteins were eluted in the same manner as the first column. The proteins eluted were pooled and passed through protein A-Sepharose (10 ml) and goat-anti-human IgM-Sepharose (20 ml) columns, respectively, to remove contaminating IgG and IgM. Effluents were pooled, dialysed against starting buffer, aliquoted and stored at − 70°C. Final preparations contained > 80% MBL as estimated by SDS-polyacrylamide gel and protein analyses, and were free (< 20 μg/ml) of detectable immunoglobulins.
Preparation of mannan-coated and haemolysin-sensitized erythrocytes
Hemolysin-sensitized sheep erythrocytes (EA) and E coated with mannan (E-M) and sensitized with MBL (E-M-MBL) were prepared as previously described [13,21,22]. Briefly, 0.5 ml sheep E (109 cells/ml) were mixed with 0.5 ml CrCl3 solution (0.5 mg/ml), mannan (0.5 ml, 200 μg/ml) was added and the mixture was incubated (5 min, 25°C) with occasional shaking. The reaction was stopped by adding 1.5 ml ice-cold GVB++, and the E-M were washed three times and resuspended to 109 cells/ml in GVB++. An aliquot (0.1 ml) was added to 0.4 ml MBL (2000 ng) in GVB++, incubated with gentle shaking for (30 min at room temperature, 30 min at 0°C), washed and resuspended to 108 cells/ml in GVB++. EA-M and EA-M-MBL always were prepared by incubation of E-M and E-M-MBL, respectively, with haemolysin in Mg-EGTA for 15 min at 37°C, followed by two washes in Mg-EGTA.
Preparation of Ig-sensitized erythrocytes (E-Ig) by chromic chloride treatment
Human IgM-, IgG- and IgA-sensitized sheep erythrocytes (E-IgM, E-IgG and E-IgA, respectively) were prepared by a modification of chromic chloride treatment method. Briefly, 0.5 ml sheep E (109 cells/ml) were mixed 0.5 ml CrCl3 solution (0.5 mg/ml), 0.5 ml Ig solution (1–2500 μg) was added and the mixture was incubated (5 min, 25°C) with occasional shaking. The reaction was stopped by adding 1.5 ml ice-cold GVB++, and the cells were washed three times and resuspended to 109 cells/ml in Mg-EGTA.
Preparation of indicator cells bearing C4, C3 and C5
Indicator cells bearing C4, C3 and C5 (E-M-MBL-C4,3,5 and EA-M-MBL-C4,3,5) were prepared as previously described [13,21,23]. Briefly, E-M-MBL and EA-M-MBL were washed three times and resuspended to 108 cells/ml in Mg-EGTA and prewarmed to 30°C. An equal volume of 1/10 absorbed C8D serum was added slowly with continuous shaking, and after 30 min at 30°C, the mixture was washed, resuspended to the original volume in Mg-EGTA and incubated for another 1 h to decay off C2; the resulting indicator cells containing C4, C3 and C5 were washed and resuspended to the original volume in Mg-EGTA.
Haemolytic assays
The 50% haemolytic titres for classical and lectin pathway activity (CH50 and LH50, respectively) were determined as previously described [13]. For these and all other results presented, a minimum of three replicate experiments was performed.
Assay of the decay rate of the lectin pathway C3 convertases
The effect of C4 bp on the intrinsic decay rate of the C3 convertases created by E-M-MBL and EA-M-MBL was determined kinetically as previously described [17]. Briefly, E-M-MBL-C4 and EA-M-MBL-C4 were prepared by incubating E-M-MBL and EA-M-MBL (108/ml in Mg-EGTA), respectively, with an equal volume of 1:10 dilution of C2D serum for 15 min at 37°C. The cells were washed, resuspended in the original volume and mixed with an equal volume of purified human C2 (2.5 μg/ml) for 5 min at 30°C. After washing, the E-M-MBL-C4,2 and EA-M-MBL-C4,2 were resuspended to original volume in Mg-EGTA in the presence or absence of C4 bp, to assay for the decay rate of the C3 convertases. The mixtures were incubated at 30°C, and 100 μl samples were withdrawn at various time intervals and added to C2D serum (1:10) in Mg-EGTA to measure haemolysis via both pathways as a reflection of residual C3 convertase activity.
Flow cytometry assay of MBL, IgM, IgG, IgA, C4, C3, C5, C4 bp and H on sheep E
The indicator cells (100 μl; 108 cells/ml) were mixed with 100 μl 1:100 biotinylated antihuman MBL, IgM, IgG, IgA, C4, C3, C5, C4 bp and H, respectively, and incubated at 4°C for 1 h. After washing three times, 100 μl 1:10 R-phycoerythrin-conjugated streptavidin solution was added and incubated 1 h at 4°C. The cells again were washed and resuspended to 1 ml in GVB++ and assayed for the above proteins using an Ortho Cytoron flow cytometer (Ortho Diagnostic Systems, Raritan, NJ, USA) to quantify orange fluorescence. The fluorescence of cells is presented as the mean channel fluorescence intensity (MCF) of 104 cells measured using logarithmic amplification, except where noted. In this configuration, a 22-channel increase represents an approximate doubling of fluorescence [24].
RESULTS
Enhancement of lectin pathway-mediated haemolysis by antisheep E haemolysin
To evaluate the effect of antibody on lectin pathway-mediated haemolytic activity, we first investigated the lysis of MBL-sensitized mannan-coated E (E-M-MBL) cosensitized with increasing amounts of anti-E haemolysin in human serum in Mg-EGTA. This presensitization resulted in significant enhancement of MBL-initiated haemolysis (Fig. 1). Enhancement increased as increasing amounts of haemolysin were used, up to a maximum threefold enhancement (from 10 to 30 LH50 U/ml) with cells presensitized with 1:1000 haemolysin per 108 E; this latter amount was used in subsequent experiments. No lysis of E, EA, E-M or EA-M was observed in human serum-Mg-EGTA in the absence of MBL.
Fig. 1.
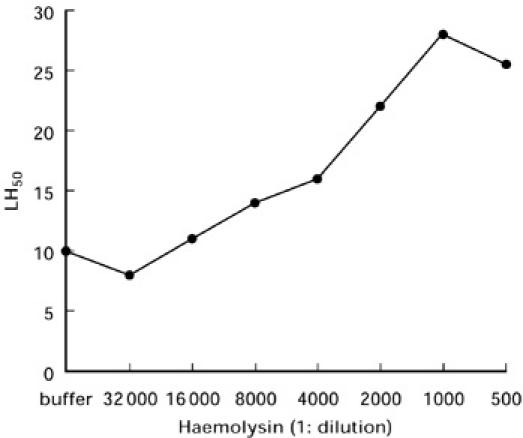
E-M-MBL were cosensitized with increasing amounts of haemolysin in Mg-ethyleneglycol tetraacetic acid (EGTA), washed, and incubated (60 min, 37°C) in an equal volume of agammaglobulinemic human serum in Mg-EGTA; the serum dilution lysing half the indicator cells (LH50) was recorded for each concentration of haemolysin tested. Enhancement increased with increasing amounts of haemolysin, up to threefold enhancement with cells presensitized with 1:1000 haemolysin per 108 E. There was no lysis of EA, or of E-M or EA-M not presensitized with mannan-binding lectin.
Binding of C4, C3 and C5 to E-M-MBL in the presence and absence of antibody
To explore the basis for antibody-based enhancement of lectin pathway activity, we first investigated the effect of presensitization with haemolysin on the binding of C4, C3 and C5 during incubation in serum-Mg-EGTA (Fig. 2). While cosensitization with haemolysin led to only minimal increase of C4 binding (from 74 to 82 MCF; P = 0.65), it significantly increased binding of C3 (74 to 122 MCF; P = 0.02) and C5 (27 to 60 MCF; P = 0.01). These experiments indicate that the presence of antibody enhances lectin pathway-mediated haemolysis, at least in part, at the level of C3 and C5.
Fig. 2.
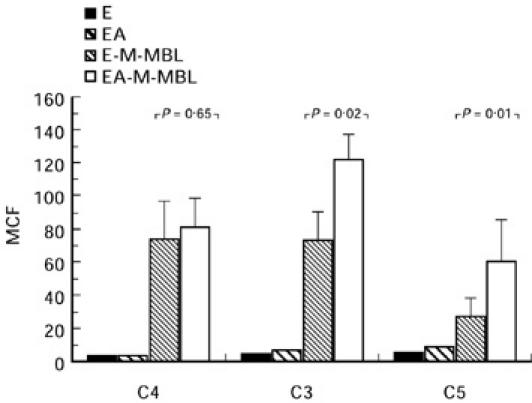
E-M-MBL and EA-M-MBL (E-M-MBL cosensitized with haemolysin) were incubated (30 min, 30°C) in an equal volume of 1:10 absorbed C8D serum, in Mg-EGTA; E and EA also were tested in an identical manner as controls. After appropriate washes the cells were reacted with an equal volume of biotinylated antihuman C4, C3 and C5, respectively (60 min, 4°C), stained with R-phycoerythrin-conjugated streptavidin, washed and assayed for bound complement components by flow cytometry as described. While cosensitization with haemolysin led to no significant increase of C4 binding, it significantly increased binding of C3 and C5.
Binding of C4 bp and H to E-M-MBL in the presence and absence of antibody
We next investigated the roles of C4 bp and factor H, the fluid phase complement regulatory proteins that control the assembly and/or stability of the classical and alternative pathway C3 convertases, respectively. The ratios of bound C4 bp to bound C4 and of bound factor H to bound C3 were used to compare the affinities of these control proteins for the bound complement proteins with which they interact when E-M-MBL and EA-M-MBL, respectively, were incubated (15 min, 37°C, Mg-EGTA) in absorbed C8D serum. As shown in Fig. 3, there was little increase in the ratio of bound C4 bp to C4b in the presence of haemolysin (0.56 to 0.63; P = 0.68). However, there was a great decrease in the ratio of bound factor H to bound C3b when E-M-MBL were presensitized with haemolysin (0.63 to 1.03; P = 0.03), indicating that the presence of immunoglobulin decreased the binding of factor H but not C4 bp.
Fig. 3.
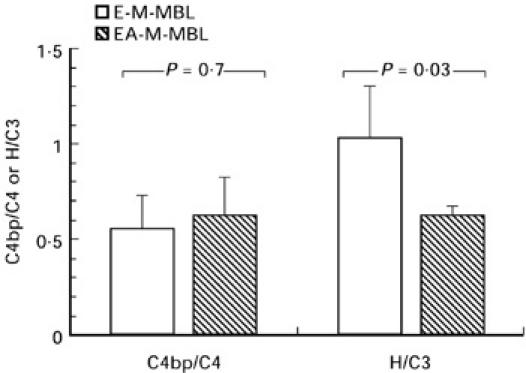
Ratios of bound C4 bp to bound C4, and bound factor H to bound C3, during lectin pathway activation in the presence of antibody. E-M-MBL and EA-M-MBL, respectively, were incubated [15 min, 37°C, Mg-ethyleneglycol tetraacetic acid (EGTA)] in absorbed C8D serum, and after washes in Mg-EGTA, tested for bound C4, C3, C4 bp and factor H, respectively, by incubation (4°C, 60 min) with equal volumes of biotinylated rabbit antisera directed against these components, staining and analysis by flow cytometry as described; results are expressed as ratios of C4 bp binding to C4 and factor H binding to C3. There was no decrease in the ratio of bound C4 bp to C4b, but a great decrease in the ratio of bound factor H to bound C3b, when E-M-MBL were presensitized with haemolysin.
Stability of the lectin pathway C3 convertase formed in the presence and absence of antibody cosensitization
To compare the stability of the lectin C3 convertase formed in the presence and absence of haemolysin, the decay rate of C3 convertase was determined. As shown in Fig. 4, the intrinsic decay rates of the C3 convertases formed on the two indicator cells, E-M-MBL and EA-M-MBL, were virtually equivalent, with half-lives (t1/2) of approximately 5.7 min. In addition, even in the presence of C4 bp the C3 convertases formed by the two cells were comparably susceptible to this control protein, with the t1/2 ≈ 2.5 min for E-M-MBL and EA-M-MBL, respectively. Thus, the presence of antibody did not influence the function of C4 bp.
Fig. 4.
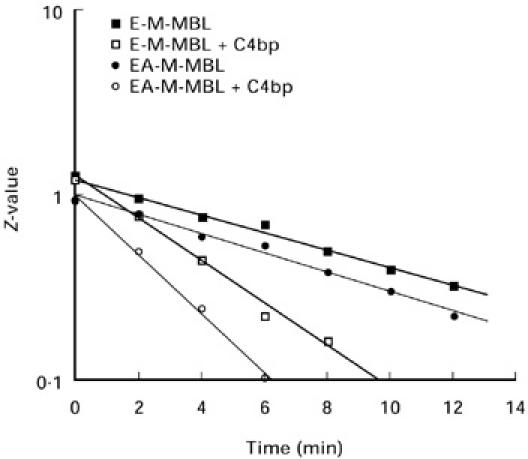
Intrinsic decay rates of the C3 convertases of the lectin pathway formed in the presence and absence of C4 bp and haemolysin. E-M-MBL and EA-M-MBL [108/ml in Mg-ethyleneglycol tetraacetic acid (EGTA)] were incubated (15 min, 37°C) with an equal volume of 1:10 C2D serum, and after washes, reacted with purified human C2 (5 min, 30°C). The E-M-MBL-C4,2 and EA-M-MBL-C4,2 were washed and resuspended in Mg-EGTA in the presence or absence of C4 bp, and the decay rate of the C3 convertases at 30°C was assayed by sampling at various time intervals for lysis upon incubation in C2D serum (1:10) in Mg-EGTA. The decay rates of the C3 convertase formed on the E-M-MBL and EA-M-MBL, were virtually equivalent (t1/2 ≈ 5.7 min) for both cells, even in the presence of C4 bp (t1/2 ≈ 2.5 min for both cells), indicating that the presence of antibody did not influence C4 bp function.
Antibody enhancement of lectin pathway-mediated haemolysis in C4 bp-and H-depleted serum
We next investigated the effect of haemolysin on the previously described [16,17] enhanced lectin pathway activity in serum depleted of C4 bp and/or factor H. The enhanced MBL-mediated haemolysis in C4 bp-depleted serum (from 12 to 28 LH50 U/ml) persisted in the presence of antibody; however, enhancement was no longer seen in factor H-depleted serum, or in serum depleted of both C4 bp and factor H (Fig. 5). These experiments indicate antibody enhances the regulatory function of factor H, but not C4 bp, during complement-mediated haemolysis via the lectin pathway.
Fig. 5.
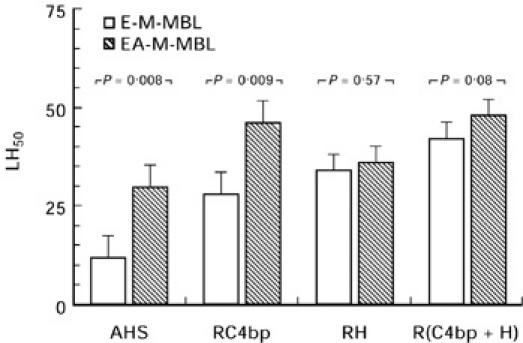
Effect of haemolysin on lectin pathway haemolytic activity in serum depleted of C4 bp and/or H. E-M-MBL and EA-M-MBL were incubated (37°C, 60 min) in unmodified agammaglobulinemic serum (AHS), AHS immunodepleted of C4 bp (RC4 bp), AHS depleted of factor H (RH) and AHS depleted of both C4 bp and factor H, and the LH50 was determined in the usual way. The enhancement of mannan-binding lectin-mediated haemolysis by antibody persisted in C4 bp-depleted serum, while enhancement was no longer seen in serum depleted factor H or of both factor H and C4 bp.
Enhancement of lectin pathway-mediated haemolysis by cosensitization with different Ig isotypes
To evaluate the effect of the three major Ig isotypes on lectin pathway-mediated haemolysis, E-M-MBL were cosensitized with human IgM, IgG or IgA using chromic chloride, prior to lysis in serum-Mg-EDTA. The presence of each of the isotypes resulted in significant enhancement of MBL-initiated haemolysis, and enhancement increased as increasing amounts of immunoglobulin were used (Fig. 6). A maximum 2.5-fold enhancement (from 9 to 26 LH50 U/ml) was observed with IgM or IgA cosensitization, while IgG led to fourfold enhancement, from 9 to 40 LH50 U/ml. We also compared the effects of IgM and IgG haemolysin on lectin pathway-mediated haemolysis by cosensitizing E-M-MBL with purified IgM or IgG anti-E. The cosensitization of each Ig isotype resulted in significant enhancement of MBL-initiated haemolysis; with enhancement increasing as increasing amounts of haemolysin were used (Fig. 7). A maximum twofold enhancement (from 9 to 20 LH50 U/ml) was observed with optimal IgM, while haemolysis in the presence of IgG anti-E increased about 4-fold (from 9 to 40 LH50 U/ml). These results using haemolysin isotypes correspond closely with those of observed when purified immunoglobulin was added to the cells by chromic chloride treatment.
Fig. 6.
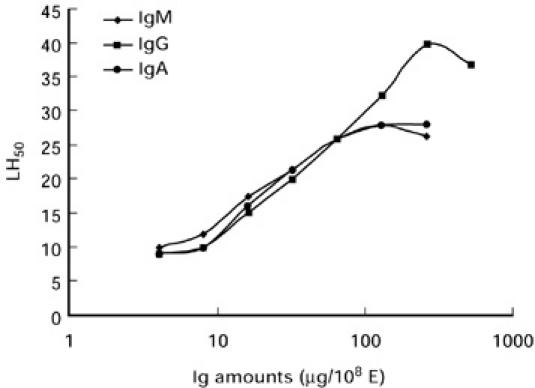
Enhancement of lectin pathway-mediated haemolysis by cosensitization with purified human IgM, IgG or IgA prior to lysis in human serum-Mg-EDTA. Each isotype resulted in significant enhancement of MBL-initiated haemolysis. E-M-MBL were cosensitized with human IgM, IgG or IgA using chromic chloride prior to incubation in serum-Mg-ethyleneglycol tetraacetic acid. A maximum 2.5-fold enhancement of haemolysis (from 9 to 26 LH50 U/ml) was observed with IgM and IgA cosensitization, while IgG led to approx. fourfold enhancement, from 9 to 40 LH50 U/ml.
Fig. 7.
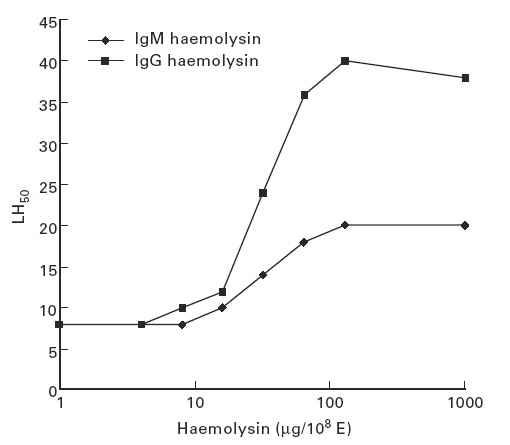
EA-M-MBL were prepared by incubation (15 min, 37°C) of E-M-MBL with IgM and IgG haemolysin, respectively, in Mg-ethyleneglycol tetraacetic acid (EGTA), washed in Mg-EGTA, and tested for lectin pathway haemolysis in human serum-Mg-EGTA. A twofold enhancement (from 9 to 20 LH50 U/ml) was observed upon presensitization with IgM, while fourfold increase (from 9 to 40 LH50 U/ml) was observed upon presensitization with IgG.
DISCUSSION
The innate immune system can react with pathogens and environmental antigens via multiple recognition molecules including natural antibody, MBL, CRP and the mannose receptor to result in their killing and elimination [1–3]. The complement system is a major effector of innate immunity through its three major activation pathways [25,26]. The classical pathway can be activated by antibodies and independent of antibody by CRP and by direct C1q recognition; the lectin pathway is activated by MBL; and the alternative pathway generally is activated by polysaccharides and appropriate cells or surfaces. There is abundant evidence that antibody can enhance alternative pathway function by conferring activating capacity to nonactivating surfaces, at least in part by protecting bound C3b from the inhibitory effects of factor H [6–9,27,28].
We recently observed that lectin pathway activation is associated with enhanced binding of C4 bp to C4 and of factor H to C3, for which the presence of MBL is required, and that these complement control proteins significantly regulate lectin pathway haemolysis [16,17]. We report here that the presence of immunoglobulin, either IgG, IgM or IgA, enhances lectin pathway haemolysis, by decreasing binding of factor H; immunoglobulin has no effect on C4 bp binding or function. The basis for the decreased binding of factor H to antibody-sensitized E-M-MBL probably reflects the ability of immunoglobulin to confer protection against breakdown of bound C3b by factors H and I, as had been shown in studies of alternative pathway activation [6–9]. The antibody-mediated enhancement of haemolysis via the lectin pathway seems much greater than antibody-mediated enhancement of haemolysis via the alternative pathway in the single report in which these have been compared [14].
The enhancing effect of Ig adds the power of antibody specificity to lectin pathway activation by MBL. Conversely, lectin pathway activation by MBL can enhance complement activation and hence inflammatory reactivity at sites where only small amounts of immunoglobulin are bound, as might occur early in the immune response. Attention previously has been directed to MBL as a nonantibody pattern recognition molecule which serves as a bridge between the innate and adaptive immune systems by its ability, shared with antibody, to activate the complement system and initiate complement-dependent host responses [29]. The synergism between bound immunoglobulin and MBL described herein, reflected in immunoglobulin enhancement of lectin pathway cytolysis initiated by MBL and enhanced lectin pathway activation at sites of immunoglobulin deposition, thus represents another manifestation of MBL bridging between the innate and adaptive systems of immunity.
Acknowledgments
This paper was presented in part to the XVII Annual International Complement Workshop in Rhodes, 11–16 October 1998 [30]. Supported by a Thai Royal Government Scholarship to C.S., and is presented by Y.Z. in partial fulfillment of the requirements for the PhD from Rush University; H.G. holds the Thomas J. Coogan Sr Chair in Immunology established by Marjorie Lindheimer Everett.
REFERENCES
- 1.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–3. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 2.Fearon DT. The complement system and adaptive immunity. Semin Immunol. 1998;10:355–61. doi: 10.1006/smim.1998.0137. [DOI] [PubMed] [Google Scholar]
- 3.Carroll MC, Prodeus AP. Linkages of innate and adaptive immunity. Curr Opin Immunol. 1998;10:36–40. doi: 10.1016/s0952-7915(98)80028-9. [DOI] [PubMed] [Google Scholar]
- 4.Fischer MB, Ma M, Goerg S, et al. Regulation of the B cell response to T-dependent antigens by classical pathway complement. J Immunol. 1996;157:549–56. [PubMed] [Google Scholar]
- 5.Ahearn JM, Fischer MB, Croix D, et al. Disruption of the Cr2 locus results in a reduction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity. 1996;4:251–62. doi: 10.1016/s1074-7613(00)80433-1. [DOI] [PubMed] [Google Scholar]
- 6.Polhill RB, Jr, Newman SL, Pruitt KM, Johnston RB. Kinetic assessment of alternative complement pathway activity in a hemolytic system. II. Influence of antibody on alternative pathway activation. J Immunol. 1978;121:371–6. [PubMed] [Google Scholar]
- 7.Ratnoff WD, Fearon DT, Austen KF. The role of antibody in the activation of the alternative complement pathway. Springer Semin Immunopathol. 1983;6:361–71. doi: 10.1007/BF02116280. [DOI] [PubMed] [Google Scholar]
- 8.Joiner KA, Goldman RC, Hammer CH, Leive L, Frank MM. Studies of the mechanism of bacterial resistance to complement-mediated killing. V. IgG and F (ab′) 2 mediate killing of E. coli 0111B4 by the alternative complement pathway without increasing C5b-9 deposition. J Immunol. 1983;131:2563–9. [PubMed] [Google Scholar]
- 9.Joiner KA, Goldman RC, Hammer CH, Leive L, Frank MM. Studies on the mechanism of bacterial resistance to complement-mediated killing. VI. IgG increases the bactericidal efficiency of C5b-9 for E. coli 0111B4 by acting at a step before C5 cleavage. J Immunol. 1983;131:2570–5. [PubMed] [Google Scholar]
- 10.Matsushita M, Endo Y, Fujita T. MASP1 (MBL-associated serine protease 1) Immunobiology. 1998;199:340–7. doi: 10.1016/S0171-2985(98)80038-7. [DOI] [PubMed] [Google Scholar]
- 11.Vorup-Jensen T, Jensenius JC, Thiel S. MASP-2, the C3 convertase generating protease of the MBLectin complement activating pathway. Immunobiology. 1998;199:348–57. doi: 10.1016/S0171-2985(98)80039-9. [DOI] [PubMed] [Google Scholar]
- 12.Ji YH, Matsushita M, Okada H, Fujita T, Kawakami M. The C4 and C2 but not C1 components of complement are responsible for the complement activation triggered by the Ra-reactive factor. J Immunol. 1988;141:4271–5. [PubMed] [Google Scholar]
- 13.Suankratay C, Zhang XH, Zhang Y, Lint TF, Gewurz H. Requirement for the alternative pathway as well as C4 and C2 in complement-dependent hemolysis via the lectin pathway. J Immunol. 1998;160:3006–13. [PubMed] [Google Scholar]
- 14.Zhang Y, Suankratay C, Zhang X-H, Lint TF, Gewurz H. Lysis via the lectin pathway of complement activation: minireview and lectin pathway enhancement of endotoxin-initiated hemolysis. Immunopharmacol. 1999;42:81–90. doi: 10.1016/s0162-3109(99)00029-6. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Suankratay C, Jones DR, Zang X-H, Lint TF, Gewurz H. Calcium independent hemolysis via the lectin pathway of complement activation in the guinea pig and other species. Immunol. 1999 doi: 10.1046/j.1365-2567.1999.00810.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suankratay C, Mold C, Zhang Y, Potempa LA, Lint TF, Gewurz H. Complement regulation in innate immunity and the acute-phase response: inhibition of mannan-binding lectin-initiated complement cytolysis by C-reactive protein (CRP) Clin Exp Immunol. 1998;113:353–9. doi: 10.1046/j.1365-2249.1998.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suankratay C, Mold C, Zhang Y, Lint TF, Gewurz H. Mechanism of complement-dependent hemolysis via the lectin pathway: role of the complement regulatory proteins. Clin Exp Immunol. 1999;117:442–8. doi: 10.1046/j.1365-2249.1999.00998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sim RB, Day AJ, Moffatt BE, Fontaine M. Complement factor I and cofactors in control of complement system convertase enzymes. Methods Enzymol. 1993;223:13–35. doi: 10.1016/0076-6879(93)23035-l. [DOI] [PubMed] [Google Scholar]
- 19.Mold C, Kingzette M, Gewurz H. C-reactive protein inhibits pneumococcal activation of the alternative pathway by increasing the interaction between factor H and C3b. J Immunol. 1984;133:882–5. [PubMed] [Google Scholar]
- 20.Kawasaki N, Kawasaki T, Yamashina I. Isolation and characterization of a mannan-binding protein from human serum. J Biochem (Tokyo) 1983;94:937–47. doi: 10.1093/oxfordjournals.jbchem.a134437. [DOI] [PubMed] [Google Scholar]
- 21.Harrison RA, Lachmann PJ. Complement technology. In: Weir RA, Lachmann DM, editors. Handbook of experimental immunology. Vol. 39. Palo Alto: Blackwell, Scientific Publications; 1986. pp. 1–39.pp. 49 [Google Scholar]
- 22.Perucca PJ, Faulk WP, Fudenberg HH. Passive immune lysis with chromic chloride-treated erythrocytes. J Immunol. 1969;102:812–20. [PubMed] [Google Scholar]
- 23.Borsos T, Rapp HJ, Mayer MM. Studies on the second component of complement. I The reaction between Eac14 and C2: evidence on single site mechanism of immune hemolysis and determination of C2 on a molecular basis. J Immunol. 1961;87:310–25. [Google Scholar]
- 24.Spear GT, Takefman DM, Sullivan BL, Landay AL, Jennings MB, Carlson JR. Anti-cellular antibodies in sera from vaccinated macaques can induce complement-mediated virolysis of human immunodeficiency virus and simian immunodeficiency virus. Virology. 1993;195:475–80. doi: 10.1006/viro.1993.1398. [DOI] [PubMed] [Google Scholar]
- 25.Volanakis JE, Fearon DT. Molecular biology of the complement system. In: Koopman WJ, editor. Arthritis and allied conditions: a textbook of rheumatology. Baltimore, MD: Williams and Wilkins; 1997. pp. 491–3. [Google Scholar]
- 26.Gewurz H, Ying SC, Jiang H, Lint TF. Nonimmune activation of the classical complement pathway. Behring Inst Mitt. 1993;93:138–47. [PubMed] [Google Scholar]
- 27.Medof ME, Lam T, Prince GM, Mold C. Requirement for human red blood cells in inactivation of C3b in immune complexes and enhancement of binding to spleen cells. J Immunol. 1983;130:1336–40. [PubMed] [Google Scholar]
- 28.Fries LF, Gaither TA, Hammer CH, Frank MM. C3b covalently bound to IgG demonstrates a reduced rate of inactivation by factors H and I. J Exp Med. 1984;160:1640–55. doi: 10.1084/jem.160.6.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fraser IP, Koziel H, Ezekowitz RA. The serum mannose-binding protein and the macrophage mannose receptor are pattern recognition molecules that link innate and adaptive immunity. Semin Immunol. 1998;10:363–72. doi: 10.1006/smim.1998.0141. [DOI] [PubMed] [Google Scholar]
- 30.Suankratay C, Mold C, Zhang Y, Jones DR, Lint TF, Gewurz H. Complement-dependent hemolysis in human serum via the lectin pathway: regulation by C4bp, factor H and immunoglobulins (abstract) Mol Immunol. 1998;35:390. [Google Scholar]


