Abstract
We examined the effect of intracolonic administration of anti-adhesion molecule antibodies on DSS-induced colitis in mice. Immunohistochemical staining in mice with colitis showed increased expression of ELAM-1 and ICAM-1 on endothelial cells of vessels in the lamina propria and submucosa at sites of inflamed lesions. Intracolonic administration of anti-ELAM-1 or anti-ICAM-1 antibody decreased bloody stools, anaemia, and histologically evident damage, as well as myeloperoxidase activity and IL-1β content. We concluded that adhesion molecule expression is important in the development of DSS-induced colitis in mice and that intracolonic administration of anti-adhesion molecule antibodies, especially anti-ELAM-1 antibody, effectively inhibits the colonic inflammation. Intracolonic administration of anti-adhesion molecule antibodies may show therapeutic promise in ulcerative colitis.
Keywords: dextran sulphate sodium, ulcerative colitis, ELAM-1, ICAM-1, IL-1β
INTRODUCTION
Circulating leucocytes adhere to vascular endothelial cells and infiltrate the affected site upon inflammatory stimulation. This process includes three steps—leucocyte rolling, adhesion, and migration—and is regulated by various humoral factors and adhesion molecules [1,2]. In the first step, ELAM-1 (or CD62E) [3] and P-selectin (CD62P) [4], members of the selectin family expressed on vascular endothelial cells following stimulation with IL-1 and tumour necrosis factor-alpha (TNF-α), bind to sialyl Lewis X-bearing ligands [5] on the leucocyte surface. This binding causes the cells to roll along vessel walls. Next, ICAM-1 (or CD54) [6], which belongs to the immunoglobulin superfamily, binds to leucocyte function-associated antigen-1 (LFA-1, or CD11a/CD18) and Mac-1 (CD11b/CD18) on leucocytes, causing the leucocytes to adhere to the vessel walls and eventually migrate beyond the vessels. Previous reports concerning ulcerative colitis (UC) support the view that adhesion molecules expressed on vascular endothelial cells play a role in mucosal immunity [7–10].
In recent years, anti-adhesion molecule therapy using specific antibodies or ligands for these molecules has been reported to inhibit inflammation [11–14]. In the present study, using a DSS-induced mouse model resembling UC, mucosal adhesion molecule expression at inflammatory sites was examined immunohistochemically and the therapeutic effect of intracolonic administration of anti-adhesion molecule antibodies was assessed.
MATERIALS AND METHODS
Animals
Nine-week-old female BALB/c mice (SLC, Shizuoka, Japan) weighing about 20 g were used. Standard mouse chow pellets and tap water were supplied in bottles ad libitum.
DSS-induced colitis model
Acute colitis was induced by providing 5% DSS solution (mol. wt 5000) for the indicated days instead of tap water.
Treatment protocol
The mice (five per cage) were weighed and randomized into three groups. A normal control group (n = 5) received tap water orally for 7 days. A colitis group (n = 5) received 5% DSS orally for 7 days and 0.2 ml of distilled water intracolonically on days 0, 2 and 4. A treatment group (n = 5, for each group) received 5% DSS orally for 7 days and a MoAb against mouse ELAM-1 or mouse ICAM-1 intracolonically in 0.2 ml of distilled water, at a dose of 1 mg/kg on each of days 0, 2 and 4. The antibodies (rat anti-mouse ELAM-1 MoAb (10E9.6) and hamster anti-mouse ICAM-1 MoAb (3E2)) were purchased from Pharmingen (San Diego, CA) and were installed slowly through a polyethylene catheter (Clea, Tokyo, Japan) carefully inserted until the tip was 3 cm proximal to the anus.
The dose-dependent effect of anti-ELAM-1 MoAb was examined. Mice were fed 5% DSS for 7 days and 0.5, 1.0 or 2.0 mg/kg of anti-ELAM-1 MoAb (n = 5, for each group) were introduced on days 2 and 4 exactly in the same way as described above. In this experiment, isotype-matched IgG (rat IgG2aλ) was used as a control (n = 5).
The effect of anti-ELAM-1 MoAb on established inflammation was assessed. Mice were fed 5% DSS for 10 days and 1.0 mg/kg of anti-ELAM-1 MoAb was administered on days 7 and 9 (n = 5). A control group (n = 5) was subjected to isotype-matched IgG treatment.
Evaluation of colitis
To reflect the general condition of mice, a disease activity index (DAI) was determined by an investigator blinded to the protocol by scoring the extent of body weight loss, stool guiac positivity or gross bleeding, and stool consistency (Table 1) according to the method of Murthy et al. [15,16]. Briefly, animals were weighed before starting DSS administration and on the day when animals were killed. Stool consistency and the degree of blood in stool were evaluated on the day when animals were killed. Simultaneously, haemoglobin levels were examined in intracardiac blood.
Table 1.
Disease activity index

The disease activity index (DAI) = (combined score of weight loss, stool consistency, and bleeding)/3.
*Normal stools, well formed pellets; loose stools, pasty stools that do not stick to the anus; diarrhoea, liquid stools that stick to the anus.
The entire colon then was removed, and the rectum, transverse colon, and caecum were separated. The segment of colon were opened longitudinally and fixed in 10% neutral buffered formalin prior to histological processing. Haematoxylin and eosin (H–E)-stained sections were examined microscopically. To evaluate the severity of inflammation, 15 randomly selected fields (magnification × 100) were inspected in each section by a pathologist blinded to the treatment protocol and graded as follows: grade 0, normal colonic mucosa; grade 1, loss of one-third of the crypts; grade 2, loss of two-thirds of the crypts; grade 3, lamina propria covered with a single layer of epithelial cells with mild inflammatory cell infiltration; and grade 4, erosions and marked inflammatory cell infiltration. After grading the 15 fields, the mean grade was calculated for each section and expressed as histological score.
Immunohistochemistry
Resected rectal specimens from animals in the normal control and colitis group were embedded in OCT compound (Tissue Tek, Elkhart, IN), frozen in liquid nitrogen, and cut into 4-μm serial sections. Expression of ELAM-1 and ICAM-1 in the rectal mucosa was examined in these sections by an indirect immunoperoxidase method. Briefly, the sections were air-dried for 30 min, fixed with acetone for 15 min, and washed twice with PBS for 5 min. Then the sections were incubated with 10% normal goat serum followed by primary antibodies for 60 min at room temperature (10 μg/ml of either anti-ELAM-1 MoAb or anti-ICAM-1 MoAb). The antibodies were the same ones administered to mice in the treatment group. Sections then were washed three times in PBS for 5 min and incubated for 30 min at room temperature with biotinylated goat anti-mouse IgG or biotinylated goat anti-hamster IgG as the respective secondary antibodies (1:200 dilution; Vector Labs, Burlingame, CA). The sections were washed three times for 5 min per wash and allowed to react with avidin–biotin complex (Vectastain Elite ABC reagent; Vector Labs) for 30 min at room temperature. The peroxidase reaction was developed in 0.03% 3′-3-diaminobenzidine (Sigma Chemical Co., St Louis, MO) for 5 min. All sections were counterstained with Mayer's haematoxylin. Endogenous peroxidase activity was inhibited by treatment with methanol containing 0.3% H2O2 for 30 min after reaction with the secondary antibody.
Myeloperoxidase activity
Myeloperoxidase (MPO) activity was measured as an indicator of neutrophil accumulation, in rectal and caecal mucosa. Tissue samples were homogenized with a rotary homogenizer in 0.1 m phosphate buffer, and tetramethylbenzidine (TMBZ; Dojin Chemicals, Tokyo, Japan) and H2O2 were added. After the mixture was incubated at room temperature for 15 min, absorbance (optical density (OD)) was measured spectrophotometrically at 630 nm. The total protein concentration was measured according to Lowry, and absorbance per milligram of protein was determined. MPO activity was measured by a guaiacol method using human MPO (Sigma) as standard [17]. Absorbance was converted to MPO activity (mU/mg) using the formula, MPO activity (mU/mg) = [absorbance/protein concentration (mg)] × 1.6.
IL-1β concentrations
IL-1β concentrations were measured in homogenized rectal and caecal tissue using an ELISA kit according to the manufacturer's protocol (R&D Systems, Abingdon, UK) and were expressed per milligram of protein.
Statistical analysis
All values are expressed as the mean ± s.e.m. Data sets were analysed using one-way analysis of variance (anova) followed by Fisher's protected least significant difference (PLSD) comparison tests for post hoc t-tests. Differences of P < 0.05 were considered statistically significant.
RESULTS
Clinical indices
DAI and haemoglobin concentration in the three groups of mice are shown in Table 2. Loose to liquid grossly bloody stools and weight loss were observed in all animals in the colitis group after administration of 5% DSS for 7 days. In both antibody treatment groups, faecal blood was less obvious, stools were better formed, and less weight was lost. Accordingly, the DAI was significantly lower in the anti-ELAM-1 treatment group than in the colitis group (P < 0.01). The DAI also was lower in the anti-ICAM-1 antibody-treated group, although the difference was not significant. Haemoglobin levels also were significantly higher in both antibody-treated groups than in the colitis group (P < 0.01).
Table 2.
Effect of intracolonic anti-adhesion molecule therapy on clinical indices of DSS-induced murine colitis

Results are expressed as mean ± s.e.m. (n = 5).
**Significantly different from colitis group at P < 0.01.
Hb, Haemoglobin; DAI, disease activity index.
Histological study
In the rectum, specimens obtained from the colitis group showed scattered erosions with marked inflammatory cell infiltration in the lamina propria. In the caecum extensive erosions with inflammatory cell infiltration were seen. However, in the transverse colon only slight inflammatory cell infiltration without erosions was observed. In both antibody-treated groups, fewer erosions were observed and inflammatory cell infiltration was almost absent in the rectum, although occasional erosions were still seen in the caecum. Histological scores for the rectum and the caecum were decreased with statistical significance by anti-ELAM-1 or anti-ICAM-1 antibody treatment (Table 3).
Table 3.
Effect of intracolonic anti-adhesion molecule therapy on histological score of DSS-induced murine colitis

Results are expressed as mean ± s.e.m. (n =5).
**Significantly different from colitis group at P < 0.01.
*Significantly different from colitis group at P < 0.05.
Immunohistochemistry
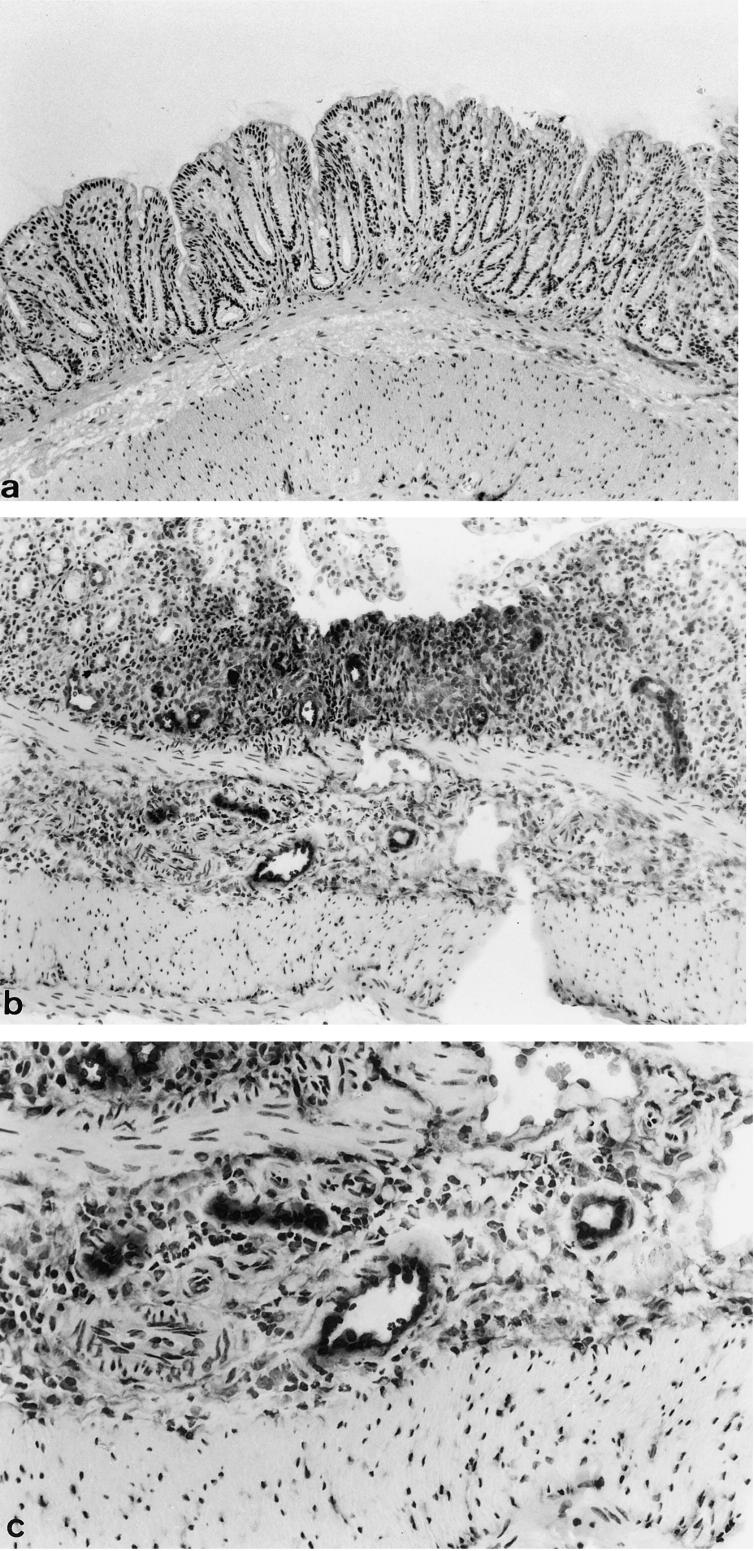
Expression of ELAM-1 and ICAM-1 was investigated by immunostaining in the normal control group and the colitis group. In the normal control group, no expression of ELAM-1 was seen on the colonic vascular endothelial surfaces (Fig. 1a). In the colitis group, scattered expression of ELAM-1 was evident on the vascular endothelium in the submucosa of inflamed sites (Fig. 1b,c). More strikingly, in the colitis group ICAM-1 was strongly expressed on the vascular endothelium in the mucosa and submucosa (Fig. 2b,c), although slight expression was seen in mucosal vessels in the normal control group (Fig. 2a).
Fig. 1.
(See next page) Expression of ELAM-1 in rectal mucosa with DSS-induced colitis. Frozen sections were incubated with anti-mouse ELAM-1 MoAb (10 μg/ml) at room temperature for 60 min (a), normal mucosa (normal control group; × 100). (b,c) Mucosa after 5% DSS consumption showing erosion (colitis group; (b) × 40, (c) × 100).
Fig. 2.
(See next page) Expression of ICAM-1 in rectal mucosa with DSS-induced colitis. Frozen sections were incubated with anti-mouse ICAM-1 MoAb (10 μg/ml) at room temperature for 60 min (a), normal mucosa (normal control group; × 100). (b,c) Mucosa after 5% DSS consumption showing erosion (colitis group; (b) × 100, (c) × 400).
Comparison of MPO activity
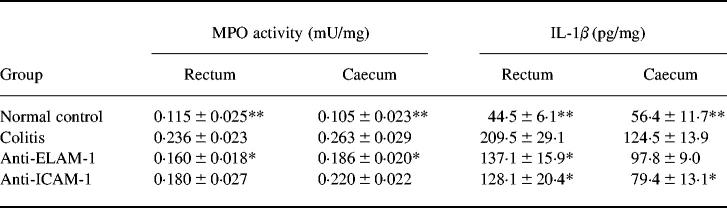
MPO activity was measured in the rectum and caecum (Table 4). In both the rectum and caecum, MPO activity in the colitis group was significantly higher than that in the normal group (P < 0.01). Anti-ELAM-1 antibody treatment significantly decreased MPO activity in both regions (P < 0.05). Anti-ICAM-1 antibody treatment also decreased MPO activity in the rectum and caecum, although the differences from the colitis group were not significant.
Table 4.
Effect of intracolonic anti-adhesion molecule therapy on myeloperoxidase (MPO) and IL-1β activity on DSS-induced murine colitis
Results are expressed as mean ± s.e.m. (n = 5).
**Significantly different from colitis group at P < 0.01.
*Significantly different from colitis group at P < 0.05.
Comparison of IL-1β concentrations
IL-1β concentrations in the rectum and caecum were compared between normal control, colitis, and antibody-treated groups (Table 4). IL-1β concentrations in the rectum for the colitis group were significantly higher than for the normal control group (P < 0.01), and the difference also was significant in the caecum (P < 0.01). Treatment with anti-ELAM-1 antibody decreased IL-1β concentrations in both the rectum and the caecum. Anti-ICAM-1 antibody also decreased IL-1β concentrations in both regions. In the rectum both decreases were significant (P < 0.05), but in the caecum only the anti-ICAM-1 antibody-associated decrease was significant (P < 0.05).
Dose-dependent effect and effect on established inflammation of anti-ELAM-1 MoAb therapy
To evaluate the effect of anti-ELAM-1 MoAb, the DAI and histological score were evaluated. As shown in Table 5, no therapeutic effect was observed in the group treated with 0.5 mg/kg of anti-ELAM-1 MoAb, although almost the same significant therapeutic effect was observed in the groups treated with 1.0 and 2.0 mg/kg of antibody. Anti-ELAM-1 MoAb was significantly effective when administered on days 2 and 4, but no effect was observed when administered on days 7 and 9.
Table 5.
Dose-dependent effect and effect on established inflammation of intracolonic anti-ELAM-1 antibody therapy on DSS-induced murine colitis
Results are expressed as mean ± s.e.m. (n = 5).
**Significantly different from control IgG group (days 2 and 4) at P < 0.01.
*Significantly different from control IgG group (days 2 and 4) at P < 0.05.
DAI, Disease activity index.
DISCUSSION
UC is a chronic recurring inflammatory condition of the colonic mucosa of unknown aetiology and pathogenesis. Oral administration of DSS induces colitis resembling UC in hamsters [18] and mice [19]. In the present study, acute colitis was induced in BALB/c mice with 5% DSS. In the colitis group, the DAI was significantly greater than in the normal control group. Histologically, erosions and marked inflammatory cell infiltration were observed in the rectum and caecum. MPO activity, which is correlated with neutrophil infiltration [20], was significantly high in the rectal and caecal mucosa in the colitis group, suggesting the involvement of neutrophils in the inflammatory process. Moreover, the concentrations of IL-1, which is produced mainly by activated macrophages, were significantly increased in both rectum and caecum in the colitis group, consistent with the previous findings in DSS-induced colitis in rats [21].
Adhesion molecules are involved in the pathogenesis of various conditions, including UC [7–10]. Oshitani et al. have reported that in the active phase of UC, the number of ELAM-1+ vessels is increased in colonic mucosa compared with mucosa in the normal and remission phases [8]. Moreover, soluble ICAM-1 and ELAM-1 in the intestinal mucosa of UC patients were found to be higher in the active phase than in the remission phase or normal mucosa [9]. In the present study, immunoreactive ELAM-1 and ICAM-1 were highly expressed in the vessels of inflamed colon mucosa, suggesting that cell–cell interaction mediated by these adhesion molecules is crucial to the pathogenesis of DSS-induced colitis.
In recent years, anti-adhesion molecule therapy utilizing antibodies or ligands for adhesion molecules has been tested in various experimental animal models, and several articles have indicated that the treatment could suppress inflammation [11–14]. The present study examined whether intracolonic administration of anti-adhesion molecule antibodies inhibited DSS-induced colitis in mice. Podolsky et al. have demonstrated that administration of an anti-α4-integrin MoAb resulted in significant attenuation of acute colitis in the cotton-top tamarin [14]. Recently, Bennet et al. found that subcutaneous injection of ICAM-1 anti-sense oligonucleotide on consecutive days prevented development of murine DSS-induced colitis [22]. However, no previous reports have examined the therapeutic effect of anti-adhesion molecule antibodies in DSS-induced colitis.
So far, i.v. administration of anti-adhesion molecule antibodies has been the route most commonly used, carrying the likelihood of systemic side-effects. We chose intracolonic administration to avoid these problems and to attain a more localized effect. Either anti-ICAM-1 or anti-ELAM-1 antibody resulted in clinical and histological improvement. MPO activity and IL-1β were also reduced in colonic tissues, suggesting intracolonic administration of the antibodies inhibited the colonic inflammation. In addition, a therapeutic effect was observed in the caecum as well as the rectum. Most likely, the antibody reached the proximal colon to act directly. Alternatively, antibodies absorbed from the distal colon could have acted indirectly on the proximal colon.
A difference in therapeutic effect was seen between the two antibodies tested. Anti-ELAM-1 antibody resulted in more marked improvement in DAI, histological score in the rectum, and MPO activity than anti-ICAM-1 antibody. As the expression of ELAM-1 on the vascular endothelium occurs at an earlier stage than ICAM-1 expression, it is thought that anti-ELAM-1 antibody more effectively inhibited the development of colonic inflammation. The effect of anti-ELAM-1 antibody on established inflammation was examined but no therapeutic effect was observed. These results suggest that anti-ELAM-1 antibody effectively inhibits the development of DSS-induced colitis when administered in the early phase of inflammation. Intracolonic administration of anti-adhesion molecules may show promise for clinical treatment of ulcerative colitis.
REFERENCES
- 1.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–6. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 2.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–34. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 3.Bevilacqua MP, Stengelin S, Gimbrone MA, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989;243:1160–5. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- 4.Johnston GI, Cook RG, McEver RP. Cloning of GMP-140, a granule membrane protein of platelets and endothelium: sequence similarity to proteins involved in cell adhesion and inflammation. Cell. 1989;56:1033–44. doi: 10.1016/0092-8674(89)90636-3. [DOI] [PubMed] [Google Scholar]
- 5.Phillips ML, Nudelman E, Gaeta FCA, Perez M, Singhal AK, Hakamori S, Paulson JC. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-LeX. Science. 1990;250:1130–5. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- 6.Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL-1 and interferon-γ: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–54. [PubMed] [Google Scholar]
- 7.Nakamura S, Ohtani H, Watanabe Y, Fukushima K, Matsumoto T, Kitano A, Kobayashi K, Nagura H. In situ expression of the cell adhesion molecules in inflammatory bowel disease—evidence of immunologic activation of vascular endothelial cells. Lab Invest. 1993;69:77–85. [PubMed] [Google Scholar]
- 8.Oshitani N, Campbell A, Bloom S, Kitano A, Kobayashi K, Jewell DP. Adhesion molecule expression on vascular endothelium and nitroblue tetrazolium reducing activity in human colonic mucosa. Scand J Gastroenterol. 1995;30:915–20. doi: 10.3109/00365529509101601. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen OH, Brynskov J, Vainer B. Increased mucosal concentrations of soluble intercellular adhesion molecule-1 (sICAM-1), sE-selectin, and interleukin-8 in active ulcerative colitis. Dig Dis Sci. 1996;41:1780–5. doi: 10.1007/BF02088745. [DOI] [PubMed] [Google Scholar]
- 10.Koizumi M, King N, Lobb R, Benjamin C, Podolsky DK. Expression of vascular adhesion molecules in inflammatory bowel disease. Gastroenterogy. 1992;103:840–7. doi: 10.1016/0016-5085(92)90015-q. [DOI] [PubMed] [Google Scholar]
- 11.Mulligan MS, Lowe JB, Larsen RD, et al. Protective effects of sialylated oligosaccharides in immune complex-induced acute lung injury. J Exp Med. 1993;178:623–31. doi: 10.1084/jem.178.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulligan MS, Varani J, Dame MK, Lane CL, Smith CW, Anderson DC, Ward PA. Role of endothelial-leukocyte adhesion molecule 1 (ELAM-1) in neutrophil-mediated lung injury in rats. J Clin Invest. 1991;88:1396–406. doi: 10.1172/JCI115446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weyrich AS, Ma XI, Lefer DJ, Albertine KH, Lefer AM. In vivo neutralization of P-selectin protects feline heart and endothelium in myocardial ischemia and reperfusion injury. J Clin Invest. 1993;91:2620–9. doi: 10.1172/JCI116501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Podolsky DK, Lobb R, King N, Benjamin CD, Pepinsky B, Sehgal P, Beaumont M. Attenuation of colitis in the cotton-top tamarin by anti-α4 integrin monoclonal antibody. J Clin Invest. 1993;92:372–80. doi: 10.1172/JCI116575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murthy SNS, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993;38:1722–34. doi: 10.1007/BF01303184. [DOI] [PubMed] [Google Scholar]
- 16.Cooper HS, Murthy SNS, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–49. [PubMed] [Google Scholar]
- 17.Desser RK, Himmelhoch SR, Evans WH, Januska M, Mage M, Shelton E. Guinea pig heterophil and eosinophil peroxidase. Arch Biochem Biophys. 1972;148:452–65. doi: 10.1016/0003-9861(72)90164-6. [DOI] [PubMed] [Google Scholar]
- 18.Ohkusa T. Production of experimental ulcerative colitis in hamsters by dextran sulfate sodium and changes in intestinal microflora. Jpn J Gastroenterol. 1985;82:1327–36. [PubMed] [Google Scholar]
- 19.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakata R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 20.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Gastroenterology. 1984;87:1344–50. [PubMed] [Google Scholar]
- 21.Shintani N, Nakajima T, Nakakubo H, Nagai H, Kagitani Y, Takizawa H, Asakura H. Intravenous immunoglobulin (IVIG) treatment of experimental colitis induced by dextran sulfate sodium in rats. Clin Exp Immunol. 1997;108:340–5. doi: 10.1046/j.1365-2249.1997.d01-1021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett CF, Kornbrust D, Henry S, et al. An ICAM-1 antisense oligonucleotide prevents and reverses dextran sulfate sodium-induced colitis in mice. J Pharmacol Exp Ther. 1997;280:988–1000. [PubMed] [Google Scholar]