Abstract
Several recent studies demonstrate that B7.2, but not B7.1, play an important role in allergic inflammation and IgE production. Agents that down-regulate B7.2 may therefore be of benefit for the treatment of Th2-driven allergic diseases. Our current study was carried out to investigate the effect of immunosuppressive agents, cyclosporin A (CsA) and dexamethasone, on B7.2 and B7.1 expression on B cells stimulated with the superantigen, toxic shock syndrome toxin-1 (TSST-1). The analysis of B7.2 and B7.1 on the same cells by flow cytometry demonstrated that TSST-1 up-regulated B7.2+B7.1− but not B7.1+B7.2− on B cells in a dose-dependent fashion. CsA and dexamethasone significantly down-regulated B7.2+B7.1− but up-regulated B7.2−B7.1+ B cells in the presence or absence of TSST-1 (100 ng/ml). Interestingly, the combination of CsA and dexamethasone was much more potent in the inhibition of B7.2 expression than either of these agents alone. As CD40 is known to up-regulate B7.2 expression on B cells, the mechanism of B7.2 down-regulation by CsA and dexamethasone was further studied by investigating the effect of these agents on CD40 expression on B cells. TSST-1 significantly increased CD40 expression on B cells. However, the addition of CsA or dexamethasone significantly down-regulated CD40 expression. Anti-CD40 MoAb significantly reversed the effects of CsA or dexamethasone on B7.2 and B7.1 expression, suggesting that T cell engagement of CD40 plays a role in the mechanisms by which CsA and dexamethasone acts on B cells. These data demonstrate the modulatory effect of CsA and dexamethasone on B7.2 and B7.1 expression on B cells and the potential role of CD40 in mediating this effect.
Keywords: allergy, costimulatory signals, cyclosporin A, dexamethasone
INTRODUCTION
The engagement of B7 on antigen-presenting cells (APC) with CD28/CTLA-4 on T cells is a well-characterized costimulatory pathway for T and B cell activation [1]. At least two members of the B7 family, B7.1 (CD80) and B7.2 (CD86), have now been identified. B7.1 is normally expressed at low levels on ‘professional’ APC, such as dendritic cells, and macrophages, and up-regulated on these APC as well as on B cells following activation by soluble factors (e.g. cytokines) or ligation of cell surface molecules (e.g. MHC class II and CD40). B7.2 is constitutively expressed on dendritic cells and is rapidly induced on B cells following activation by cross-linking of the immunoglobulin receptor or the addition of various cytokines [2]. Several studies have demonstrated distinct kinetics and interaction sites between the B7.1/B7.2 and the CD28/CTLA-4 receptor-counter ligand system [3–6]. Such differential binding may have unique signalling properties that affect T cell activation and subsequent Th1/Th2 development. Studies in mice have suggested that the generation of Th2 cells depends mainly on the interaction of CD28 with B7.2 [7]. In vitro studies of human T cells have demonstrated that B7.2 transfectants preferentially activate Th2-type cytokines, whereas B7.1 transfectants skew responses towards the production of Th1-type cytokines [8]. Other reports, however, have not confirmed that B7.2 is involved in Th2 responses. These studies found that both B7.1 and B7.2 were able to costimulate IL-4 and interferon-gamma (IFN-γ) production from murine T cells [9,10].
Despite the controversy over the role of B7.1 versus B7.2 in the regulation of Th1/Th2 immune responses, recent studies have demonstrated a role for B7.2 in atopic diseases, which are driven by Th2-type cytokines. In asthmatic patients, B7.2 expression on B cells was higher in atopic asthmatic subjects exposed to allergen than atopic asthmatic subjects not exposed to allergen or non-atopic control subjects [11]. In atopic dermatitis, we demonstrated that B7.2, but not B7.1 expression on fresh B cells is significantly higher than psoriasis or normal controls. Serum IgE level correlated well with B7.2 expression on B cells in atopic dermatitis patients and in vitro IgE production was blocked by anti-human B7.2, but not anti-human B7.1 MoAb [12]. These findings support the hypothesis that B7.2, but not B7.1, is an important costimulatory molecule involved in the pathogenesis of allergic response.
Therapeutic agents that down-regulate B7.2 molecule could therefore be of benefit for the treatment of Th2-driven allergic diseases. In the current study, we examined the effect of corticosteroids as well as cyclosporin A (CsA), two agents that have been used for the treatment of chronic asthma and atopic dermatitis, on the expression of B7.2 and B7.1 on B cells. We demonstrate suppressive effect of these two agents on B7.2+B7.1− but not on B7.2−B7.1+ B cells, and that the mechanism for this down-regulation is CD40-dependent.
SUBJECTS AND METHODS
Subjects
We obtained blood from six normal healthy adults (three males and three females, aged 27–36 years) who were skin prick test-negative for a panel of common allergens and had serum IgE levels <200 U/ml. Informed consent was obtained from all subjects before entry into the study.
Reagents
The following MoAbs were obtained from Becton Dickinson Immunocytometry Systems (San Jose, CA): anti-CD19 PerCP (peridinin chlorophyll-α protein), anti-B7.1 PE, G1CL (mouse IgG1 control; FITC, PE, PerCP) and G2CL (mouse IgG2 control; FITC, PE, PerCP). The anti-B7.2 PE-conjugated MoAb was obtained from Ancell (Bayport, MN). The anti-CD40 FITC- and anti-B7.1 FITC-conjugated MoAb was obtained from Pharmingen (San Diego, CA). Toxic shock syndrome toxin (TSST-1) was a kind gift from P. M. Schlievert (University of Minnesota) and prepared from Staphylococcus aureus by isoelectric focusing as previously described [13].
Cell preparation and cell cultures
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized venous blood from study subjects by density gradient centrifugation on Ficoll–Paque (Pharmacia, Uppsala, Sweden), and washed three times in Hanks' balanced salt solution (HBSS; Gibco, Grand Island, NY).
PBMC (at 1 × 106 cells/ml) were cultured in RPMI 1640 (Bio-Whitaker, Walkersville, MD) supplemented with 10% heat-inactivated fetal calf serum (FCS; Gibco), 20 mm HEPES buffer (Gibco), antibiotics and 2 mml-glutamine (Gibco) in flat-bottomed 24-well plates (Costar, Cambridge, MA) at 37°C in a humidified 5% CO2 atmosphere for 48 h. A range of TSST-1 concentrations was added into cell cultures in the presence or absence of dexamethasone (Sigma, St Louis, MO) plus or minus CsA (Sigma). Anti-human CD40 MoAb (Pharmingen) at 1 μg/ml was added in certain experiments to cross-link the CD40 molecule on B cells.
Cell staining and flow cytometric analysis
Five-parameter analysis was performed using a FACScalibur flow cytometer (Becton Dickinson) using FITC, PE and PerCP as the three fluorescent parameters. Immunofluorescence staining for this multiparameter analysis and methods of cytometer set up and data acquisition were performed as described previously [14]. List mode multiparameter data files (each file with forward scatter, side scatter, and three fluorescent parameters) were analysed using the Cell Quest MacIntosh program (Becton Dickinson). Analysis was performed using a light scatter gate including only viable lymphocytes and a gate based on expression of CD19+ B cells. Negative control reagents were used to verify the staining specificity of experimental antibodies.
Statistical analysis
Data are expressed as individual values and geometric mean for each subject groups. Data differences between paired groups were tested for normality. A paired Student's t-test was used for normally distributed data differences. In contrast, a Wilcoxon signed rank test was used for non-parametric data differences. Differences between groups were considered significant at P < 0.05.
RESULTS
TSST-1 increases B7.2+B7.1− but not B7.2−B7.1+ B cells
PBMC from six normal donors were analysed by flow cytometry for the expression of B7.2 and B7.1 on their B cells after culture for 48 h with a range of TSST-1 concentrations. As shown in Fig. 1, both B7.2 and B7.1 expression on B cells were up-regulated by TSST-1 in a dose-dependent fashion. As B7.2 and B7.1 have differential functions and can be expressed on the same cells, we further analysed for B7.2+B7.1−, B7.2+B7.1+ and B7.2−B7.1+ B cells by using anti-B7.1 FITC, anti-B7.2 PE and anti-CD19 PerCP. As shown in Fig. 2, B7.2+B7.1− and B7.2+B7.1+ B cells were up-regulated by TSST-1 in a dose-dependent fashion in the same way as B7.2+ B cells. In contrast, B7.2−B7.1+ B cells were down-regulated by TSST-1 in a dose-dependent fashion. Of interest, the majority of B7.2+ B cells were B7.2+B7.1−, while the majority of B7.1+ B cells were B7.2+B7.1+. For the remainder of this study, we used TSST-1 at 100 ng/ml as it provided optimal B7.2 induction.
Fig. 1.
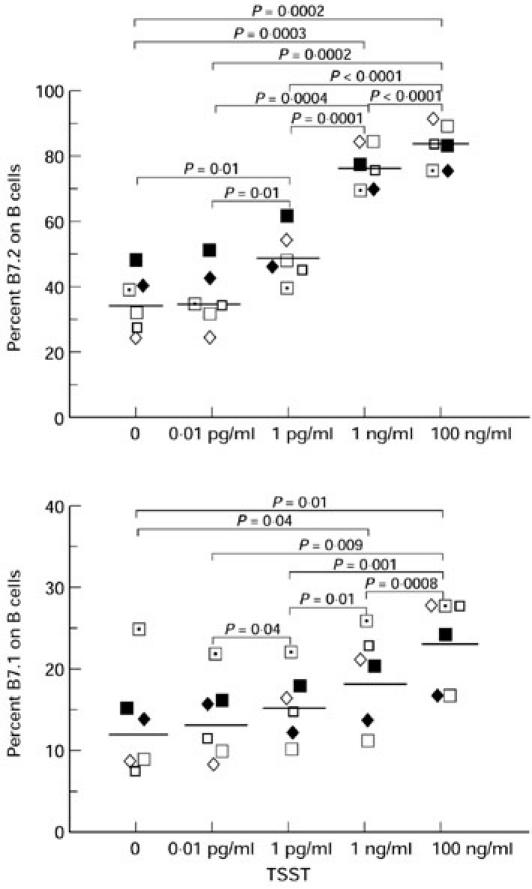
Toxic shock syndrome toxin-1 (TSST-1) up-regulates total B7.2 and B7.1 expression on B cells. Peripheral blood mononuclear cells (PBMC) from six normal donors were cultured with a range of TSST-1 concentrations for 48 h and analysed for B7.2 and B7.1 expression on B cells by flow cytometry. Results from each individual and geometric mean values are shown.
Fig. 2.
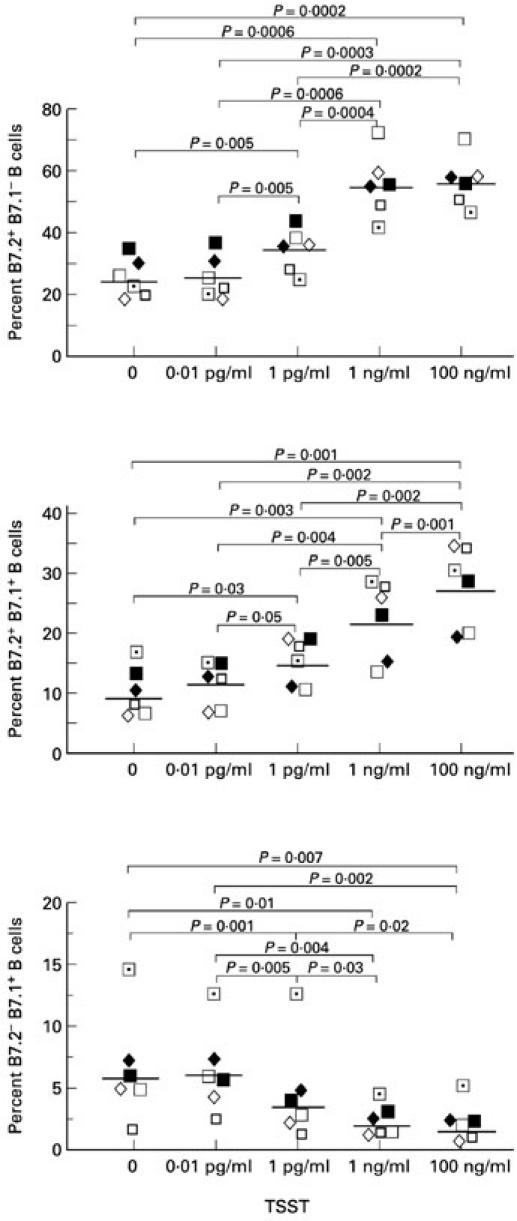
Toxic shock syndrome toxin-1 (TSST-1) up-regulates B7.2+B7.1− but not B7.2−B7.1+ B cells. Peripheral blood mononuclear cells (PBMC) from six normal donors were analysed by flow cytometry for the expression of B7.2 and B7.1 on the same B cells after culture for 48 h with a range of TSST-1 concentrations. Results from each individual and geometric mean values are shown.
CsA significantly decreases B7.2+B7.1− but not B7.2−B7.1+ B cells
In these experiments, PBMC were cultured for 48 h with or without TSST-1 in the presence or absence of 1 μg/ml CsA. As shown in Fig. 3, CsA significantly decreased the percentage of B7.2+B7.1− B cells in the presence or absence of TSST-1. In contrast, CsA significantly increased the percentage of B7.2−B7.1+ B cells in the presence or absence of TSST-1. CsA did not demonstrate any significant effect on B7.2+B7.1+ B cells (data not shown).
Fig. 3.
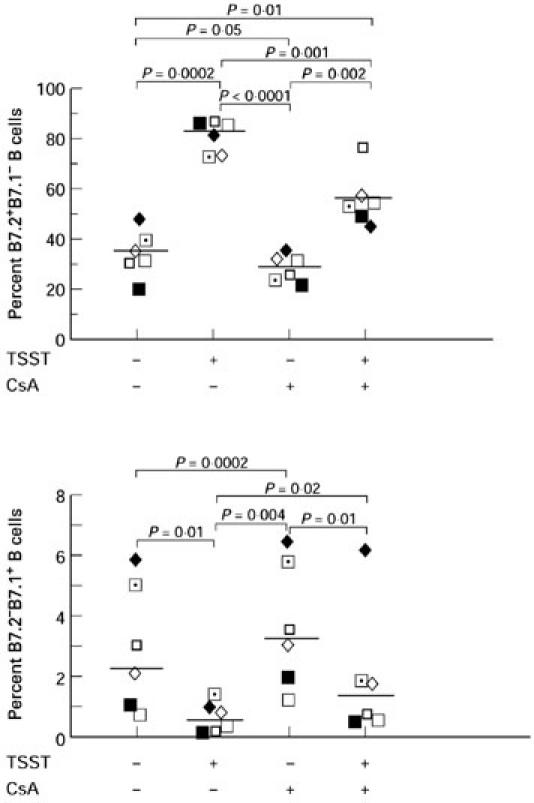
Cyclosporin A (CsA) significantly decreases B7.2+B7.1− but not B7.2−B7.1+ B cells. Peripheral blood mononuclear cells (PBMC) from six normal donors were cultured for 48 h with or without 100 ng/ml toxic shock syndrome toxin-1 (TSST-1) in the presence or absence of 1 μg/ml CsA. Cells were analysed by flow cytometry for the expression of B7.2 and B7.1 on the same B cells. Results from each individual and geometric mean values are shown.
Dexamethasone significantly decreases B7.2+B7.1− but not B7.2−B7.1+ B cells
In these experiments, PBMC were cultured for 48 h with or without TSST-1 in the presence or absence of 10−6m dexamethasone. As shown in Fig. 4, dexamethasone significantly decreased B7.2+B7.1− B cells in the presence or absence of TSST-1. In contrast, dexamethasone significantly increased B7.2−B7.1+ B cells in the presence or absence of TSST-1. Dexamethasone did not demonstrate any significant effect on B7.2+B7.1+ B cells (data not shown).
Fig. 4.
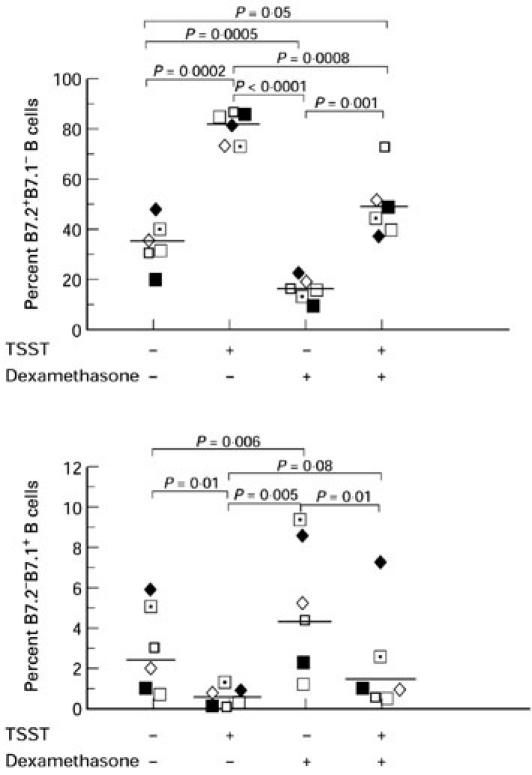
Dexamethasone significantly decreases B7.2+B7.1− but not B7.2−B7.1+ B cells. Peripheral blood mononuclear cells (PBMC) from six normal donors were cultured for 48 h with or without 100 ng/ml toxic shock syndrome toxin-1 (TSST-1) in the presence or absence of 10−6m dexamethasone. Cells were analysed by flow cytometry for the expression of B7.2 and B7.1 on the same B cells. Results from each individual and geometric mean values are shown.
CsA and dexamethasone reduce B7.2+B7.1− B cells in a synergistic manner
As both CsA and dexamethasone can create significant adverse effects in patients who use high doses of these agents, we examined whether they had a synergistic effect when used in combination at one log lower concentrations than in the previous experiments. In the left panel of Fig. 5, the combination of 100 ng/ml CsA and 10−6m dexamethasone significantly decreased B7.2+B7.1− B cells and increased B7.2−B7.1+ compared with either agent alone. This synergistic effect was also demonstrated in B7.2+B7.1+ B cells, which was significantly down-regulated by the combination of both agents.
Fig. 5.
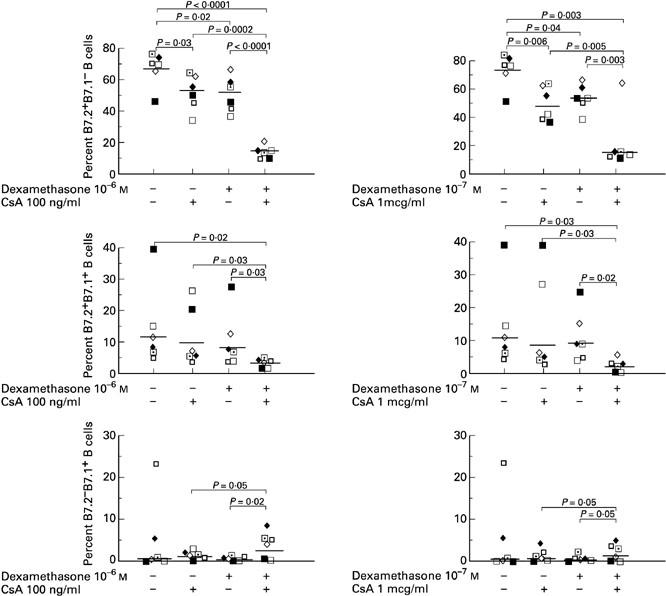
Cyclosporin A (CsA) and dexamethasone have synergistic effects on the down-regulation of B7.2+B7.1− B cells. Toxic shock syndrome toxin-1 (TSST-1; 100 ng/ml) was added in cell culture with or without 100 ng/ml CsA in the absence or presence of 10−6m dexamethasone (left panel), and with or without 1 μg/ml CsA in the absence or presence of 10−7m dexamethasone (right panel). In both panels, the combination of both agents showed significant synergistic effects compared with either agent alone in the down-regulation of B7.2+B7.1− B cells and up-regulation of B7.2−B7.1+ B cells. The synergistic effect can also be demonstrated in B7.2+B7.1+ B cells, which were significantly down-regulated by the combination of both agents. Results from individuals and geometric mean values are shown.
In the right panel of Fig. 5, the combination of 1 μg/ml CsA and 10−7m dexamethasone significantly decreased B7.2+B7.1− B cells and increased B7.2−B7.1+ compared with either agent alone. This synergistic effect was also demonstrated in B7.2+B7.1+ B cells, which was significantly down-regulated by the combination of both agents.
CsA and dexamethasone decrease CD40 expression on B cells
As CD40 is known to regulate B7.2 expression on B cells [15], we investigated whether CD40 expression on B cells was affected by CsA or dexamethasone, as this might explain the down-regulatory effects of CsA or dexamethasone on B7.2 expression on B cells. Figure 6a demonstrates that CD40 was up-regulated by TSST-1, and 1 μg/ml CsA significantly decreased the effect of TSST-1-induced CD40 expression on B cells. In Fig. 6b, 10−6m dexamethasone demonstrates significant reversal of the effects of TSST-1 on CD40 expression.
Fig. 6.
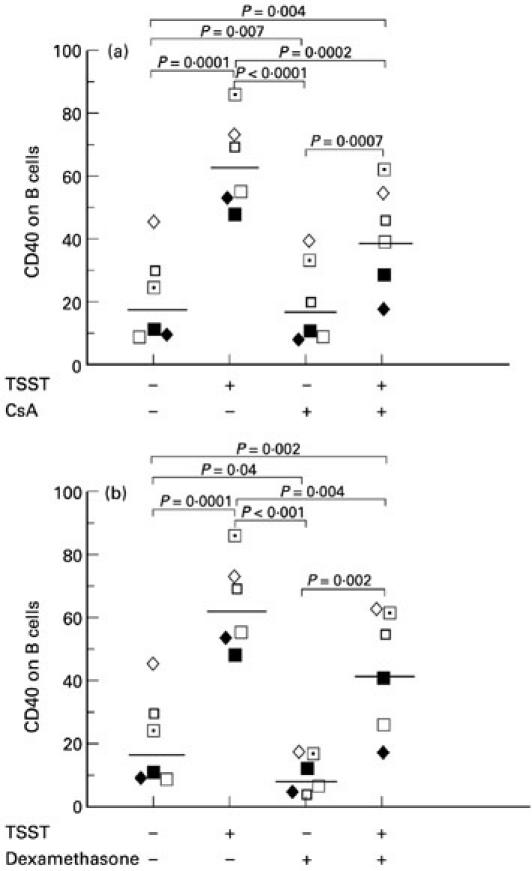
Cyclosporin A (CsA) and dexamethasone decrease CD40 expression on B cells. Peripheral blood mononuclear cells (PBMC) were cultured for 48 h with or without 100 ng/ml toxic shock syndrome toxin-1 (TSST-1) in the presence or absence of 1 μg/ml CsA (a) and in the presence or absence of 10−6m dexamethasone (b). These experiments demonstrate that CD40 was up-regulated by 100 ng/ml TSST-1, and 1 μg/ml CsA as well as dexamethasone significantly decreased the effect of TSST-1-induced CD40 expression on B cells. Results from individuals and geometric mean values are shown.
Anti-CD40 MoAb significantly increases B7.2+B7.1− and decreases B7.2−B7.1+ B cells when cultured with CsA or dexamethasone
To provide further support for the hypothesis that CsA or dexamethasone inhibit B7.2 expression on B cells through suppression of CD40 molecule, we added anti-CD40 MoAb, which is known to crosslink CD40 molecule on the B cell surface, into cell cultures with CsA or dexamethasone and analysed for B7.2+B7.1−, B7.2+B7.1+ and B7.2−B7.1+ B cells. As shown in Fig. 7, the combination of anti-CD40 MoAb plus CsA or dexamethasone significantly increased B7.2+B7.1−, decreased B7.2−B7.1+, but had no significant effects on B7.2+B7.1+ B cells (data not shown), compared with either CsA or dexamethasone alone without anti-CD40 MoAb.
Fig. 7.
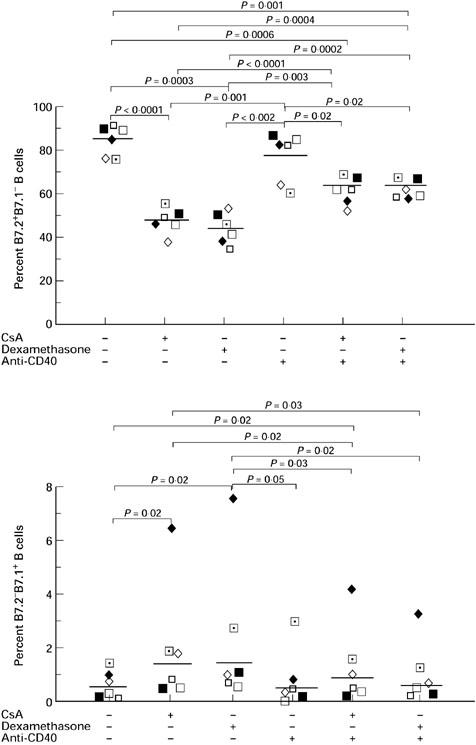
Anti-CD40 MoAb significantly increases B7.2+B7.1− and decreases B7.2−B7.1+ B cells in culture with cyclosporin A (CsA) or dexamethasone. Peripheral blood mononuclear cells (PBMC) were cultured for 48 h with 100 ng/ml toxic shock syndrome toxin-1 (TSST-1) in the presence or absence of 1 μg/ml CsA or 10−6m dexamethasone. Anti-CD40 MoAb at 1 μg/ml was added to activate CD40 molecule on B cells. These experiments demonstrate that anti-CD40 MoAb significantly reversed the effect of CsA and dexamethasone on B7.2+B7.1− and B7.2−B7.1+ B cells in the presence of TSST-1. Results from individuals and geometric mean values are shown.
DISCUSSION
Several recent studies support the concept that B7.2, but not B7.1, plays a significant role in atopic diseases. In murine models of allergen-induced airway hyperresponsiveness, administration of anti-B7.2, but not anti-B7.1, MoAb leads to the reduction of airway hyperresponsiveness and IgE as well as IL-4 and IL-5 levels in bronchoalveolar lavage fluid [16]. In studies of human PBMC cultured in vitro with house dust mite antigen, B7.2 but not B7.1 on B cells was significantly and selectively up-regulated in atopic patients, but not in normal subjects [17]. Moreover, antigen-specific T cell proliferation is effectively blocked by CTLA4-Ig and anti-B7.2 MoAb, but not by anti-B7.1 MoAb [18]. Our recent studies demonstrate that B7.2 but not B7.1 is up-regulated in atopic dermatitis and atopic asthmatics compared with control subjects [11,12]. IgE production in atopic dermatitis patients also correlated well with B7.2 expression on B cells [12].
TSST-1 is a staphylococcal toxin that can induce polyclonal T and B cell activation [19,20]. Several studies have shown that CD28/B7 counter-receptor molecules are important as a costimulatory signal in superantigen-induced T cell activation. CD28/B7-regulated signal transduction pathway is activated during superantigen stimulation of T cells and plays an important role in T cell proliferation and IL-2 gene expression [21–23]. These effects are blocked by CTLA-Ig [24]. In in vitro studies of anergic T cells stimulated by staphylococcal enterotoxin B (SEB), cross-linking of CD28 molecule on these T cells is critical for reversal of anergy [25]. The addition of antibodies to CD28 or B7 transfected cell lines increased the sensitivity of naive T cells to lower doses of superantigens [26]. Moreover, recent studies have demonstrated that TSST-1 significantly up-regulates B7 in a dose-dependent fashion [27]. We therefore used TSST-1 in this study as an agent to stimulate B7 molecules expressed on B cells and investigated the effects of immunosuppressive agents CsA and dexamethasone on B7.2 and B7.1 expression on B cells.
Although the importance of B7/CD28 interactions in superantigen-induced T and B cell activation has been identified, whether the B7.2 or B7.1 molecule is more involved in this process is still not resolved. Previous studies have reported that the inhibition of naive T cell responses in vitro to superantigens required simultaneous blocking of B7.1 and B7.2, suggesting that either B7.1 or B7.2 is sufficient to provide a costimulatory signal [28]. However, in vivo studies by the same group examining SEB-induced toxic shock syndrome in BALB/c mice demonstrated that a single dose of anti-B7.2, but not anti-B7.1, antibodies significantly inhibited T cell activation and lowered systemic IL-2 release, blastogenesis and IL-2 receptor expression [29]. Hofer et al. showed that TSST significantly induced B7.2 but not B7.1 on B cells [27].
Our current study demonstrates that TSST-1 up-regulated both B7.1 and B7.2 on B cells. However, the simultaneous analysis of both molecules on the same B cells revealed that the majority of B7.1+ cells were B7.2+B7.1+ cells, while the majority of B7.2+ cells were B7.2+B7.1− cells, and TSST-1 up-regulated B7.2+B7.1− and B7.2+B7.1+ cells but down-regulated B7.2−B7.1+ cells. To our knowledge, the current study is the first to demonstrate differential regulation of B7.2 and B7.1 by staining both molecules on the same cells. This finding might partially explain the controversy in B7.2 and B7.1 regulation as well as their diverse biological functions.
In this study, we investigated the effects of immunoregulatory agents on B7.2 and B7.1 molecules on B cells. We used dexamethasone as a prototype for corticosteroids, an agent commonly used for controlling allergic diseases. CsA is frequently used as an alternative agent in steroid-resistant asthma and recalcitrant atopic dermatitis unresponsive to corticosteroids. Our study showed that both CsA and dexamethasone significantly down-regulated B7.2+B7.1− B cells but up-regulated B7.2−B7.1+ B cells. Both agents demonstrated synergistic effects when used in combination, even though the concentration used was decreased by one log concentration.
To better understand the mechanism by which CsA and corticosteroid regulate B7.2 versus B7.1 expression on B cells, we investigated the effect of these agents on CD40 expression on B cells, since it has been demonstrated that CD40 signals an up-regulation of B7.2 on APC [15]. Our study reveals that CsA and dexamethasone suppressed CD40 expression on B cells after high-dose TSST-1 stimulation. The addition of anti-CD40 to cultures containing TSST-1 with CsA or dexamethasone reversed the suppressive effect of these two agents on B7 expression. Since anti-CD40 is known to crosslink and activate CD40 molecule on B cells, these results suggest that CsA and dexamethasone modulate B7 molecule by the suppression of CD40 expression on B cells or CD40L expression on T cells. Indeed, both mechanisms are likely to be operative.
In support of a direct effect of CsA or dexamethasone on B cells, several reports have demonstrated that transcription factor NF-κB is induced during B cell stimulation through CD40 receptor [30–33], and the ability of CD40 cross-linking to induce IgE germ-line transcription in B cells is mediated by activation of NF-κB [34,35]. Moreover, ligation of the CD40 receptor in conjunction with IL-4 induces the nuclear factor of activated T cells (NF-AT) [36]. It is well known that one of the mechanisms of corticosteroid action is mediated by blocking the induction of NF-κB [37,38], while CsA's action is mediated by blocking the induction of NF-AT [39]. In this regard, recent studies have demonstrated that CsA has direct effect on B cell proliferation and V(D)J recombination [40–42]. We propose that the mechanism of corticosteroid- and CsA-regulated B7 molecules on B cells is mediated through the inhibition of CD40-induced NF-κB or NF-AT signalling pathway. This finding may account for the synergistic action of these two immunosuppressive agents as they each act on a different component of the CD40 signalling pathway.
The addition of anti-CD40 into cell cultures with CsA or dexamethasone did not bring B7.2+B7.1− B cells back to the levels observed in cultures without CsA or dexamethasone. This suggests that other mechanisms, in addition to the blocking of CD40 expression, might also play a role in the mechanism by which CsA or dexamethasone regulate B7 molecule. It has been demonstrated that B7.2 is up-regulated by Th2 cytokines such as IL-4 and IL-13 [11]. Since both CsA and dexamethasone down-regulate IL-4 [43,44], it is possible that suppressing these T cell-derived cytokines might be another mechanism for B7 regulation by these immunosuppressive agents. Furthermore, the known ability of these two immunosuppressive agents to suppress CD40L expression by T cells provides an additional mechanism for reduced CD40-mediated B7.2 expression [45,46].
In conclusion, this study demonstrates that both corticosteroid and CsA regulate B7.2 and B7.1 on B cells. The results are more distinct if analyses for both molecules are done on the same B cells. Both agents demonstrate synergistic effect on B cells, leading to the potential to decrease doses and clinical side-effects. These findings provide new insights into potential mechanisms of these immunosuppressive agents and their ability to control expression of B7.2 in allergic diseases.
Acknowledgments
Supported by NIH grants AR41256, HL37260, HL3577, 5M01 RR00051 and the University of Colorado Cancer Center. The authors wish to thank Maureen Plourd-Sandoval for preparation of this manuscript. The work of O.J. was supported in part by the Florence Myers Goldhamer Fellowship in Pediatric Allergy and Immunology funded through National Jewish Medical and Research Center.
REFERENCES
- 1.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–58. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 2.Bluestone JA. New perspectives of CD28-B7-mediated T cell costimulation. Immunity. 1995;2:555–9. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 3.Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) B 7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 4.Ellis JH, Burden MN, Vinogradov DV, Linge C, Crowe JS. Interactions of CD80 and CD86 with CD28 and CTLA4. J Immunol. 1996;156:2700–9. [PubMed] [Google Scholar]
- 5.Morton PA, Fu XT, Stewart JA, et al. Differential effects of CTLA-4 substitutions on the binding of human CD80 (B7-1) and CD86 (B7-2) J Immunol. 1996;156:1047–54. [PubMed] [Google Scholar]
- 6.Kariv I, Truneh A, Sweet RW. Analysis of the site of interaction of CD28 with its counter-receptors CD80 and CD86 and correlation with function. J Immunol. 1996;157:29–38. [PubMed] [Google Scholar]
- 7.Kuchroo VK, Das MP, Brown JA, et al. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–18. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 8.Freeman GJ, Boussiotis VA, Anumanthan A, et al. B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL- 4. Immunity. 1995;2:523–32. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 9.Lanier LL, O'Fallon S, Somoza C, Phillips JH, Linsley PS, Okumura K, Ito D, Azuma M. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J Immunol. 1995;154:97–105. [PubMed] [Google Scholar]
- 10.Natesan M, Razi-Wolf Z, Reiser H. Costimulation of IL-4 production by murine B7-1 and B7-2 molecules. J Immunol. 1996;156:2783–91. [PubMed] [Google Scholar]
- 11.Hofer MF, Jirapongsananuruk O, Trumble AE, Leung DYM. Upregulation of B7.2, but not B7.1, on B cells from patients with allergic asthma. J Allergy Clin Immunol. 1998;101:96–102. doi: 10.1016/S0091-6749(98)70199-X. [DOI] [PubMed] [Google Scholar]
- 12.Jirapongsananuruk O, Hofer MF, Trumble AE, Norris DA, Leung DYM. Enhanced expression of B7.2 (CD86) in patients with atopic dermatitis: a potential role in the modulation of IgE synthesis. J Immunol. 1998;160:4622–7. [PubMed] [Google Scholar]
- 13.Blomster-Hautamaa DA, Schlievert PM. Preparation of toxic shock syndrome toxin-1. Methods Enzymol. 1988;165:37–43. doi: 10.1016/s0076-6879(88)65009-9. [DOI] [PubMed] [Google Scholar]
- 14.Leung DYM, Gately M, Trumble A, Ferguson-Darnell B, Schlievert PM, Picker LJ. Bacterial superantigens induce T cell expression of the skin-selective homing receptor, the cutaneous lymphocyte-associated antigen, via stimulation of interleukin 12 production. J Exp Med. 1995;181:747–53. doi: 10.1084/jem.181.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y, Wilson JM. CD40 ligand-dependent T cell activation: requirement of B7-CD28 signaling through CD40. Science. 1996;273:1862–4. doi: 10.1126/science.273.5283.1862. [DOI] [PubMed] [Google Scholar]
- 16.Keane-Myers AM, Gause WC, Finkelman FD, Xhou XD, Wills-Karp M. Development of murine allergic asthma is dependent upon B7-2 costimulation. J Immunol. 1998;160:1036–43. [PubMed] [Google Scholar]
- 17.Nakada M, Nishizaki K, Yoshino T, Okano M, Masuda Y, Ohta N, Akagi T. CD86 (B7-2) antigen on B cells from atopic patients shows selective, antigen-specific upregulation. Allergy. 1998;53:527–31. doi: 10.1111/j.1398-9995.1998.tb04091.x. [DOI] [PubMed] [Google Scholar]
- 18.Van Neerven RJ, Van de Pol MM, Van der Zee JS, Stiekema FE, De Boer M, Kapsenberg ML. Requirement of CD28–CD86 costimulation for allergen-specific T cell proliferation and cytokine expression. Clin Exp Allergy. 1998;28:808–16. doi: 10.1046/j.1365-2222.1998.00306.x. [DOI] [PubMed] [Google Scholar]
- 19.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–11. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 20.Mourad W, Scholl P, Diaz A, Geha R, Chatila T. The staphylococcal toxic shock syndrome toxin 1 triggers B cell proliferation and differentiation via major histocompatibility complex-unrestricted cognate T/B cell interaction. J Exp Med. 1989;170:2011–22. doi: 10.1084/jem.170.6.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraser JD, Newton ME, Weiss A. CD28 and T cell antigen receptor signal transduction coordinately regulate interleukin 2 gene expression in response to superantigen stimulation. J Exp Med. 1992;175:1131–4. doi: 10.1084/jem.175.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldbach-Mansky R, King PD, Taylor AP, Dupont B. A co-stimulatory role for CD28 in the activation of CD4+ T lymphocytes by staphylococcal enterotoxin B. Int Immunol. 1992;4:1351–60. doi: 10.1093/intimm/4.12.1351. [DOI] [PubMed] [Google Scholar]
- 23.Lando PA, Olsson C, Kalland T, Newton D, Kotb M, Dohlsten M. Regulation of superantigen-induced T cell activation in the absence and the presence of MHC class II. J Immunol. 1996;157:2857–63. [PubMed] [Google Scholar]
- 24.Saha B, Jaklic B, Harlan DM, Gray GS, June CH, Abe R. Toxic shock syndrome toxin-1-induced death is prevented by CTLA4Ig. J Immunol. 1996;157:3869–75. [PubMed] [Google Scholar]
- 25.Heeg K, Wagner H. Induction of responsiveness in superantigen-induced anergic T cells. Role of ligand density and costimulatory signals. J Immunol. 1995;155:83–92. [PubMed] [Google Scholar]
- 26.Muraille EM, De Becker G, Bakkus M, Thielemans K, Urbain J, Moser M, Leo O. Co-stimulation lowers the threshold for activation of naive T cells by bacterial superantigens. Int Immunol. 1995;7:295–304. doi: 10.1093/intimm/7.2.295. [DOI] [PubMed] [Google Scholar]
- 27.Hofer MF, Harbeck RJ, Schlievert PM, Leung DYM. Staphylococcal toxins augment specific IgE responses by atopic patients exposed to allergen. J Invest Dermatol. 1999;112:171–6. doi: 10.1046/j.1523-1747.1999.00492.x. [DOI] [PubMed] [Google Scholar]
- 28.Muraille E, De Smedt T, Thielemans K, Urbain J, Moser M, Leo O. Activation of murine T cells by bacterial superantigens requires B7- mediated costimulation. Cell Immunol. 1995;162:315–20. doi: 10.1006/cimm.1995.1084. [DOI] [PubMed] [Google Scholar]
- 29.Muraille E, De Smedt T, Urbain J, Moser M, Leo O. B7. 2 provides co-stimulatory functions in vivo in response to staphylococcal enterotoxin B. Eur J Immunol. 1995;25:2111–4. doi: 10.1002/eji.1830250747. [DOI] [PubMed] [Google Scholar]
- 30.Berberich I, Shu GL, Clark EA. Cross-linking CD40 on B cells rapidly activates nuclear factor-kappa B. J Immunol. 1994;153:4357–66. [PubMed] [Google Scholar]
- 31.Lalmanach-Girard AC, Chiles TC, Parker DC, Rothstein TL. T cell-dependent induction of NF-kappa B in B cells. J Exp Med. 1993;177:1215–9. doi: 10.1084/jem.177.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsing Y, Hostager BS, Bishop GA. Characterization of CD40 signaling determinants regulating nuclear factor-kappa B activation in B lymphocytes. J Immunol. 1997;159:4898–906. [PubMed] [Google Scholar]
- 33.Francis DA, Karras JG, Ke XY, Sen R, Rothstein TL. Induction of the transcription factors NF-kappa B, AP-1 and NF-AT during B cell stimulation through the CD40 receptor. Int Immunol. 1995;7:151–61. doi: 10.1093/intimm/7.2.151. [DOI] [PubMed] [Google Scholar]
- 34.Yanagihara Y, Basaki Y, Ikizawa K, Kajiwara K. Possible role of nuclear factor-kappa B activity in germline C epsilon transcription in a human Burkitt lymphoma B cell line. Cell Immunol. 1997;176:66–74. doi: 10.1006/cimm.1996.1071. [DOI] [PubMed] [Google Scholar]
- 35.Iciek LA, Delphin SA, Stavnezer J. CD40 cross-linking induces Ig epsilon germline transcripts in B cells via activation of NF-kappaB: synergy with IL-4 induction. J Immunol. 1997;158:4769–79. [PubMed] [Google Scholar]
- 36.Choi MS, Brines RD, Holman MJ, Klaus GG. Induction of NF-AT in normal B lymphocytes by anti-immunoglobulin or CD40 ligand in conjunction with IL-4. Immunity. 1994;1:179–87. doi: 10.1016/1074-7613(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 37.Mukaida N, Morita M, Ishikawa Y, Rice N, Okamoto S, Kasahara T, Matsushima K. Novel mechanism of glucocorticoid-mediated gene repression. Nuclear factor-kappa B is target for glucocorticoid-mediated interleukin 8 gene repression. J Biol Chem. 1994;269:13289–95. [PubMed] [Google Scholar]
- 38.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–90. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 39.Flanagan WM, Corthesy B, Bram RJ, Crabtree GR. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991;352:803–7. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- 40.Venkataraman C, Muthusamy N, Muthukkumar S, Bondada S. Activation of lyn, blk, and btk but not syk in CD72-stimulated B lymphocytes. J Immunol. 1998;160:3322–9. [PubMed] [Google Scholar]
- 41.Newton JS, Li J, Ning ZQ, Schoendorf DE, Norton JD, Murphy JJ. B cell early response gene expression coupled to B cell receptor, CD40 and interleukin-4 receptor co-stimulation: evidence for a role of the egr-2/krox 20 transcription factor in B cell proliferation. Eur J Immunol. 1996;26:811–6. doi: 10.1002/eji.1830260413. [DOI] [PubMed] [Google Scholar]
- 42.Dobbeling U. The effects of cyclosporin A on V(D)J recombination activity. Scand J Immunol. 1997;45:494–8. doi: 10.1046/j.1365-3083.1997.d01-426.x. [DOI] [PubMed] [Google Scholar]
- 43.Guyre PM, Girard MT, Morganelli PM, Manganiello PD. Glucocorticoid effects on the production and actions of immune cytokines. J Steroid Biochem. 1988;30:89–93. doi: 10.1016/0022-4731(88)90080-5. [DOI] [PubMed] [Google Scholar]
- 44.Andersson J, Nagy S, Groth CG, Andersson U. Effects of FK506 and cyclosporin A on cytokine production studied in vitro at a single-cell level. Immunology. 1992;75:136–42. [PMC free article] [PubMed] [Google Scholar]
- 45.Fuleihan R, Ramesh N, Horner A, et al. Cyclosporin A inhibits CD40 ligand expression in T lymphocytes. J Clin Invest. 1994;93:1315–20. doi: 10.1172/JCI117089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bischof F, Melms A. Glucocorticoids inhibit CD40 ligand expression of peripheral CD4+ lymphocytes. Cell Immunol. 1998;187:38–44. doi: 10.1006/cimm.1998.1308. [DOI] [PubMed] [Google Scholar]


