Abstract
Cytokines play a crucial role in initiating and perpetuating EAE, an animal model of multiple sclerosis (MS). A low dose of IL-4, administered by the nasal route over 5 days (100 ng/rat per day) prior to immunization, improved clinical scores of EAE induced in Lewis rats with myelin basic protein (MBP) peptide 68–86 (MBP 68–86). We examined whether dendritic cells (DC) may have contributed to the amelioration of the disease process. These professional antigen-presenting cells (APC) not only activate T cells, but also tolerize T cells to antigens, thereby minimizing autoimmune reactions. We found that IL-4 administration enhanced proliferation of DC. In comparison with DC of PBS-treated rats, DC from IL-4-treated rats secreted high levels of interferon-gamma (IFN-γ) and IL-10. Nitric oxide (NO) production by DC was also strongly augmented in IL-4-treated rats. In vitro studies showed that IL-4 stimulated DC expansion and that IFN-γ enhanced NO production by DC. DC-derived NO promoted apoptosis of autoreactive T cells. These results indicate that nasal administration of IL-4 promotes activation of DC and induces production of IFN-γ and IL-10 by DC. IL-10 suppresses antigen presentation by DC, while IFN-γ induces NO production by DC which leads to apoptosis in autoreactive T cells. Such a DC-derived negative feedback loop might contribute to the clinical improvement observed in EAE.
Keywords: experimental allergic encephalomyelitis, IL-4, dendritic cells, nitric oxide
INTRODUCTION
Multiple sclerosis (MS) is a chronic demyelinating disease of the central nervous system (CNS). Its precise aetiology is unknown and therapeutic interventions are limited. However, the perivenous distribution of the demyelinating lesions, the presence of macrophages, lymphocytes, and plasma cells within them [1], and the similarities between MS and experimentally induced EAE [2], suggest an autoimmune component in pathogenesis. It is thus likely that targeting of the immune system, either to divert it along a more benign pathway, or to directly block tissue-damaging effector mechanisms, may result in disease control.
Cytokines play a key role in immunity because of their functional pleiotropism and redundancy. They have been widely used in disease prevention and treatment. However, systemic administration of cytokines has resulted in some severe adverse effects and toxicity [3]. Recent studies suggest that administration of cytokines via mucosal routes offers an exciting alternative to systemic application. Increasing evidence indicated that oral administration of certain cytokines is not only safe and effective, but also avoids the deleterious consequences of systemic administration [4]. However, inactivation by gastric acid and digestion by proteases may affect orally administered molecules before they reach the target cells. Concerning this aspect, nasal administration is more effective than the intragastric route, generating earlier and stronger mucosal immune responses. Nasal lymphoid tissue and its local draining lymph nodes may also retain long-term immune memory [5]. We have previously shown that nasal administration of low doses of IL-10 can effectively suppress both acute and protracted-relapsing EAE in rats [6]. In this study, we found that nasal administration of as low as 500 ng/rat of IL-4 suppressed EAE in Lewis rats. IL-4 promoted activation of dendritic cells (DC) and induced production of IL-10 and interferon-gamma (IFN-γ) by DC. IFN-γ triggered nitric oxide (NO) production by DC, which together with IL-10, suppressed the development of EAE by inducing apoptosis of CD4+ T cells and inhibiting antigen presentation of DC. Such a DC-derived negative feedback loop might contribute to improvement of EAE.
MATERIALS AND METHODS
Reagents
Guinea pig myelin basic protein (MBP) peptide covering the amino acid residues 68–86 (MBP 68–86) (YGSLPQKSQRSQDENPV) was synthesized in an automatic Tecan/Syro Synthesizer (Multisyntech, Bochum, Germany). Murine IL-4 (specific activity 5.98 × 107–1.98 × 108 U/mg) was from Schering-Plough Research Institute (Kenilworth, NJ), and can act in rats. Recombinant rat IFN-γ (rrIFN-γ) and mouse anti-rat IFN-γ MoAb (DB1) were from Innogenetics (Ghent, Belgium). Anti-rat CD4 and MHC class II MoAbs were purified from culture supernatant of hybridoma [7]. PE-conjugated mouse anti-rat CD45RA MoAb, anti-rat macrophage MoAb (ED1), anti-rat CD3, CD11c, Mac-1, and isotype control antibodies were purchased from SeroTec (Oxford, UK). Monoclonal anti-rat B7-2 and OX-42 were from PharMingen (Becton Dickinson, Stockholm, Sweden). The modified Griess reagent, Nω-nitrol-l-arginine methylester (L-NAME) and lipopolysaccharide (LPS) were from Sigma (St Louis, MO), polyclonal goat anti-rat IL-10 from Santa Cruz Biotechnology (Santa Cruz, CA), and biotin-conjugated anti-goat secondary antibody from Dakopatts (Copenhagen, Denmark).
Animals
Lewis rats, 6–8 weeks old, were from Zentralinstitut fur Versuchstierzucht (Hannover, Germany).
Nasal administration of IL-4
Four groups of rats received into nostrils 60 μl PBS pH 7.4 containing IL-4 at amounts of 0, 10 ng, 100 ng, and 1000 ng, respectively, using a micropipette. At each administration, rats were gently anaesthetized with ether. The administration was performed daily for 5 consecutive days. Thus, the total amount of IL-4 received by each rat in the four groups was: 0, 50 ng, 500 ng, 5000 ng/rat, respectively.
To examine whether the IL-4 entered the bloodstream, it was labelled with deoxyadenosine-5′-thiotriphosphate (35S) (New England Nuclear, Cambridge, MA) and administered to rats nasally. Blood was collected from the heart at 6 h, 12 h and 24 h afterwards. Radioactivity was measured in a liquid β-scintillation counter.
Induction of EAE
Seven days after the last nasal administration, each rat was immunized subcutaneously in hind footpads with 200 μl inoculum containing 25 μg MBP 68–86, 2 mg Mycobacterium tuberculosis (strain H37RA; Difco, Detroit, MI), 100 μl saline and 100 μl Freund's incomplete adjuvant (FIA; Difco). Rats were weighed and evaluated in a blinded fashion by at least two investigators every day for the presence of clinical signs. Clinical scores of EAE were graded according to the following criteria: 0, asymptomatic; 1, flaccid tail; 2, loss of righting reflex with or without partial hind limb paralysis; 3, complete hind limb paralysis; 4, moribund; 5, dead.
Culture and purification of DC
The culture medium used was Dulbecco's modification of Eagle's medium (Gibco, Paisley, UK) supplemented with 1% modified Eagle's medium (MEM; Gibco), 2 mm glutamine (Flow Labs, Irvine, UK), 50 U/ml penicillin and 50 μg/ml streptomycin (Gibco), 10 mm HEPES and 5% fetal calf serum (FCS; Gibco). DC were prepared according to Steinman et al. [8] with modifications. Mononuclear cell (MNC) suspensions were prepared from rat spleen by pressing the tissues through a sterile wire mesh to obtain a single-cell suspension. DC were further enriched by differential adherence by incubating cells in 75-mm2 Falcon culture flasks (Becton Dickinson, Franklin Lakes, NJ) in serum-free culture medium for 2 h at 37°C in a 5% CO2incubator, removing the non-adherent fraction and culturing the remaining cells overnight in culture medium containing 5% FCS. By the next day, DC became non-adherent. These transiently adherent cells were harvested, resuspended in culture medium, and used as a splenic DC-enriched fraction. Enrichment of DC was verified by morphological appearance, immunocytochemistry and flow cytometric analysis.
Measurement of nitrite
NO was assayed by measuring the end product nitrite, which was determined by a colourimeter assay based on the Griess reaction. Aliquots of cell culture supernatant (100 μl) were mixed with 100 μl of Griess reagent at room temperature for 10 min. The absorbance was measured at 540 nm in an automated plate reader. Concentration of nitrite was determined by reference to a standard curve of sodium nitrite (Sigma).
Proliferation assay of DC
Proliferative responses of DC were examined by 3H-thymidine incorporation. Briefly, 200 μl of DC suspensions (1 × 106/ml) were incubated in 96-well polystyrene microtitre plates (Nunc, Roskilde, Denmark) at 37°C in a 5% CO2incubator with or without LPS (10 μg/ml). After 60 h, cells were pulsed with 3H-thymidine (1 μCi/well; Amersham, Aylesbury, UK) for 12 h. Cells were harvested and 3H-thymidine incorporation was measured in a liquid β-scintillation counter.
Assay of IFN-γ-secreting DC
An ELISPOT assay for detection of IFN-γ secretion at the single-cell level was used [9]. Nitrocellulose-bottomed microtitre plates (Millititre-HAM plates; Millipore Co., Bedford, UK) were coated with 100-μl aliquots of mouse anti-rat IFN-γ MoAb (DB1) at 15 μg/ml. DC suspensions (1 × 105 cells/200 μl) were added to individual wells, and incubated with or without LPS (10 μg/ml). After 48 h of culture, the wells were extensively washed. The plates were incubated with 100 μl of polyclonal rabbit anti-rat IFN-γ antibody (Innogenetics) diluted 1:500 for 4 h at room temperature. After washing, the plates were incubated with biotinylated swine anti-rabbit IgG (1:500; Dakopatts) and then avidin-biotin peroxidase complex (1:200; Vector, Burlingame, CA) followed by peroxidase staining. The red-brown immunospots which corresponded to the cells that had secreted IFN-γ were counted in a dissection microscope.
Preparation of T cells from draining lymph nodes
T cells were prepared according to Julius et al. [10] with modifications. In brief, on day 14 p.i., MNC suspensions from popliteal and inguinal lymph nodes of EAE rats were prepared by grinding through a wire mesh. Cells were washed three times, then incubated in serum-free medium in culture dishes for 2 h at 37°C in a 5% CO2incubator. The non-adherent fraction was collected and passed through a 20-ml nylon wool column. T cells were enriched in the non-adherent fraction.
Immunohistochemistry
On day 14 p.i., the spinal cords from PBS- and IL-4 (500 ng/rat)-treated EAE rats were dissected and frozen in liquid nitrogen. Cryostat sections were cut at 10 μm and fixed in acetone for 10 min. Endogenous peroxidase activity was inactivated with 0.3% H2O2for 20 min. Non-specific binding sites were further blocked with 1% blocking reagent (Boehringer Mannheim, Mannheim, Germany). The sections were incubated overnight in primary anti-CD4 and macrophage (ED1) antibodies at a dilution of 1:100. Reactivity was detected using the ABC (Vector) reactive system. The specificity of the staining was tested by incubating sections without the primary antibodies. For each animal, four spinal cord sections were observed in a blinded fashion. Positive cells were counted by automatic video scanning using Leica Q500MC.
Intracellular staining of cytokines
Intracellular cytokine staining of DC was performed according to de Boer et al. [11] with modifications. Briefly, DC were spun onto a glass slide and stored at −20°C until use. Slides were dried, fixed with acetone, lysed with 0.2% Triton, and subsequently incubated with polyclonal goat anti-rat IL-10 (1:100) and biotin-conjugated anti-goat secondary antibody (1:100). All incubations were performed in PBS containing 1% bovine serum albumin (BSA). The specificity of staining was tested by incubating slides without the primary antibodies. Percentage of positive cells was counted by automatic video scanning using Leica Q500MC.
In vitro stimulation of DC with IL-4 and IFN-γ
Naive DC were prepared from spleens of non-immunized rats as described before. Cells (1 × 106/ml) were incubated without or with IL-4 at a final concentration of 10 ng/ml, or IFN-γ at 100 U/ml.
Assays of apoptosis
Apoptosis was determined by using In Situ Cell Death Detection Kit (Boehringer Mannheim). At day 14 p.i., DC from PBS- or IL-4 (500 ng/rat)-treated EAE rats as well as T cells from non-treated EAE rats were prepared and co-cultured (DC:T ratio at 1:20) with or without MBP 68–86 (10 ng/ml), or withMBP 68–86 (10 ng/ml) plus L-NAME (0.5 mm) for 24 h. Cell suspensions were fixed with 4% paraformaldehyde for 30 min at room temperature and then permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate for 2 min on ice. TUNEL reaction mixture (50 μl) was added to samples incubated in a humidified chamber for 60 min at 37°C in the dark. Cell suspensions were then incubated with anti-rat CD4 and PE-conjugated secondary MoAb (Serotec). The cells were analysed with a FACScan flow cytometer (Becton Dickinson).
Statistical analysis
Differences between pairs of groups were tested with Student's t-test.
RESULTS
Nasal administration of IL-4 suppresses EAE in Lewis rats
To study whether nasal IL-4 administration could prevent EAE, Lewis rats received PBS or IL-4 before induction of EAE. Rats receiving PBS or 50 ng/rat IL-4 developed severe EAE, with no difference between the two groups in mean maximal clinical scores. In contrast, rats receiving the middle dose (500 ng/rat) or high dose (5000 ng/rat) of IL-4 exhibited only mild EAE (Fig. 1).
Fig. 1.
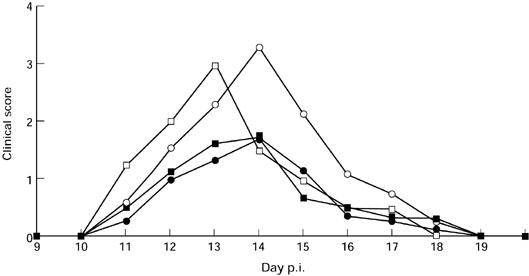
Nasal administration of IL-4 suppresses EAE in Lewis rats. Four groups of rats (n = 5 in each group) received IL-4 at amounts of 0, 10 ng, 100 ng, and 1000 ng daily for 5 consecutive days before EAE induction. Two independent experiments revealed similar results. ○, PBS; □, IL-4 50 ng; •, IL-4 500 ng; ▪, IL-4 5000 ng.
To examine whether the IL-4 entered the bloodstream, it was labelled with 35S and administered to Lewis rats nasally. The radioactivity levels in blood were high 6 h after administration and declined over the next 18 h (Fig. 2).
Fig. 2.
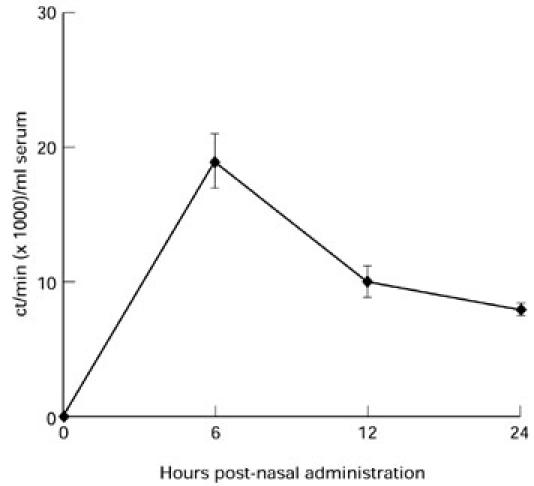
IL-4 delivered by the nasal route rapidly entered the blood stream. IL-4 was labelled with deoxyadenosine-5′-thiotriphosphate (35S) and administered nasally to Lewis rats. Blood was collected afterwards and radioactivity was measured. Data are expressed as the mean of triplicate samples ± s.d. Data are representative of two independent experiments with similar results.
IL-4 reduces inflammatory CD4+ T cell and macrophage infiltration within the CNS
In EAE, large numbers of infiltrating cells have been found within the CNS and are thought to contribute to disease pathogenesis [12]. In this study, we found that at day 14 p.i., infiltrating ED1+ macrophages and CD4+ T cells were significantly reduced in sections of spinal cords from IL-4-treated rats (500 ng/rat) compared with PBS-treated control rats (Fig. 3). This is consistent with the clinical signs in the animals.
Fig. 3.
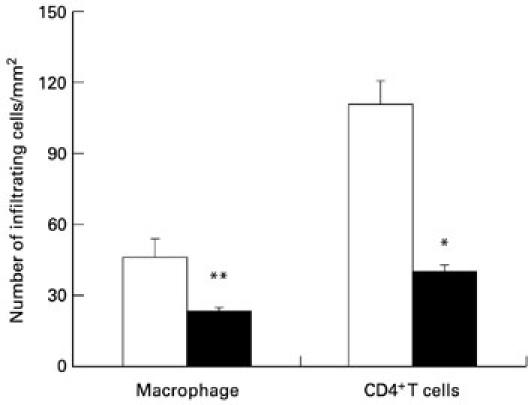
IL-4 reduced CD4+ T cell and macrophage infiltration into the central nervous system (CNS). On day 14 p.i., the spinal cords from PBS- (□) and IL-4 (500 ng/rat) (▪)-treated rats were dissected. Numbers of infiltrating cells were detected by immunohistochemical staining. Data are representative of two independent experiments with similar results. *P < 0.05; **P < 0.01.
Characterization of spleen DC-enriched fraction
DC have been characterized from different lymphoid and non-lymphoid organs, and from several species of mammals. In each instance, the cells exhibit the following features: (i) large size and irregular shape with many mobile veils (lamellipodia); (ii) abundant surface MHC class I and II; (iii) high levels of costimulatory molecules that enhance T cell responses; (iv) absence or low levels of critical macrophage markers; and (v) presence of certain markers that, while not entirely DC-specific, help to distinguish DC from other leucocytes [13].
In our DC preparations, when isolated and dropped onto slides, the cells showed characteristic Giemsa and surface MHC class II labelling patterns. We used several surface markers to assess the purity of fresh splenic DC preparations. They showed high frequencies of CD11c+ (81.3 ± 1.9%; mean ± s.d.), MHC class II+ (86.3 ± 2.0%) and B7-2+ (89.0 ± 2.5%) cells. In order to distinguish DC from macrophages, we chose two macrophage markers, OX-42 and Mac-1 [14,15]. Their expressions were uncommon, amounting to 13.3 ± 1.2% for OX-42, and 14.4 ± 0.8% for Mac-1. Contamination by CD3+ cells (2.3 ± 0.5%) and CD45RA+ cells (1.98 ± 0.6%) was also very low. Taken together, morphological and phenotypic studies revealed that the DC preparations used in this study had a purity of >85%. No significant difference in the number, phenotype and purity of DC was found after IL-4 administration compared with PBS-treated rats.
Nasal administration of IL-4 induces DC activation
DC can capture, process and present antigens to T cells, and secrete cytokines and mediators to participate in immune responses. To elucidate the mechanisms in the suppression of EAE after IL-4 administration, splenic DC were obtained from PBS- and IL-4 (500 ng/rat)-treated EAE rats at day 14 p.i., and their activities were evaluated. Compared with PBS-treated rats, DC from IL-4-treated rats showed higher proliferative responses (Fig. 4a), produced more NO (Fig. 4b), and secreted higher levels of IFN-γ (Fig. 4c), both spontaneously and upon stimulation with LPS. IL-4 administration thus triggered DC activation. At the same time, intracellular IL-10 staining of DC showed increased percentages of IL-10+ DC after IL-4 treatment (Fig. 4d).
Fig. 4.
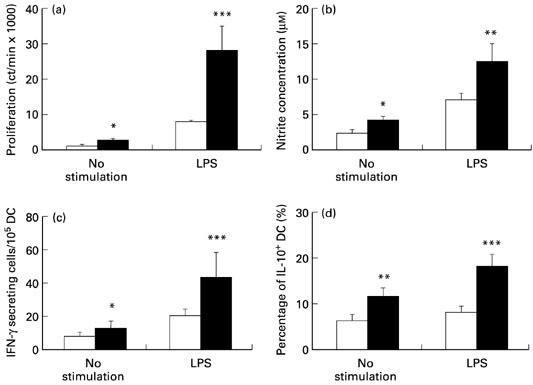
Nasal IL-4 activates splenic dendritic cells (DC). DC were enriched from PBS- (□) and IL-4 (500 ng/rat) (▪)-treated rats on day 14 p.i., and their lipopolysaccharide (LPS) responses were evaluated by measuring (a) 3H-thymidine incorporation; (b) nitric oxide (NO) production; (c) IFN-γ-secreting DC; (d) intracellular IL-10+ DC, both spontaneously and upon stimulation with LPS (10 μg/ml). Data are representative of two independent experiments with similar results. *P < 0.05; **P < 0.01; ***P < 0.001.
We compared the levels of proliferation, NO production, IFN-γ-secreting cells and IL-10+ cells among spleen MNC from PBS- and IL-4 (500 ng/rat)-treated rats. There was no difference between these two groups, indicating that the augmentations seen in Fig. 4 were mainly generated from DC. However, the contaminating macrophages, T cells and B cells, although in small proportions, could possibly contribute to the LPS responses and cytokine productions.
In vitro studies of effects of IL-4 and IFN-γ on DC
To analyse further the effects of IL-4 on DC activities, naive DC were incubated in the presence or absence of IL-4 (10 ng/ml). This significantly enhanced proliferation of DC (Fig. 5a), whereas IFN-γ-secreting cells were not augmented at this concentration of IL-4 (Fig. 5b). When DC were cultured with IFN-γ (100 U/ml) for 48 h, NO production was dramatically augmented (Fig. 5c). These data suggest that IL-4 induced DC expansion, and IFN-γ promoted NO production by DC.
Fig. 5.
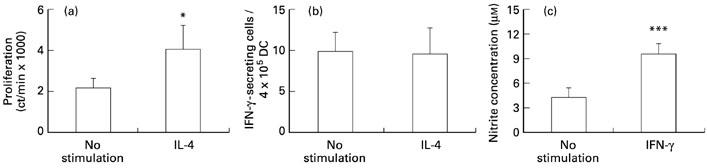
Effects of IL-4 and IFN-γ on dendritic cells (DC). Naive DC were incubated in the absence or presence of IL-4 (10 ng/ml) and assayed for 3H-thymidine incorporation (a) and IFN-γ production (b). Naive DC were incubated and detected for nitric oxide (NO) production in response to IFN-γ (100 U/ml) (c). Two independent experiments revealed similar results. *P < 0.05; ***P < 0.001.
DC-derived NO mediates apoptosis of CD4+ T cells
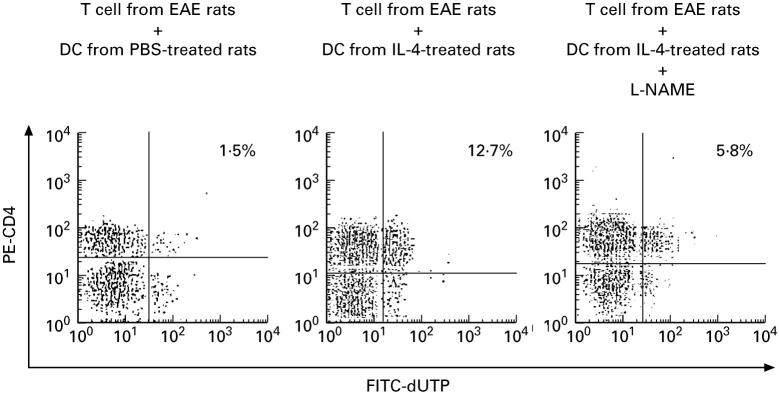
It has been reported that DC-derived NO is associated with impaired T cell responses [16]. In the present study, on day 14 p.i., T cells from EAE rats were incubated with DC from PBS- or IL-4-treated (500 ng/rat) rats. In the absence of MBP 68–86 stimulation, the levels of apoptosis were quite low in both co-cultures (0.21 ± 0.07% for T + DC from PBS-treated rats; 0.26 ± 0.07% for T + DC from IL-4-treated rats; P > 0.05), which could be bystander effects such as Fas/FasL-mediated apoptosis. Upon stimulation with MBP 68–86 (10 ng/ml), as shown in Fig. 6, apoptotic CD4+ T cells amounted to only 1.5% after 24 h of incubation in co-cultures of T cells and DC from PBS-treated rats. In co-cultures of T cells and DC from IL-4-treated rats, much higher levels of apoptosis (12.7%) of CD4+ T cells were observed; if the nitric oxide synthase (NOS) inhibitor L-NAME was added into the co-cultures, the percentage of apoptotic CD4+ T cells was reduced to 5.8%. Therefore, DC-induced apoptosis of CD4+ T cells is partly NO-dependent.
Fig. 6.
Dendritic cell (DC)-derived nitric oxide (NO) induces apoptosis of CD4+ T cells. On day 14 p.i., T cells obtained from EAE rats immunized with myelin basic protein (MBP) 68–86 were incubated with DC from PBS- or IL-4 (500 ng/rat)-treated rats plus MBP 68–86 (10 ng/ml) for 24 h in the absence or presence of Nω-nitrol-l-arginine methylester (L-NAME; 0.5 mm). At least 10 000 cells per sample were analysed by FACScan. The value given in each panel is the percentage of double-labelled cells. Data are representative of two independent experiments with similar results.
DISCUSSION
IL-4 has been called the ‘prototypic immunoregulatory cytokine’ [17] and extensively studied [18]. It influences a variety of target cells in multiple ways, and has been widely used in immunotherapies for cancers, infectious diseases, transplantation and autoimmune diseases [19–22]. Racke et al. reported that i.p. injection of IL-4 (1 μg per 8 h from day 0 to 11 or 6 to 11 p.i.) ameliorated EAE in mice [23]. In our study, we found that nasal administration of as little as 500 ng/rat of IL-4 suppressed MBP 68–86-induced EAE in Lewis rats. Immunohistochemical staining revealed considerably lower numbers of infiltrating cells in spinal cords from IL-4-treated rats compared with PBS-treated rats. Racke et al. explained the suppressive effect of IL-4 in EAE as ‘cytokine-induced immune deviation’. In our study, we found that IL-4 might affect the antigen-presenting functions of DC, thereby influencing the subsequent immune cascade during the disease course.
DC are very potent antigen-presenting cells (APC) and are critically involved in the initiation of immune responses. They constitute a small subpopulation of bone marrow-derived leucocytes that are widely distributed throughout the body. In response to infection, entrance of toxic chemicals or necrosis, DC rapidly undergo a maturation process, migrate into peripheral immune organs and initiate immune responses. All these processes appear to be dependent on the influence of cytokines. IL-4 is an essential factor for in vitro growth and differentiation of DC precursors [24,25]. Here we reported that both in vivo administration and in vitro addition of IL-4 enhanced DC activation and proliferation.
Nasal administration of IL-4 increased the number of intracellular IL-10-positive DC. IL-10 has been identified as critical for suppression of multiple activities of immune responses, including antigen presentation and T cell responses [26]. IL-10 inhibits the stimulatory capacity of APC by down-regulating MHC class II molecules and the costimulatory molecules B7-1/B7-2 and intercellular adhesion molecule-1 (ICAM-1) [27–29]. It can also directly inhibit T cell proliferation and growth [30,31], and reduce the release of a variety of inflammatory cytokines, including tumour necrosis factor-alpha (TNF-α) and TNF-β [32–35]. DC precultured with IL-10 induced a state of alloantigen-specific anergy among CD4+ T cells, suggesting that IL-10 converts immature DC into tolerogenic APC, which might be a useful tool in the therapy of patients with autoimmune diseases [36].
Although IFN-γ is a proinflammatory cytokine and is suggested to be involved in the pathogenesis of EAE and MS [37], there are also data implying that IFN-γ could contribute to disease resistance and recovery [38,39]. Therefore, the role of IFN-γ in disease induction and perpetuation may be rather complex. IFN-γ is a known NO inducer. Our data showed that short-term in vitro stimulation of DC with IFN-γ dramatically increased NO production.
NO is a major modulator of physiological and pathophysiological processes, and plays a critical role in inflammation and autoimmunity [40]. NO derived from DC is associated with impaired T cell responses and enhances apoptosis of DC themselves [16,41]. CD4+ T cell apoptosis was enhanced when cultured with DC from IL-4-treated rats. This induction of apoptosis was reduced by inhibiting NOS activity, suggesting that DC-derived NO may suppress autoactive T cell functions through mechanisms of apoptosis. We postulate that nasal administration of IL-4 triggers DC activation; the activated DC generate immunoregulatory molecules such as IL-10, IFN-γ and NO, which in turn further influence the direction of subsequent immune responses and divert them in a benign, disease-inhibiting direction.
Acknowledgments
This work was supported by grants from the Swedish MS Society (NHR), the Swedish Medical Research Council and Karolinska Institute Research Funds.
REFERENCES
- 1.Prineas JW, Wright RG. Macrophages, lymphocytes, and plasma cells in the perivascular compartment in chronic multiple sclerosis. Lab Invest. 1978;38:409–21. [PubMed] [Google Scholar]
- 2.Swanborg RH. Experimental autoimmune encephalomyelitis in rodents as a model for human demyelinating disease. Clin Immunol Immunopathol. 1995;77:4–13. doi: 10.1016/0090-1229(95)90130-2. [DOI] [PubMed] [Google Scholar]
- 3.Miossec P. Cytokine-induced autoimmune disorders. Drug Safety. 1997;17:93–104. doi: 10.2165/00002018-199717020-00002. [DOI] [PubMed] [Google Scholar]
- 4.Rollwagen FM, Baqar S. Oral cytokine administration. Immunol Today. 1996;17:548–50. doi: 10.1016/s0167-5699(96)30065-0. [DOI] [PubMed] [Google Scholar]
- 5.Wu HY, Russell MW. Nasal lymphoid tissue, intranasal immunization, and compartmentalization of the common mucosal immune system. Immunol Res. 1997;16:187–201. doi: 10.1007/BF02786362. [DOI] [PubMed] [Google Scholar]
- 6.Xiao BG, Bai XF, Zhang GX, Link H. Suppression of acute and protracted-relapsing experimental allergic encephalomyelitis by nasal administration of low-dose IL-10 in rats. J Neuroimmunol. 1998;84:230–7. doi: 10.1016/s0165-5728(97)00264-6. [DOI] [PubMed] [Google Scholar]
- 7.Holmdahl R, Moran T, Andersson M. A rapid and efficient immunization protocol for production of monoclonal antibodies reactive with autoantigens. J Immunol Methods. 1985;83:379–84. doi: 10.1016/0022-1759(85)90260-1. [DOI] [PubMed] [Google Scholar]
- 8.Steinman RM, Kaplan G, Witman MD, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. J Exp Med. 1979;149:1–16. doi: 10.1084/jem.149.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Link H, Olsson O, Sun J, et al. Acetylcholine receptor-reactive T and B cells in myasthenia gravis and controls. J Clin Invest. 1991;87:2191–5. doi: 10.1172/JCI115253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Julius MH, Simpson E, Herzenberg LA. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973;3:645–9. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 11.de Boer BA, Fillie YE, Kruize YC, Yazdanbakhsh M. Antigen-stimulated IL-4, IL-13 and IFN-γ production by human T cells at a single-cell level. Eur J Immunol. 1998;28:3154–60. doi: 10.1002/(SICI)1521-4141(199810)28:10<3154::AID-IMMU3154>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 12.Eng LF, Ghirnikar RS, Lee YL. Inflammation in EAE: role of chemokine/cytokine expression by resident and infiltrating cells. Neurochem Res. 1996;21:511–25. doi: 10.1007/BF02527717. [DOI] [PubMed] [Google Scholar]
- 13.Steinman RM, Pack M, Inaba K. Dendritic cells in the T cell area of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 14.Rhodes JM, Agger R. Comparison of membrane antigens of mouse dendritic cell types. Immunol Letters. 1987;16:107–12. doi: 10.1016/0165-2478(87)90116-7. [DOI] [PubMed] [Google Scholar]
- 15.Savary CA, Grazziutti ML, Melichar B, et al. Multidimensional flow-cytometric analysis of dendritic cells in peripheral blood of normal donors and cancer patients. Cancer Immunol Immunother. 1998;45:234–40. doi: 10.1007/s002620050438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonham CA, Lu L, Hoffman RA, Simmons RL, Thomson AW. Nitric oxide production by dendritic cells is associated with impairment of T cell responses. Transplant Proc. 1997;29:1116–7. doi: 10.1016/s0041-1345(96)00458-7. [DOI] [PubMed] [Google Scholar]
- 17.Paul WE. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991;77:1859–70. [PubMed] [Google Scholar]
- 18.Brown MA, Hural J. Functions of IL-4 and control of its expression. Crit Rev Immunol. 1997;17:1–32. doi: 10.1615/critrevimmunol.v17.i1.10. [DOI] [PubMed] [Google Scholar]
- 19.Vokes EE, Figlin R, Hochster H, Lotze M, Rybak ME. A phase II study of recombinant human interleukin-4 for advanced or recurrent non-small cell lung cancer. Cancer J Sci Am. 1998;4:46–51. [PubMed] [Google Scholar]
- 20.Stevens DA. Combination immunotherapy and antifungal chemotherapy. Clin Inf Dis. 1998;26:1266–9. doi: 10.1086/516362. [DOI] [PubMed] [Google Scholar]
- 21.He XY, Chen J, Verma N, Plain K, Tran G, Hall BM. Treatment with interleukin-4 prolongs allogeneic neonatal heart graft survival by inducing T helper 2 responses. Transplantation. 1998;65:1145–52. doi: 10.1097/00007890-199805150-00001. [DOI] [PubMed] [Google Scholar]
- 22.Rocken M, Racke M, Shevach EM. IL-4-induced immune deviation as antigen-specific therapy for inflammatory autoimmune disease. Immunol Today. 1996;17:225–31. doi: 10.1016/0167-5699(96)80556-1. [DOI] [PubMed] [Google Scholar]
- 23.Racke MK, Bonomo A, Scott DE, Cannella B, Levine A, Raine CS, Shevach EM, Rocken M. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J Exp Med. 1994;180:1961–6. doi: 10.1084/jem.180.5.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi Y. Developmental regulation by cytokines of bone marrow-derived dendritic cells and epidermal Langerhans cells. Microbiol Immunol. 1998;42:639–50. doi: 10.1111/j.1348-0421.1998.tb02334.x. [DOI] [PubMed] [Google Scholar]
- 25.Gieseler R, Heise D, Soruri A, Schwartz P, Peters JH. In-vitro differentiation of mature dendritic cells from human blood monocytes. Dev Immunol. 1998;6:25–39. doi: 10.1155/1998/72054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Waal Malefyt R, Yssel H, Roncarolo MG, Spits H, de Vries JE. Interleukin-10. Curr Opin Immunol. 1992;4:314–20. doi: 10.1016/0952-7915(92)90082-p. [DOI] [PubMed] [Google Scholar]
- 27.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–51. [PubMed] [Google Scholar]
- 28.Willems F, Marchant A, Delville JP, Gerard C, Delvaux A, Velu T, de Boer M, Goldman M. Interleukin-10 inhibits B7 and intercellular adhesion molecule-1 expression on human monocytes. Eur J Immunol. 1994;24:1007–9. doi: 10.1002/eji.1830240435. [DOI] [PubMed] [Google Scholar]
- 29.de Waal Malefyt R, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sieg S, King C, Huang Y, Kaplan D. The role of interleukin-10 in the inhibition of T-cell proliferation and apoptosis mediated by parainfluenza virus type 3. J Virol. 1996;70:4845–8. doi: 10.1128/jvi.70.7.4845-4848.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taga K, Mostowski H, Tosato G. Human interleukin-10 can directly inhibit T-cell growth. Blood. 1993;81:2964–71. [PubMed] [Google Scholar]
- 32.Nicod LP, el Habre F, Dayer JM, Boehringer N. Interleukin-10 decreases tumor necrosis factor alpha and beta in alloreactions induced by human lung dendritic cells and macrophages. Am J Respir Cell Mol Biol. 1995;13:83–90. doi: 10.1165/ajrcmb.13.1.7598941. [DOI] [PubMed] [Google Scholar]
- 33.Bogdan C, Nathan C. Modulation of macrophage function by transforming growth factor beta, interleukin-4, and interleukin-10. Ann N Y Acad Sci. 1993;685:713–39. doi: 10.1111/j.1749-6632.1993.tb35934.x. [DOI] [PubMed] [Google Scholar]
- 34.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 35.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–80. [PubMed] [Google Scholar]
- 37.Popko B, Corbin JG, Baerwald KD, Dupree J, Garcia AM. The effects of interferon-gamma on the central nervous system. Mol Neurobiol. 1997;14:19–35. doi: 10.1007/BF02740619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223–7. [PubMed] [Google Scholar]
- 39.Heremans H, Dillen C, Groenen M, Martens E, Billiau A. Chronic relapsing experimental autoimmune encephalomyelitis (CREAE) in mice: enhancement by monoclonal antibodies against interferon-gamma. Eur J Immunol. 1996;26:2393–8. doi: 10.1002/eji.1830261019. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt HHHW, Walter U. NO at work. Cell. 1994;78:919–25. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 41.Lu L, Bonham CA, Chambers FG, Watkins SC, Hoffman RA, Simmons RL, Thomson AW. Induction of nitric oxide synthase in mouse dendritic cells by IFN-gamma, endotoxin, and interaction with allogeneic T cells: nitric oxide production is associated with dendritic cell apoptosis. J Immunol. 1996;157:3577–86. [PubMed] [Google Scholar]