Abstract
Retinal pigment epithelial (RPE) cells, situated between the neurosensory retina and the vascularized choroid, form part of the blood–eye barrier and are important for homeostasis of the outer retina. These cells are able to produce a variety of cytokines which may play a role in the maintenance of the immunosuppressive milieu inside the eye and in intraocular inflammatory responses. In the present study, we investigated whether RPE cells secreted the anti-inflammatory cytokine TGF-β2 and the proinflammatory cytokine MCP-1 in a polarized manner. Monolayers of human donor RPE cells were cultured on transwell filters. Secretion of TGF-β2 and MCP-1 at either the apical or basal side of the RPE cell monolayers, that were not treated or stimulated with IL-1β (200 U/ml), was analysed by ELISA. All three cell lines examined had a different TGF-β2 secretion pattern. In two of the three donor RPE cell lines tested, TGF-β2 secretion was polarized, but not in the same direction. TGF-β2 secretion was not up-regulated by stimulation with IL-1β. In contrast, IL-1β strongly induced MCP-1 secretion preferentially into the basal compartment of all RPE monolayers tested. These data indicate that human RPE cells are able to secrete TGF-β2 and MCP-1 in a polarized fashion. Our results suggest that MCP-1 can be secreted by RPE cells in the direction of choroidal vessels during inflammatory responses in the posterior part of the eye, which may limit damage to the neurosensory retina.
Keywords: retinal pigment epithelium, polarized secretion, cytokine, monocyte chemotactic protein-1, transforming growth factor-beta
INTRODUCTION
The eye is an immune privileged site where immunological processes are highly regulated. Retinal pigment epithelial (RPE) cells appear to play a pivotal role in the maintenance of this immune privileged status. RPE cells are situated between the vascular bed of the eye and the neurosensory retina and form part of the blood–eye barrier, which prevents access of blood cells and plasma proteins. Furthermore, RPE cells constitutively express Fas ligand (FasL) [1], which may induce apoptosis of extravasated activated T cells. Moreover, RPE cells are able to produce the anti-inflammatory cytokines TGF-β [2], mainly the β2-isoform [3], and IL-1 receptor antagonist (IL-1Ra) [4,5], which may contribute to the immunosuppressive milieu inside the eye.
RPE cells may also play a role in intraocular inflammation. In response to IL-1 and other proinflammatory cytokines, RPE cells express MHC class II [6], adhesion molecules [7,8] and a variety of cytokines, including IL-1 [4,9], IL-6 [10–12], IL-8 [13], MCP-1 [14] and granulocyte-macrophage colony-stimulating factor (GM-CSF) [9].
Recently, we reported that monolayers of RPE cells, cultured on transwell filters, secrete the proinflammatory cytokines IL-6 and IL-8 predominantly into the basal compartment [15]. We hypothesized that polarized secretion in the direction of the choriocapillaris may represent a mechanism to reduce damage of the neurosensory retina during inflammation.
In the present study we investigated whether RPE cells secrete both immunosuppressive and proinflammatory cytokines in a polarized manner. As a model we investigated the secretion of TGF-β2 and MCP-1. TGF-β2 possesses a variety of anti- inflammatory activities, including inhibition of B and T cell proliferation [16,17], inhibition of granulocyte adherence to endothelial cells [18], deactivation of macrophages [19] and down-regulation of MHC class II antigen expression on human cells (including RPE cells) [20–22]. Moreover, TGF-β appears to be pivotal in the induction of deviated immune responses in the eye [23]. MCP-1 is a member of the C-C family of chemokines and is a potent chemoattractant for monocytes, activated T cells, basophils and natural killer (NK) cells [24–27]. MCP-1 is also an activator of monocytes and induces oxidative burst and cytokine release [28,29]. Furthermore, MCP-1 has been implied to play a role in inflammation of the posterior part of the eye [30,31]. The data in the present study indicate that RPE cells are capable of secreting TGF-β2 and MCP-1 in a polarized manner.
MATERIALS AND METHODS
RPE cell cultures
Human donor eyes (age of the donors 9–35 years) obtained from the eye bank were used as a source of primary RPE cells. These RPE cells were isolated within 24 h post mortem. Isolation and characterization of the RPE cells was performed as described earlier [15]. In short, after removal of the cornea (for transplantation) and the anterior segment, the optic nerve was cut and vitreous plus neural retina were washed out of the eye cup. RPE cells were removed from the sclera by trypsin dissociation and plated in 24-well plates (Costar, Cambridge, MA) at 105 cells/well in Iscove's modified Dulbecco's medium (IMDM; Gibco BRL, Breda, The Netherlands) supplemented with 20% fetal calf serum (FCS; Gibco BRL), penicillin 100 U/ml (Gibco BRL) and streptomycin 100 μg/ml (Gibco BRL). Non-adherent cells were removed after 2 days by refreshment of the medium. At confluence, cells were passed to culture flasks at 4 × 104 cells/cm2.
Cells between passages 3 and 8 were used. Morphological examination and immunohistochemical staining using an antibody against cytokeratin 8/18 (CAM 5:2; Becton Dickinson, San Jose, CA) revealed that the cultured RPE cells were not contaminated by other cell types.
Monolayers of RPE cells on transwell filters
RPE cell monolayers were generated by culturing the cells on transwell filters (Costar; 12 mm diameter, 0.4 μm pore size) as described earlier [15]. The filters were coated with 160 μl of a 1:40 dilution of Matrigel in medium and air-dried overnight. The RPE cells were seeded on the filters at a concentration of 1.6 × 105 cell/cm2 in IMDM medium supplemented with 1% normal human serum (NHS; CLB, Amsterdam, The Netherlands). Medium was changed twice a week. The filters were used 19 days after seeding and when the transepithelial resistance (TER) reached 30 Ω/cm2. TER was measured using an Endohm chamber and an ohmmeter (World Precision Instruments, Sarasota, FL). At this time approximately 2 × 105 cells were present on the filters.
The integrity of the monolayer was determined by light microscopic examination of (i) haematoxylin–eosin (H–E)-stained sections of filters, and (ii) immunofluorescent stained filters using an antibody against ZO-1, a tight junction-associated protein, as previously described [15].
Stimulation of the RPE cells
To determine whether RPE cells produced sufficient amounts of TGF-β2 for transwell experiments, RPE cells were cultured in 24-well plates until confluence (approximately 3 × 105 cells/well). The RPE cells were either not treated or stimulated with 200 U/ml IL-1β (specific activity 109 U/mg; Genzyme, Cambridge, MA) or 200 U/ml tumour necrosis factor-alpha (TNF-α; specific activity 108 U/mg; Boehringer Mannheim, Almere, The Netherlands) in IMDM supplemented with 0.1% FCS for 48 h. High responder RPE cell lines (producing > 150 pg/ml) were used for transwell filters experiments.
RPE cell monolayers on transwell filters were cultured for 24 h in IMDM supplemented with 0.1% NHS before stimulation. RPE cell monolayers were not treated or stimulated with 200 U/ml IL-1β in the upper compartment (apical side of the RPE cells) or in the lower compartment (basal side of the RPE cells). At the time points 24 h, 48 h and 72 h after stimulation the medium was isolated and snap-frozen until assay.
Cytokine assays
Measurement of TGF-β2 and MCP-1 was performed using commercially available ELISAs. The TGF-β2 assay was a sandwich ELISA with a detection limit of 5 pg/ml (R&D Systems, Abingdon, UK). Using this ELISA only mature TGF-β2 could be measured. Latent TGF-β2 can be converted into the mature form by heat activation. To determine the total (mature plus latent) TGF-β2 concentration of the supernatants, the samples were diluted (minimal 1:2) with PBS supplemented with 0.5% bovine serum albumin (BSA; Gibco BRL) and 0.012% human serum albumin (HSA; CLB) and incubated at 80°C for 8 min. The concentration of mature TGF-β2 was determined using samples that were not heat-activated. The MCP-1 assay was also a sandwich ELISA (PharMingen, San Diego, CA) with a detection limit of 25 pg/ml.
Statistical analysis
Statistical analysis was performed after log transformation of the data. The difference between cytokine levels measured in the upper or lower compartment and time was tested by the anova method (SPSS software; SPSS Inc., Chicago, IL). Differences were considered significant at P < 0.05.
RESULTS
TGF-β2 production by RPE cells
In view of the relatively small number of cells and large culture volume used for transwell filter experiments, first experiments were carried out to detect high TGF-β2-producing cell lines. Five RPE cell lines, cultured in 24-well plates, were either not stimulated or stimulated for 48 h with IL-1β or TNF-α to determine TGF-β2 production. All cell lines secreted TGF-β2 constitutively (Fig. 1). The amount of TGF-β2 produced by the cell lines 372, 364 and 605 was sufficient for transwell filter experiments; the other two were excluded for further studies. Treatment with IL-1β or TNF-α did not up-regulate, or only moderately so, TGF-β2 secretion by RPE cells (Fig. 1). Other stimuli, including IL-1β in combination with TNF-α, 4-phorbol-12 myristate-13 acetate (PMA) or increased concentrations of glucose, did not affect TGF-β2 secretion (data not shown).
Fig. 1.

TGF-β2 production by human donor retinal pigment epithelial (RPE) cell lines that were not treated or stimulated with IL-1β or tumour necrosis factor-alpha (TNF-α) for 48 h. RPE cells were seeded in 24-well plates and cultured until confluence in Iscove's modified Dulbecco's medium (IMDM) supplemented with 20% fetal calf serum (FCS). Before stimulation the RPE cells were cultured for 24 h in IMDM with 0.1% FCS. Cells were stimulated with IL-1β 200 U/ml or TNF-α 200 U/ml in 1 ml IMDM with 0.1% FCS for 48 h. The total TGF-β2 content (mature and latent) was determined by ELISA. Data are expressed as the mean of two or four wells ± s.e.m.
To investigate whether the RPE cells secreted TGF-β2 in the mature or in the biological non-active (latent) form, supernatants were tested with or without heat activation. With heat activation the total amount of TGF-β2 (mature + latent) was measured and without heat activation only the mature form of TGF-β2 was measured (see Materials and Methods). In supernatants of unstimulated cells, approximately one-third of the produced amount of TGF-β2 was of the mature form (101 pg/ml TGF-β2 before and 333 pg/ml TGF-β2 after heat activation; Table 1). The ratio mature/total TGF-β2 was not changed by treatment with either IL-1β or TNF-α.
Table 1.
TGF-β2 production by human retinal pigment epithelial (RPE) cells cultured in 24-well plates*

*Data represent mean ± s.e.m. of a representative experiment performed in quadruplicate using one RPE cell line.
TGF-β2 and MCP-1 secretion by RPE cell monolayers on transwell filter
To investigate the direction of TGF-β2 and MCP-1 secretion by RPE cell monolayers, three donor RPE cell lines were cultured on transwell filters. The TER of the RPE cell monolayers was 33.9 ± 0.9 Ω/cm2, 32.3 ± 1.1 Ω/cm2, and 32.9 ± 1.2 Ω/cm2 (mean ± s.e.m., for the cell cultures with number 605, 372 and 364, respectively) after correction for the resistance of a filter without cells. Light microscopy of sections of the filters with RPE cells stained with H–E showed a monolayer of cells, similar to our previous study [15]. Immunofluorescent staining of the RPE cell monolayers on filter with the antibody against ZO-1 revealed tight junctions around every cell, indicating that the RPE cell layer had obtained barrier properties [15].
Different TGF-β2 secretion patterns were found for each of the three cell lines (Fig. 2). Cell line 372 secreted similar amounts of TGF-β2 into the apical and basal compartment (Fig. 2a). Cell line 605 secreted approximately two times more TGF-β2 into the apical compartment than into the basal compartment. In contrast, cell line 364 secreted TGF-β2 in a highly polarized manner into the basal compartment. At 72 h approximately nine times more TGF-β2 was found at the basal side than at the apical side of the RPE cell layer(158 pg versus 18 pg/filter). As expected, stimulation with IL-1β at either the basal or apical side did not markedly up-regulate the secretion of TGF-β2 by the three RPE cell lines (Fig. 2b,c). Strikingly, basal TGF-β2 secretion by the cell lines 372 and 605 appeared to be transiently inhibited by the IL-1β treatment (at t = 48 h).
Fig. 2.
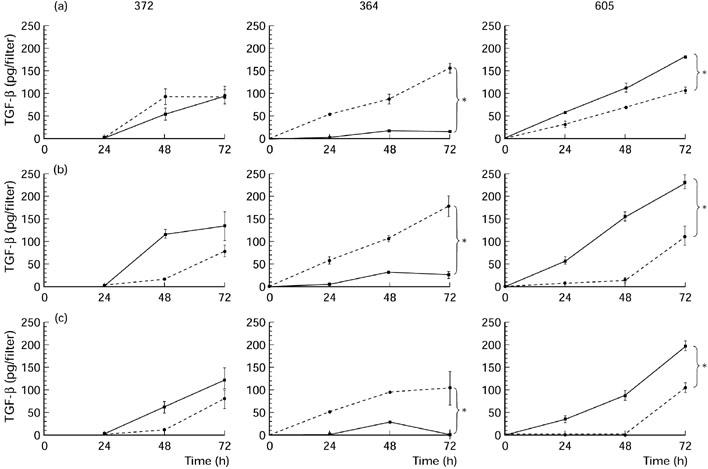
Secretion of TGF-β2 by three donor retinal pigment epithelial (RPE) cell lines (372, 364, 605) into the basal compartment (segmented line) and into the apical compartment (continuous line) without stimulation (a) or after stimulation with IL-1β from the apical (b) or basal (c) side. RPE cells were seeded on transwell filters at a concentration of 1.6 × 105 cells/cm2 and cultured for at least 19 days in Iscove's modified Dulbecco's medium (IMDM) with 1% normal human serum (NHS) and had a transepithelial resistance (TER) > 30 Ω/cm2. Filters were stimulated with 200 U/ml IL-1β in IMDM with 0.1% NHS. Data are expressed as absolute amounts of TGF-β2 per filter and are the mean of three or five filters ± s.e.m. *Statistically significant differences between upper and lower compartments.
Two of the three RPE cell monolayers also secreted MCP-1 constitutively in a polarized manner. The level of MCP-1 varied between 1.6 ± 0.4 ng (mean ± s.e.m.) and 2 ± 0.1 ng/filter in the upper compartment, and 1.8 ± 0.6 ng and 4.6 ± 0.1 ng/filter in the lower compartment of unstimulated RPE cells (Fig. 3a). In contrast to TGF-β2 secretion, the secretion of MCP-1 in both the upper and lower compartment of all RPE cell monolayers examined was markedly increased (in total approximately 25 times) by IL-1β stimulation (Fig. 3). The amounts of MCP-1 detected in the lower compartments were significantly higher then the amounts found in the upper compartments. This indicates that MCP-1 is secreted by the RPE cells in a polarized manner, at the basal side. No difference was detected in MCP-1 levels between stimulation with IL-1β in either the upper or lower compartment of the RPE cell monolayers.
Fig. 3.
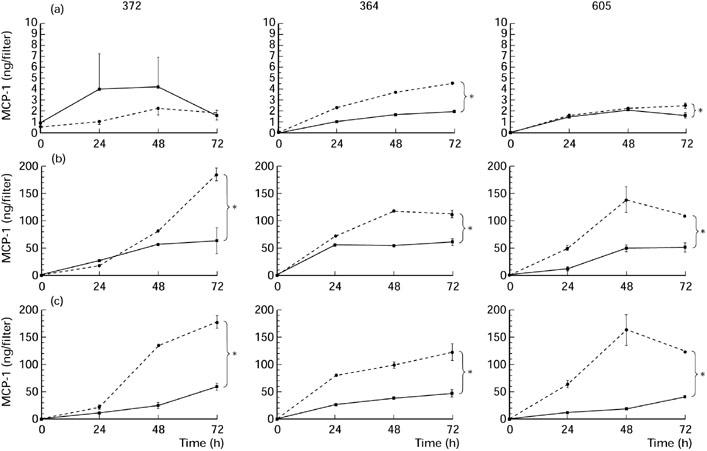
Secretion of MCP-1 by three donor retinal pigment epithelial (RPE) cell lines (372, 364, 605) into the basal compartment (segmented line) and into the apical compartment (continuous line) without stimulation (a) or after stimulation with IL-1β from the apical (b) or basal (c) side. RPE cells were seeded on transwell filters at a concentration of 1.6 × 105 cells/cm2 and cultured for at least 19 days in Iscove's modified Dulbecco's medium (IMDM) with 1% normal human serum (NHS) and had a transepithelial resistance (TER) > 30 Ω/cm2. Filters were stimulated with 200 U/ml IL-1β in IMDM with 0.1% NHS. Data are expressed as absolute amounts of MCP-1 per filter and are the mean of three to five filters ± s.e.m. *Statistically significant differences between upper and lower compartments.
Thus, despite some heterogeneity in TGF-β2 secretion, IL-1β induced MCP-1 secretion mainly into the basal compartment, in all RPE cell lines tested.
DISCUSSION
RPE cells are able to produce a variety of cytokines and thus may contribute to the regulation of immune responses in the posterior segment of the human eye. In a previous study, we have shown that RPE cells secrete the proinflammatory cytokines IL-6 and IL-8 in a polarized fashion at the basal side, which represents the side of the choriocapillaris. We hypothesized that this may be a mechanism to limit damage to the neurosensory retina during inflammatory processes [15]. In the present study we showed that MCP-1 was also secreted preferentially into the basal compartment. Moreover, we showed that the anti-inflammatory cytokine TGF-β2 was secreted in a polarized manner, but that the direction of secretion varied between the RPE cell donors.
One of the three RPE cell lines tested for polarized secretion did not show any difference in the amount of TGF-β2 secreted into the apical or basal compartment. The other two RPE cell lines showed polarized secretion of TGF-β2, but strikingly the secretion was directed into the apical compartment for one cell line and into the basal compartment for the other cell line. Thus no clear pattern for the direction of TGF-β2 secretion could be observed. Variability in the direction of TGF-β2 secretion caused by diffusion of proteins across the monolayer of cells can not be excluded. Earlier experiments in which we analysed diffusion of IL-8 across the RPE cell monolayers revealed < 20% diffusion over a time period of 8 h [15]. Heterogeneity between RPE cell lines obtained from different donors has been described for the production of other cytokines, including IL-1Ra [4,5], IL-6 and IL-8 [15]. Furthermore, differences between RPE cell lines have also been described for growth rate [32] and phenotypic appearance [33]. It has been suggested that RPE cells are a source of TGF-β2 in vitreous fluid, which fills the posterior part of the eye. With regard to the heterogeneity of RPE cells of different donors, it is of interest that a considerable variation (from 230 to 5060 pg/ml) was found in TGF-β2 levels in vitreous fluid of 16 eye bank eyes [34].
TGF-β2 secretion was not up-regulated in response to IL-1β or TNF-α, at concentrations known to induce secretion of other cytokines by RPE cells. Others found increased levels of TGF-β2 in supernatants of RPE cells after photocoagulation treatment [35] or in response to monocyte conditioned medium [36]. In the latter study it was reported that monocyte conditioned medium did not alter TGF-β2 mRNA expression, but increased the protein level by a post-transcriptional mechanism. Interestingly, addition of antibodies against IL-1α, IL-1β and TNF-α to the monocyte conditioned medium blocked this effect [36]. The discrepancy between these results and those presented here may be attributed to the other factors present in the monocyte conditioned medium that act in combination or in synergy with IL-1β or TNF-α. It has been shown that interferon-gamma (IFN-γ) potentiated the IL-1β- or TNF-α-induced MCP-1 production by RPE cells [37]. The amounts of TGF-β2 secreted by human RPE cells described in the present study are comparable to the amounts detected by others who studied RPE cells in vitro [35,36]. Due to the low level of TGF-β2 (50–100 pg per 4×105 cells) secreted by some RPE cell lines and the relatively low number of RPE cells at confluence on transwell filters, only three of the five donor-derived RPE cell lines were suitable for filter experiments. This observation further underlines the heterogeneity found between various RPE cell lines.
Secretion of MCP-1 by RPE cells has been thoroughly studied by Elner and co-workers [14,37–41]. Recently, they found that the majority of MCP-1 produced by RPE cells was secreted and that only a small amount (approximately 4%) of the total MCP-1 remained cell-associated [40]. Furthermore, it was shown that MCP-1 secreted by RPE cells was bioactive and that antibodies directed against MCP-1 strongly inhibited monocyte chemotaxis elicited by supernatants of RPE cells [40]. Our results add to these studies and clearly show that RPE cells stimulated with IL-1β secrete MCP-1 in a polarized manner at their basal side. A similar secretion pattern of MCP-1 was shown by others who studied rat alveolar epithelial cells [42]. Our findings imply that during an inflammatory response in the posterior part of the eye, monocytes and other MCP-1-responsive cells will be attracted from choroidal vessels, rather than from retinal blood vessels. Moreover, due to a lower concentration of MCP-1 at the retinal side, cells may be prevented from crossing the RPE barrier. Thus, polarized secretion of MCP-1 by RPE cells may limit damage to the neurosensory retina.
Taken together, our results show that RPE cells are capable of secreting TGF-β2 and MCP-1 in a polarized manner. Consistent with our previous study, IL-1β induced secretion of the proinflammatory cytokine MCP-1 mainly into the basal compartment of all RPE cell monolayers tested. This may represent a mechanism to prevent damage to the fragile retinal tissue.
Acknowledgments
The RPE cells used in this study were isolated from the remainder of tissues donated and used for transplantation purposes or which could not be used for transplantation. The authors wish to thank the BIS foundation (Leiden, The Netherlands) and Dr L. Pels (Cornea Bank, Amsterdam, The Netherlands) for assistance in obtaining the tissue. A.F.d.V. was supported by the Dutch foundation ‘Vrienden MS Research’.
REFERENCES
- 1.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–92. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 2.Tanihara H, Yoshida M, Matsumoto M, Yoshimura N. Identification of transforming growth factor-beta expressed in cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1993;34:413–9. [PubMed] [Google Scholar]
- 3.Pfeffer BA, Flanders KC, Guerin CJ, Danielpour D, Anderson DH. Transforming growth factor beta 2 is the predominant isoform in the neural retina, retinal pigment epithelium-choroid and vitreous of the monkey eye. Exp Eye Res. 1994;59:323–33. doi: 10.1006/exer.1994.1114. [DOI] [PubMed] [Google Scholar]
- 4.Jaffe GJ, Van Le L, Valea F, et al. Expression of interleukin-1 alpha, interleukin-1 beta, and an interleukin-1 receptor antagonist in human retinal pigment epithelial cells. Exp Eye Res. 1992;55:325–35. doi: 10.1016/0014-4835(92)90197-z. [DOI] [PubMed] [Google Scholar]
- 5.Holtkamp GM, de Vos AF, Kijlstra A, Peek R. Expression of multiple forms of IL-1 receptor antagonist (IL-1ra) by human retinal pigment epithelial cells: identification of a new IL-1ra exon. Eur J Immunol. 1999;29:215–24. doi: 10.1002/(SICI)1521-4141(199901)29:01<215::AID-IMMU215>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Liversidge JM, Sewell HF, Forrester JV. Human retinal pigment epithelial cells differentially express MHC class II (HLA, DP, DR and DQ) antigens in response to in vitro stimulation with lymphokine or purified IFN-gamma. Clin Exp Immunol. 1988;73:489–94. [PMC free article] [PubMed] [Google Scholar]
- 7.Elner SG, Elner VM, Pavilack MA, et al. Modulation and function of intercellular adhesion molecule-1 (CD54) on human retinal pigment epithelial cells. Lab Invest. 1992;66:200–11. [PubMed] [Google Scholar]
- 8.Platts KE, Benson MT, Rennie IG, Sharrard RM, Rees RC. Cytokine modulation of adhesion molecule expression on human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1995;36:2262–9. [PubMed] [Google Scholar]
- 9.Planck SR, Huang XN, Robertson JE, Rosenbaum JT. Retinal pigment epithelial cells produce interleukin-1 beta and granulocyte-macrophage colony-stimulating factor in response to interleukin-1 alpha. Curr Eye Res. 1993;12:205–12. doi: 10.3109/02713689308999465. [DOI] [PubMed] [Google Scholar]
- 10.Planck SR, Dang TT, Graves D, Tara D, Ansel JC, Rosenbaum JT. Retinal pigment epithelial cells secrete interleukin-6 in response to interleukin-1. Invest Ophthalmol Vis Sci. 1992;33:78–82. [PubMed] [Google Scholar]
- 11.Elner VM, Scales W, Elner SG, Danforth J, Kunkel SL, Strieter RM. Interleukin-6 (IL-6) gene expression and secretion by cytokine- stimulated human retinal pigment epithelial cells. Exp Eye Res. 1992;54:361–8. doi: 10.1016/0014-4835(92)90048-w. [DOI] [PubMed] [Google Scholar]
- 12.Benson MT, Shepherd L, Rees RC, Rennie IG. Production of interleukin-6 by human retinal pigment epithelium in vitro and its regulation by other cytokines. Curr Eye Res. 1992;11(Suppl.):173–9. doi: 10.3109/02713689208999529. [DOI] [PubMed] [Google Scholar]
- 13.Elner VM, Strieter RM, Elner SG, Baggiolini M, Lindley I, Kunkel SL. Neutrophil chemotactic factor (IL-8) gene expression by cytokine-treated retinal pigment epithelial cells. Am J Pathol. 1990;136:745–50. [PMC free article] [PubMed] [Google Scholar]
- 14.Elner SG, Strieter RM, Elner VM, Rollins BJ, Del Monte MA, Kunkel SL. Monocyte chemotactic protein gene expression by cytokine-treated human retinal pigment epithelial cells. Lab Invest. 1991;64:819–25. [PubMed] [Google Scholar]
- 15.Holtkamp GM, Van Rossem M, de Vos AF, Willekens B, Peek R, Kijlstra A. Polarized secretion of IL-6 and IL-8 by human retinal pigment epithelial cells. Clin Exp Immunol. 1998;112:34–43. doi: 10.1046/j.1365-2249.1998.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kehrl JH, Roberts AB, Wakefield LM, et al. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986;137:3855–60. [PubMed] [Google Scholar]
- 17.Kehrl JH, Wakefield LM, Roberts AB, et al. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986;163:1037–50. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gamble JR, Vadas MA. Endothelial adhesiveness for blood neutrophils is inhibited by transforming growth factor-beta. Science. 1988;242:97–9. doi: 10.1126/science.3175638. [DOI] [PubMed] [Google Scholar]
- 19.Tsunawaki S, Sporn M, Ding A, Nathan C. Deactivation of macrophages by transforming growth factor-beta. Nature. 1988;334:260–2. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- 20.Czarniecki CW, Chiu HH, Wong GH, McCabe SM, Palladino MA. Transforming growth factor-beta 1 modulates the expression of class II histocompatibility antigens on human cells. J Immunol. 1988;140:4217–23. [PubMed] [Google Scholar]
- 21.Schluesener HJ. Transforming growth factors type beta 1 and beta 2 suppress rat astrocyte autoantigen presentation and antagonize hyperinduction of class II major histocompatibility complex antigen expression by interferon-gamma and tumor necrosis factor-alpha. J Neuroimmunol. 1990;27:41–47. doi: 10.1016/0165-5728(90)90134-9. [DOI] [PubMed] [Google Scholar]
- 22.Gabrielian K, Osusky R, Sippy BD, Ryan SJ, Hinton DR. Effect of TGF-beta on interferon-gamma-induced HLA-DR expression in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1994;35:4253–9. [PubMed] [Google Scholar]
- 23.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 24.Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989;169:1485–90. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baggiolini M, Dahinden CA. CC chemokines in allergic inflammation. Immunol Today. 1994;15:127–33. doi: 10.1016/0167-5699(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 26.Wang XC, Jobin C, Allen JB, Roberts WL, Jaffe GJ. Suppression of NF-kappaB-dependent proinflammatory gene expression in human RPE cells by a proteasome inhibitor. Invest Ophthalmol Vis Sci. 1999;40:477–86. [PubMed] [Google Scholar]
- 27.Rollins BJ, Walz A, Baggiolini M. Recombinant human MCP-1/JE induces chemotaxis, calcium flux, and the respiratory burst in human monocytes. Blood. 1991;78:1112–6. [PubMed] [Google Scholar]
- 28.Rollins BJ. Chemokines. Blood. 1997;90:909–28. [PubMed] [Google Scholar]
- 29.Jiang Y, Beller DI, Frendl G, Graves DT. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. J Immunol. 1992;148:2423–8. [PubMed] [Google Scholar]
- 30.Elner SG, Elner VM, Jaffe GJ, Stuart A, Kunkel SL, Strieter RM. Cytokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Curr Eye Res. 1995;14:1045–53. doi: 10.3109/02713689508998529. [DOI] [PubMed] [Google Scholar]
- 31.Abu el-Asrar AM, Van Damme J, Put W, et al. Monocyte chemotactic protein-1 in proliferative vitreoretinal disorders. Am J Ophthalmol. 1997;123:599–606. doi: 10.1016/s0002-9394(14)71072-4. [DOI] [PubMed] [Google Scholar]
- 32.Flood MT, Gouras P, Kjeldbye H. Growth characteristics and ultrastructure of human retinal pigment epithelium in vitro. Invest Ophthalmol Vis Sci. 1980;19:1309–20. [PubMed] [Google Scholar]
- 33.Burke JM, Skumatz CM, Irving PE, McKay BS. Phenotypic heterogeneity of retinal pigment epithelial cells in vitro and in situ. Exp Eye Res. 1996;62:63–73. doi: 10.1006/exer.1996.0008. [DOI] [PubMed] [Google Scholar]
- 34.de Boer JH, Limpens J, Orengo-Nania S, de Jong PT, La Heij E, Kijlstra A. Low mature TGF-beta 2 levels in aqueous humor during uveitis. Invest Ophthalmol Vis Sci. 1994;35:3702–10. [PubMed] [Google Scholar]
- 35.Matsumoto M, Yoshimura N, Honda Y. Increased production of transforming growth factor-beta 2 from cultured human retinal pigment epithelial cells by photocoagulation. Invest Ophthalmol Vis Sci. 1994;35:4245–52. [PubMed] [Google Scholar]
- 36.Jaffe GJ, Roberts WL, Wong HL, Yurochko AD, Cianciolo GJ. Monocyte-induced cytokine expression in cultured human retinal pigment epithelial cells. Exp Eye Res. 1995;60:533–43. doi: 10.1016/s0014-4835(05)80068-5. [DOI] [PubMed] [Google Scholar]
- 37.Elner SG, Elner VM, Bian ZM, et al. Human retinal pigment epithelial cell interleukin-8 and monocyte chemotactic protein-1 modulation by T-lymphocyte products. Invest Ophthalmol Vis Sci. 1997;38:446–55. [PubMed] [Google Scholar]
- 38.Elner VM, Elner SG, Standiford TJ, Lukacs NW, Strieter RM, Kunkel SL. Interleukin-7 (IL-7) induces retinal pigment epithelial cell MCP-1 and IL-8. Exp Eye Res. 1996;63:297–303. doi: 10.1006/exer.1996.0118. [DOI] [PubMed] [Google Scholar]
- 39.Kurtz RM, Elner VM, Bian ZM, Strieter RM, Kunkel SL, Elner SG. Dexamethasone and cyclosporin A modulation of human retinal pigment epithelial cell monocyte chemotactic protein-1 and interleukin-8. Invest Ophthalmol Vis Sci. 1997;38:436–45. [PubMed] [Google Scholar]
- 40.Elner VM, Burnstine MA, Strieter RM, Kunkel SL, Elner SG. Cell-associated human retinal pigment epithelium interleukin-8 and monocyte chemotactic protein-1: immunochemical and in-situ hybridization analyses. Exp Eye Res. 1997;65:781–9. doi: 10.1006/exer.1997.0380. [DOI] [PubMed] [Google Scholar]
- 41.Bian ZM, Elner SG, Strieter RM, et al. Glycated serum albumin induces chemokine gene expression in human retinal pigment epithelial cells. J Leuk Biol. 1996;60:405–14. doi: 10.1002/jlb.60.3.405. [DOI] [PubMed] [Google Scholar]
- 42.Paine R, Rolfe MW, Standiford TJ, et al. MCP-1 expression by rat type II alveolar epithelial cells in primary culture. J Immunol. 1993;150:4561–70. [PubMed] [Google Scholar]


