Abstract
The influence of pharmacological treatment on the immune response of patients with Echinococcus granulosus infection was evaluated by reverse transcriptase-polymerase chain reaction (RT-PCR) to determine mRNA expression for IL-12 p35, IL-12 p40, interferon-gamma (IFN-γ), tumour necrosis factor-alpha (TNF-α) and IL-4 in PBMC from 12 patients before and after chemotherapy and from seven uninfected controls. Most patients' PBMC showed measurable amounts of IL-12 p35, IL-4, IFN-γ and TNF-α mRNA in parasite antigen-stimulated and unstimulated cultures. Conversely, IL-12 p40 mRNA was detected almost exclusively in successfully treated patients (86%) after therapy. In these patients semiquantitative analysis of RT-PCR products showed a significant difference between IL-12 p40 mRNA mean levels before and after therapy (P = 0.03 in parasite antigen-stimulated cultures; P = 0.001 in unstimulated cultures). IL-4 mRNA was weakly expressed before therapy and more highly so after treatment in both groups of patients and under both culture conditions; IL-4 mRNA reached its highest level in post-therapy PBMC from patients in whom therapy failed (stimulated cultures). IFN-γ and TNF-α mRNA expression increased in patients who responded to therapy and decreased in patients who did not. In contrast to IL-12 p35, IFN-γ and TNF-α mRNAs, IL-12 p40 and IL-4 mRNAs were detected exclusively in patients, suggesting a close relationship between these two cytokines and cystic echinococcosis. Our findings indicate that chemotherapy influences the immune response, thus determining changes in Th1/Th2 cytokine mRNA patterns, predominantly in IL-12 p40 and IL-4 mRNA expression.
Keywords: cystic echinococcosis, Th1/Th2 cytokine gene expression, chemotherapy, follow up
INTRODUCTION
Cystic echinococcosis (CE) is a severe chronic parasitic disease caused by the cestode Echinococcus granulosus. The preferred treatment for cystic echinococcosis is surgical excision even if chemotherapy now offers alternatives, especially for inoperable cysts and patients at high surgical risk [1]. Although benzimidazole carbamates (albendazole and mebendazole) are effective against CE, whether and how these drugs influence the immune response remains unclear. As the drug penetrates the cyst, it kills the protoscoleces, brood capsules and germinal membrane, causing the release of parasitic materials [2]. The host immune system may therefore be exposed to varying antigen types or concentrations, thus inducing a Th2 response (chronic antigenic stimulation) or a Th1 response (small parasitic lesions or reduced antigen output/recognition) [3]. Because in most parasitic diseases a predominantly cellular (Th1) or humoral (Th2) immune response offers the best control over pathogens, the induction of an appropriate T-helper cell response is essential in determining a successful immune reaction. Th1 cells are effective mediators of DTH and secrete IL-2 and interferon-gamma (IFN-γ)—the prime effectors of cell-mediated immunity [4]. In contrast, Th2 cells do not transfer DTH, but they produce IL-4, IL-5, IL-6 and IL-10 and co-operate with B cells to generate IgM, IgG, IgA and IgE responses [5]. The presence of IL-4 during priming results in a Th2 phenotype; the antagonist cytokines that depress a Th2 response are IL-12 and IFN-γ. IL-12 is a heterodimer 70-kD protein composed of two covalently linked chains, p35 and p40, encoded by separate genes. The light chain (p35) is constitutively expressed and only partially regulated; conversely, the heavy chain (p40) is expressed only in cell types that produce the bioactive heterodimer p70 (macrophages, dendritic cells and other accessory cells) and is highly regulated. As well as inducing IFN-γ, IL-12 induces production of tumour necrosis factor-alpha (TNF-α) by natural killer (NK) cells and T cells. TNF-α, released mostly by macrophages, in turn represents an important cofactor for IL-12 in inducing IFN-γ production by T and NK cells.
In a previous in vitro study of CE patients at the end of pharmacological treatment we related Th1 cell activation to protective immunity and Th2 cell activation to susceptibility to the disease [6]. More recently we investigated cytokine concentrations in CE patients' sera before and after chemotherapy [7]. The results of this in vivo study, highlighting an evident association between high IL-4/IL-10 cytokine concentrations and the failure of therapy, prompted us to study this association in depth.
We designed this in vitro study to expand and confirm our initial in vivo observations suggesting that chemotherapy leads to changes in the immune response to CE. For this purpose we used the reverse transcriptase-polymerase chain reaction (RT-PCR) to determine mRNA expression for IL-12 p35, IL-12 p40, IFN-γ and TNF-α (Th1 cell activation) and IL-4 (Th2 cell activation) in PBMC isolated from CE patients before and after chemotherapy and from uninfected controls.
PATIENTS AND METHODS
Blood samples
Blood samples were obtained from 12 patients with clinically and serologically diagnosed CE (nine with hepatic cysts, two with pulmonary cysts and one with cysts in multiple locations) and from seven sex- and age-matched uninfected controls. CE patients were selected on the basis of cyst morphology before therapy. All patients had type I and II cysts, which indicate active and progressive disease according to the sonographic classification of Caremani et al. [8]. In brief, type I cysts are simple cysts (Ia: overall echo-free, without echoes or internal structures; Ib: echo-free but with fine and suspended echoes). Type II are multiple cysts (IIa: multiple contiguous cysts, with or without thin echoes; IIb: septate cysts, with a rosette, honeycomb or wheel-like pattern). None of the subjects studied had a history of atopic manifestation; three patients had peripheral blood eosinophilia. All patients received a 3-month cycle of albendazole 10–12 mg/kg per day as allowed by WHO [9]. Three months after therapy ended, its effectiveness was assessed by objective criteria, mainly based on imaging techniques and, in particular, on ultrasound monitoring of hepatic and abdominal cysts. In this assessment the following changes were considered: the volume of the cyst (with a decrease of at least 10% up to complete disappearance), the morphology of the cyst, the increase of the solid component of the cyst (partial or total solidification corresponding to the pseudosolid ultrasonic pattern), the decrease in the number and/or size of daughter cysts, detachment and/or collapse of membranes (split wall or water lily sign), and calcification. The patients were divided into two groups according to the outcome of therapy (seven patients who responded and five who did not) (Table 1). All procedures were approved by the local Ethical Committee and all subjects gave their informed consent to the study. All blood samples were obtained before and after the end of pharmacological treatment.
Table 1.
Clinical features of the 12 patients with cystic echinococcosis
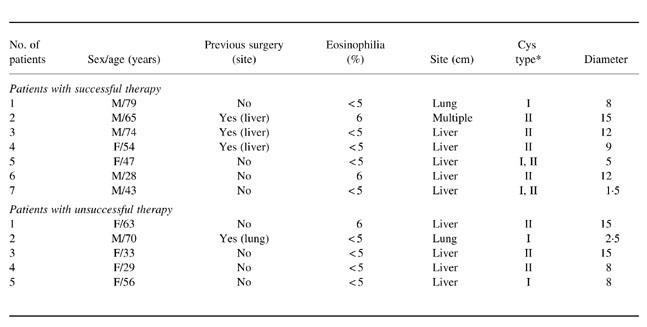
*According to the sonographic classification of Caremani et al. [8].
Antigen
Sheep hydatid fluid was collected from fertile cysts, centrifuged at 16 320 g, lyophilized and stored at −80°C until use.
PBMC isolation and cell culture experiments
PBMC were separated from plasma by Lymphoprep (Nyegaard & Co., Oslo, Norway) density gradient centrifugation and cultured as described by Riganòet al. [10] in the presence of 100 μg/ml of crude concentrated sheep hydatid fluid or medium alone.
RT-PCR
Cells were harvested after 16 h of culture as established by preliminary studies (data not shown) and RNA was isolated by RNeasy silica-gel membrane (RNeasy Mini Kits; Qiagen, Hilden, Germany) following the manufacturer's instructions. In brief, cells were centrifuged, lysed in the culture plate, homogenized in the presence of guanidinium-isothiocyanate buffer and the sample was then applied to an RNeasy mini spin column to obtain total RNA. Reverse transcription was performed by mixing 1 μg of total RNA, 200 U of Superscript RNase H− Reverse Transcriptase (Gibco BRL, Life Technologies, Paisley, UK) and 0.5 μg of oligo(dT) primer (Gibco BRL). The cDNAs were stored at −20°C until use. Samples were tested for the presence of the cytokine gene sequences by PCR. Primer pairs for β-actin (sense, 5′-ATCTGGCACCA CACCTTCTACAATGAGCTGCG-3′; anti-sense 5′-CGTCATA CTCCTGCTTGCTGATCCACATCTGC-3′), IFN-γ (sense, 5′-GCATCGTTTTGGGTTCTCTTGGCTGTTACTGC-3′; anti-sense 5′-CTCCTTTTTCGCTTCCCTGTTTTAGCTGCTGG-3′;), TNF-α (sense 5′-GAGTGACAAGCCTGTAGCCCATGTTGTAGCA-3′; anti-sense 5′-GCAATGATCCCAAAGTAGACCTGCCCAGACT-3′) and IL-4 (sense 5′-CGGCAACTTTGACCACGGACACAA GTGCGATA-3′; anti-sense 5′-ACGTACTCTGGTTGGCTTCCT CACAGGACAG-3′) were purchased from Clontech (Palo Alto, CA). Primer pairs for IL-12 p35 (sense 5′-CCTC AGTTTGGCCAG AAACC-3′; anti-sense 5′-GGTCTTTCTGGAGG CCAGGC-3′) and IL-12 p40 (sense 5′-CCAAGAACTTGCAGCTGAAG-3′; anti-sense 5′-TGGGTCTATTCCGTTGTGTC-3′) were designed as described by Sieling et al. [11]. cDNA (2 μl) was added to 48 μl of a solution containing 10 mm Tris–HCl pH 8.3, 50 mm KCl, 1.5 mm MgCl2, 0.2 mm each deoxynucleoside triphosphate, 1 U Taq polymerase (Amplitaq; Perkin-Elmer Cetus, Chester, UK), 0.4 μm each primer for β-actin, IFN-γ, TNF-α and IL-4 and 0.5 μm each primer for IL-12 p35 and IL-12 p40. We used a hot-start technique and PCR conditions were as follows: one cycle of 94°C for 7 min, followed by 35 cycles of 94°C for 45 s, 60°C for 45 s, 72°C for 2 min for β-actin, IFN-γ, TNF-α and IL-4; one cycle of 94°C for 7 min followed by 33 cycles of 94°C for 40 s, 62°C for 40 s and 72°C for 1 min for IL-12 p35 and IL-12 p40. Positive control DNAs for β-actin IFN-γ, TNF-α, IL-4 were obtained from Clontech. Positive control DNAs for IL-12 p35 and IL-12 p40 were obtained from the Epstein–Barr virus (EBV)-transformed cell line RPMI 8866 cultured in the presence of phorbol 12-myristate 13-acetate (PMA; 50 ng/ml) plus ionomycin (1 μg/ml) (Sigma Chemical Co., St Louis, MO) for 16 h [12]. The cDNA samples were amplified in the Gene Amp PCR System 9600 (Perkin-Elmer Cetus). Ten microlitres of the amplified products were fractionated in 1.5% agarose gel containing 0.5 μg/ml ethidium bromide and visualized by an ultraviolet transilluminator. The negatives were analysed by laser densitometry GS 700 (BioRad, Richmond, CA).
Semiquantitative analysis of PCR products
To normalize the amounts of RNA in each sample we amplified the housekeeping gene β-actin. The uniformity of the amplification process was verified by comparing the intensity of the cytokine signal with that of a simultaneously amplified positive control. The results were expressed in arbitrary units (AU). Semiquantitative PCR procedures quantify the relative mRNA for any given cytokine but they do not allow for direct comparison of exact levels (i.e. units) of mRNA between different cytokines, nor do they determine the exact amount of cytokine mRNA present in each sample.
Statistical analysis
All results are expressed as arithmetic means and ranges. Wilcoxon signed-rank test for paired and non-paired data was used to compare mRNA cytokine levels in samples from patients and uninfected controls. Differences with a confidence level of 95% or higher were considered statistically significant (P < 0.05).
RESULTS
Positivity to cytokine mRNA
Parasite antigen-stimulated and unstimulated PBMC obtained and cultured before and after therapy from most CE patients expressed mRNA for IL-12 p35, IL-4, IFN-γ and TNF-α (Fig. 1). In contrast, a high percentage of samples (86%) expressed IL-12 p40 mRNA, only after therapy and only samples from patients who responded to therapy. Percentages of positivity for IL-12 p35, IFN-γ and TNF-α mRNA were similar in patients and uninfected controls. None of the uninfected control samples showed detectable IL-4 mRNA expression, and only one control subject resulted positive for IL-12 p40 mRNA.
Fig. 1.
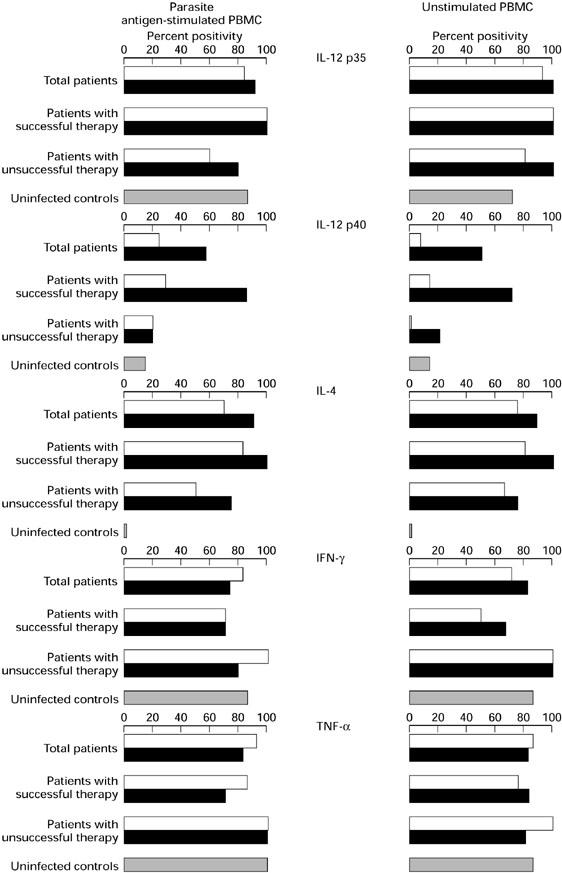
Percentages of positivity for cytokine mRNA expression in PBMC from cystic echinococcosis (CE) patients before and after therapy and from uninfected controls. □, Pre-therapy; ▪, post-therapy.
Semiquantitative analysis of RT-PCR products
IL-12 p35 mRNA
Parasite antigen-stimulated and unstimulated PBMC obtained before and after therapy from patients with successful therapy and from those with unsuccessful therapy showed high IL-12 p35 mRNA expression: expression was highest before therapy in patients with successful therapy. PBMC from uninfected controls showed the lowest mean IL-12 p35 mRNA expression and the difference with pretreatment expression in successfully treated patients reached statistical significance (P = 0.03 in parasite antigen-stimulated cultures; P = 0.04 in unstimulated cultures) (Table 2).
Table 2.
Cytokine mRNA expression in patients with cystic echinococcosis and in uninfected controls
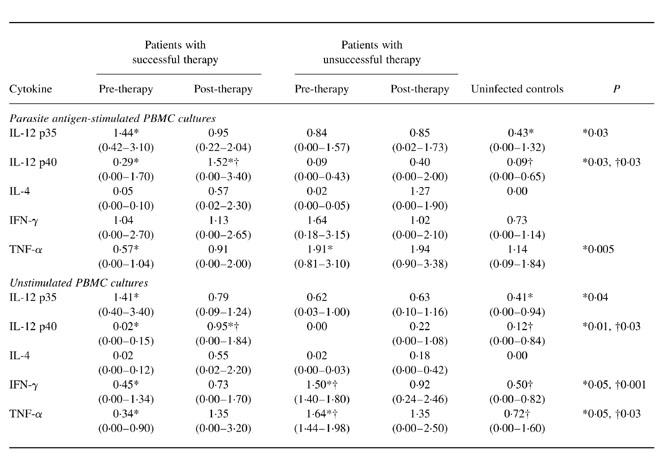
All values are arithmetic means and (ranges) expressed in arbitrary units (AU).
IL-12 p40 mRNA
In stimulated and unstimulated cultures, PBMC from patients who were IL-12 p40-positive before therapy showed minimal IL-12 p40 mRNA expression. IL-12 p40 mRNA expression increased after therapy so that in patients who responded, pre-therapy and post-therapy values differed significantly (0.29 AU versus 1.52 AU in parasite antigen-stimulated cultures: P = 0.03; 0.02 AU versus 0.95 AU in unstimulated cultures: P = 0.01). Stimulated and unstimulated PBMC obtained from uninfected controls showed significantly lower IL-12 p40 expression than PBMC from successfully treated patients after therapy (P = 0.03).
IL-4 mRNA
Under both culture conditions and in both groups of patients IL-4 mRNA expression was very weak before therapy and increased after treatment; notably, mean expression peaked after therapy in parasite antigen-stimulated PBMC from patients who failed to respond.
IFN-γ mRNA
In stimulated PBMC obtained after therapy from patients who responded, IFN-γ mRNA expression remained unchanged; in unstimulated PBMC, expression slightly but not significantly increased. Under both culture conditions, in PBMC obtained after therapy from patients who failed to respond, IFN-γ mRNA expression decreased. Interestingly, pre-therapy IFN-γ mRNA expression was higher in patients who did not respond than in those who responded (unstimulated cultures, P = 0.05). PBMC from uninfected controls had lower IFN-γ mRNA expression than PBMC from patients and differed significantly from expression in unstimulated PBMC from patients in whom therapy failed (0.50 AU versus 1.50 AU; P = 0.001).
TNF-α mRNA
In stimulated and unstimulated PBMC obtained after therapy from patients who responded, TNF-α mRNA expression increased; in stimulated PBMC from patients who did not respond it remained unchanged and in unstimulated PBMC it slightly but not significantly decreased. Again, pre-therapy TNF-α mRNA expression was significantly higher in patients who did not respond than in those who responded (stimulated cultures, P = 0.005; unstimulated cultures, P = 0.05). PBMC from uninfected controls had lower TNF-α mRNA expression than PBMC from patients and differed significantly from expression in unstimulated PBMC from patients in whom therapy failed (0.72 AU versus 1.64 AU; P = 0.03).
Cytokine mRNA expression in the individual CE patients before and after therapy
Analysis of individual pre- and post-therapy cytokine mRNA patterns in parasite antigen-stimulated PBMC showed that IL-12 p35, IFN-γ and TNF-α mRNA expression varied widely among patients (Fig. 2). Before therapy, both groups of patients had very low or undetectable IL-12 p40 and IL-4 mRNA expression. After therapy, only successfully treated patients had detectable IL-12 p40 mRNA expression and especially unsuccessfully treated patients had increased IL-4 mRNA expression.
Fig. 2.
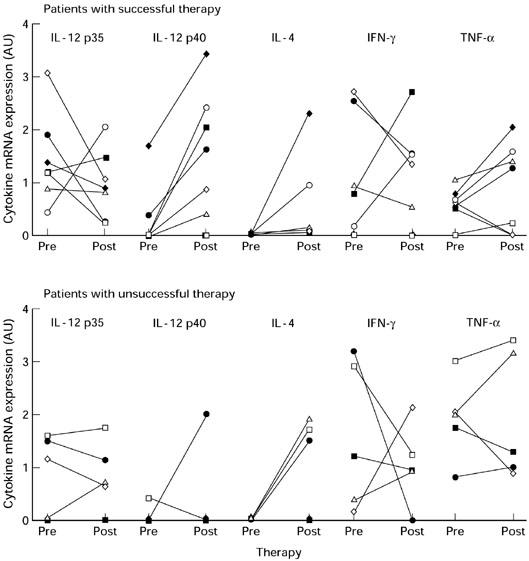
Parasite antigen-stimulated cytokine mRNA expression in the individual cystic echinococcosis (CE) patients before and after therapy.
DISCUSSION
In many chronic infections the clinical outcome of the disease is associated with Th1 or Th2 cell activation. In most helminthic infections the healing or protection is related to Th2 cell activation [13–15], even if some infections, including trichinellosis, schistosomiasis, and cystic and alveolar echinococcosis, display Th2-mediated susceptibility [6,7,16–19].
The current study of 12 pharmacologically treated CE patients suggests that chemotherapy influences the immune response, thus altering Th1/Th2 cytokine mRNA patterns. The main distinguishing finding is that these changes mainly involved IL-12 p40 and IL-4 mRNA expression. In contrast to IL-12 p35 mRNA, only partially regulated, we detected IL-12 p40 mRNA almost exclusively in successfully treated patients at the end of chemotherapy. IL-4 mRNA was weakly expressed before therapy, and increased thereafter, especially in PBMC from patients in whom therapy failed. IFN-γ and TNF-α mRNA expression, apart from semiquantitative individual variations, increased in patients who responded to therapy and decreased in those who did not. Whereas we detected IL-12 p35, IFN-γ and TNF-α mRNAs in patients and in uninfected controls, we found IL-12 p40 and IL-4 mRNAs in patients alone, suggesting a close association between these two cytokines and CE. Further investigations are necessary to verify whether the up-regulation of IL-12 p40 and IL-4 is due to specific hydatid antigenic epitopes. In a recent study, Fauser & Kern have already shown that PBMC from CE patients can be reactivated only by antigenic stimulus of a closely related species and not by a distantly related species [20]. Using a qualitative RT-PCR, they have reported enhanced Th2 cytokine mRNA expression (especially IL-5) by PBMC from CE patients after stimulation with parasite antigens. In contrast to us, they found that PBMC from healthy controls invariably expressed IL-4 mRNA. The discrepancy could depend either on differences in the control populations or on experimental conditions. In patients with chronic alveolar echinococcosis a Th2-type immune response has also been reported and in particular, a specific up-regulation of IL-5 mRNA by CD4+ correlates with severity of the disease [18,19]. In contrast to Jenne et al., in a previous in vitro study in CE patients we found no association between IL-5 production and the outcome of therapy [21]. In the present study, as a prototype of the Th2 response we used IL-4, because we had previously found it useful as an immunological marker of therapeutic success. We also found it more specific and generally more informative than IL-10.
In previous in vitro and in vivo studies we found high Th1 cytokine concentrations (IFN-γ) in patients who responded to chemotherapy and high Th2 cytokine concentrations (IL-4 and IL-10) in patients who did not [6,7]. In the present in vitro study of cytokine expression at the molecular level, including TNF-α and IL-12, we confirm these results, especially for Th1 cell activation. The effective production of TNF-α and IL-12 awaits confirmation from studies now underway in our laboratory. A recent study of T cell activity in BALB/c mice infected with protoscoleces of E. granulosus or with implanted cysts [22] adds further information on the association of the Th2-type response (IL-4 and IL-10 production) with advanced hydatid infections and of the Th1-type response (IFN-γ production) with protection. In particular, it relates IFN-γ activity with the death of the protoscoleces and the disappearance of the implanted E. granulosus cyst.
To our knowledge, this is the first study to investigate the role of IL-12 in human CE. The association of IL-12 with pharmacological treatment seemingly suggests a role of this cytokine in resolving the disease—probably by promoting Th1 cell activation (IFN-γ). IL-12 may also prove useful in predicting the clinical course of E. granulosus infection. Further investigation on a larger population of patients with a longer follow up will clarify the relation of IL-12 and chemotherapeutic strategies. In alveolar echinococcosis, in vivo treatment with recombinant IL-12 shows that this cytokine is of crucial importance in inhibiting larval growth (mainly through production of IFN-γ), suggesting its usefulness in therapy [23].
Because IL-12 and TNF-α are mainly produced by macrophages, our preliminary results, showing that chemotherapy may induce changes in cytokine mRNA expression, suggest a selective modulation of macrophages themselves by parasite antigens. Further studies are necessary to clarify the functional activity of IFN-γ and TNF-α in the induction of anti-parasite mechanisms, including nitric oxide production and chemokine expression. Until now the induction of nitric oxide production, as a defence mechanism, has been demonstrated in vitro and in vivo in E. multilocularis but not in E. granulosus infection [24,25]. Another unanswered question is why pre-therapy expression of TNF-α and IFN-γ mRNA was more intense in patients in whom therapy failed than in those in whom it succeeded. Multiple clinical and experimental studies have demonstrated the important role of TNF-α in the pathogenesis of various parasitic diseases. A moderate production of TNF-α is beneficial to the host, as it is in infection with Plasmodium and Tripanosoma; conversely, excessive production is deleterious to the host because it leads to immunosuppression [26,27].
Several mechanisms might explain why cytokine expression varied so widely in our patients. One is a drug-induced antigenic variation. These cytokine differences may also reflect the individual's expanding peripheral repertoire during chronic infection. Another explanation involves host genetic factors. Our observation that many of our patients' Th1/Th2 cytokine profiles differed from the expected pattern, implies that the Th1/Th2 model cannot be oversimplified, neither can the interaction between host and parasite always be addressed in the context of Th1 and Th2 cells. The Th1/Th2 concept cannot entirely account for the true complexity in vivo. Survival of the parasite depends on various mechanisms rather than on a generic ability to ignore the Th1- or Th2-type responses [28]. The failure to control or resolve infectious diseases often results from an inappropriate rather than an insufficient immune response [29]. Immunotherapy for infections should therefore aim at developing a successful immune response with minimum adverse effects.
In conclusion, this preliminary study suggests that in patients with CE, pharmacological treatment with benzimidazole carbamates induces changes in cytokine mRNA expression, probably modifying the host–parasite relationship. Our results require the analysis of a larger group of patients with a longer follow up to draw firm conclusions and prompt further studies to assess the role of IL-12 and of macrophages in protecting humans against E. granulosus infection. Once the protective effects of IL-12 have been ascertained, a promising clinical application could be combined therapy with IL-12 and an anti-parasitic drug to promote Th1 cell activation [23,30].
REFERENCES
- 1.Teggi A, Lastilla MG, De Rosa F. Therapy of human hydatid disease with Mebendazole and Albendazole. Antimicrob Agents Chemother. 1993;37:1679–84. doi: 10.1128/aac.37.8.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chinnery JB, Morris DL. Effect of albendazole sulphoxide on viability of hydatid protoscoleces in vitro. Trans R Soc Trop Med Hyg. 1986;80:815–7. doi: 10.1016/0035-9203(86)90392-5. [DOI] [PubMed] [Google Scholar]
- 3.Abbas AK, Murphy M, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 4.Perez VL, Lederer JA, Lichtman AH, Abbas AK. Stability of Th1 and Th2 populations. Int Immunol. 1995;7:869–75. doi: 10.1093/intimm/7.5.869. [DOI] [PubMed] [Google Scholar]
- 5.Finkelman FD, Holmes J, Katona IM, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Ann Rev Immunol. 1990;8:303–33. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 6.Riganò R, Profumo E, Ioppolo S, Notargiacomo S, Ortona E, Teggi A, Siracusano A. Immunological markers indicating the effectiveness of pharmacological treatment in human hydatid disease. Clin Exp Immunol. 1995;102:281–5. doi: 10.1111/j.1365-2249.1995.tb03778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riganò R, Profumo E, Ioppolo S, Notargiacomo S, Teggi A, Siracusano A. Serum cytokine detection in the clinical follow up of patients with cystic echinococcosis. Clin Exp Immunol. 1999;115:503–7. doi: 10.1046/j.1365-2249.1999.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caremani M, Benci A, Maestrini R, Rossi G, Menchetti D. Abdominal cystic hydatid disease (CHD): classification of sonographic appearance and response to treatment. J Clin Ultrasound. 1996;24:491–500. doi: 10.1002/(SICI)1097-0096(199611/12)24:9<491::AID-JCU1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 9.WHO Informal Working Group on Echinococcosis. Guidelines for treatment of cystic and alveolar echinococcosis in humans. Bull World Health Org. 1996;74:231–42. [PMC free article] [PubMed] [Google Scholar]
- 10.Riganò R, Profumo E, Di Felice G, Ortona E, Teggi A, Siracusano A. In vitro production of cytokines by peripheral blood mononuclear cells from hydatid patients. Clin Exp Immunol. 1995;99:433–9. doi: 10.1111/j.1365-2249.1995.tb05569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sieling PA, Wang X, Gately MK, et al. IL-12 regulates T helper type 1 cytokine responses in human infectious disease. J Immunol. 1994;153:3639–47. [PubMed] [Google Scholar]
- 12.Wolf SF, Temple PA, Kobayashi M, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991;146:3074–81. [PubMed] [Google Scholar]
- 13.Folkard SG, Hogarth PJ, Taylor MJ, Bianco AE. Eosinophils are the major effector cells of immunity to microfilariae in a mouse model of onchocerchiasis. Parasitology. 1996;112:323–7. doi: 10.1017/s0031182000065847. [DOI] [PubMed] [Google Scholar]
- 14.Folkard SG, Taylor MJ, Butcher GA, Bianco AE. Protective responses against skin-dwelling microfilariae of Onchocerca lienalis in severe combined immunodeficient mice. Infect Immun. 1997;65:2846–9. doi: 10.1128/iai.65.7.2846-2851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finkelman FD, Shea-Donohue T, Goldhil J. Cytokine regulation of host defence against parasitic gastrointestinal nematodes: lessons from studies with rodent models. J Exp Med. 1997;15:505–30. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 16.Pond L, Wassom DL, Hayes CE. Influence of resistant and susceptible genotype, IL-1, and lymphoid organ on Trichinella spiralis-induced cytokine secretion. J Immunol. 1992;149:957–65. [PubMed] [Google Scholar]
- 17.Kaplan MH, Whitfield JR, Boros DL, Grusby MJ. Th2 cells are required for the Schistosoma mansoni egg-induced granulomatous response. J Immunol. 1998;160:1850–6. [PubMed] [Google Scholar]
- 18.Sturm D, Menzel J, Gottstein B, Kern P. Interleukin-5 is the predominant cytokine produced by peripheral blood mononuclear cells in alveolar echinococcosis. Infect Immun. 1995;63:1688–95. doi: 10.1128/iai.63.5.1688-1697.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenne L, Kilwinski J, Scheffold W, Kern P. IL-5 expressed by CD4+ lymphocytes from Echinococcus multilocularis-infected patients. Clin Exp Immunol. 1997;109:90–97. doi: 10.1046/j.1365-2249.1997.4031299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fauser S, Kern P. T-lymphocyte cytokine mRNA expression in cystic echinococcosis. Acta Tropica. 1997;64:35–51. doi: 10.1016/s0001-706x(96)00638-9. [DOI] [PubMed] [Google Scholar]
- 21.Rigano R, Profumo E, Teggi A, Siracusano A. Production of IL-5 and IL-6 by peripheral blood mononuclear cells (PBMC) from patients with Echinococcus granulosus infection. Clin Exp Immunol. 1996;105:456–9. doi: 10.1046/j.1365-2249.1996.d01-796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogan MT. T-cell activity associated with secondary infections and implanted cysts of Echinococcus granulosus in Balb/c mice. Parasite Immunol. 1998;20:527–33. doi: 10.1046/j.1365-3024.1998.00180.x. [DOI] [PubMed] [Google Scholar]
- 23.Emery I, Leclerc C, Sengphommachanh K, Vuitton DA, Liance M. In vivo treatment with recombinant IL-12 protects C57BL/6J mice against secondary alveolar echinococcosis. Parasite Immunol. 1998;20:81–91. doi: 10.1046/j.1365-3024.1998.00131.x. [DOI] [PubMed] [Google Scholar]
- 24.Kanazawa T, Asahi H, Hata H, Mochida K, Kagei N, Stadecker MJ. Arginine-dependent generation of reactive nitrogen intermediates is instrumental in the in vitro killing of protoscoleces of Echinococcus multilocularis by activated macrophages. Parasite. 1993;15:619–23. doi: 10.1111/j.1365-3024.1993.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 25.Bories C, Liance M, Bories PN, Sengphommachanh K, Simon F, Houin R. No evidence for increased production of nitric oxide in C57BL/6J mice infected with Echinococcus multilocularis. Ann Trop Med Parasitol. 1996;90:641–4. doi: 10.1080/00034983.1996.11813095. [DOI] [PubMed] [Google Scholar]
- 26.Schaffer N, Grau GE, Hedberg K, Davachi F, Lyamba B, Hightower AW, Breman JG, Nguyen-Dinh P. Tumor necrosis factor and severe malaria. J Infect Dis. 1991;163:96–101. doi: 10.1093/infdis/163.1.96. [DOI] [PubMed] [Google Scholar]
- 27.Okomo-Assoumou MC, Daulouede S, Lemsre JL, N'Zila-Mouanda A, Vincendeau P. Correlation of high serum levels of tumor necrosis factor-α with disease severity in human African trypanosomiasis. Am J Trop Med Hyg. 1995;53:539–43. doi: 10.4269/ajtmh.1995.53.539. [DOI] [PubMed] [Google Scholar]
- 28.Allen JE, Maizel RM. Th1-Th2: reliable paradigm or dangerous dogma? Immunol Today. 1997;18:387–92. doi: 10.1016/s0167-5699(97)01102-x. [DOI] [PubMed] [Google Scholar]
- 29.Powrie F, Coffman RL. Cytokine regulation of T-cell function: potential for therapeutic intervention. Immunol Today. 1993;14:270–4. doi: 10.1016/0167-5699(93)90044-L. [DOI] [PubMed] [Google Scholar]
- 30.Romani L, Puccetti P, Bistoni F. Interleukin-12 in infectious diseases. Clin Microb Rev. 1997;10:611–36. doi: 10.1128/cmr.10.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]


