Abstract
Highly active anti-retroviral therapy (HAART) is associated with reduction in the morbidity and mortality of patients with advanced HIV-1 disease. The ability of such treatment to improve immune responses against HIV-1 and opportunistic pathogens is variable and limited. Addition of cytokine immunotherapy to this treatment may improve immune responses. IL-2 with or without granulocyte-macrophage colony-stimulating factor (GM-CSF) was administered to HIV-1+ individuals receiving HAART with undetectable viral loads, and CD4 counts < 100 cells/μl. In one patient presenting with Mycobacterium avium complex (MAC) infection, we evaluated the effect of cytokine immunotherapy on lymphocyte phenotype; plasma viral load; proliferative responses to mitogens, recall and HIV-1 antigens; cytokine production and message in response to non-specific and specific stimuli; and natural killer (NK) cell activity. Proliferation assays were performed in two similar patients. Before cytokine immunotherapy the predominant CD8+ population was mainly CD28−. No proliferation or IL-2 production was seen in response to mitogens, recall or HIV-1 antigens; and no HIV-1 peptide-specific interferon-gamma (IFN-γ)-secreting cells were present. Low levels of IL-4 were detected in response to antigens to which patients had been exposed, associated with up-regulated expression of costimulatory molecules influenced by IL-4. Following IL-2 administration, loss of IL-4 was associated with increased NK cell activity and HIV-1 peptide-specific and non-specific IFN-γ-producing cells. Proliferative responses associated with IL-2 production and responsiveness were only seen after subsequent concomitant administration of GM-CSF with IL-2. These changes mirrored clinical improvement. An imbalance of lymphocyte subsets may account for immune unresponsiveness when receiving HAART. Restoration of responses following immunotherapy suggests a shift towards a lymphocyte profile with anti-pathogen activity.
Keywords: HIV-1, T cell responses, anti-retroviral therapy, cytokines, immune reconstitution
INTRODUCTION
Treatment of HIV-1-infected persons with highly active anti-retroviral therapy (HAART) may result in remission of opportunistic infections as well as HIV-1-related symptoms. This effect is not always observed and specific opportunistic infections may persist or recur [1–4]. Type 1 cytokine-secreting cells synthesize IL-2 and interferon-gamma (IFN-γ), which have anti-viral activity, and C-C chemokines which may contribute towards CD4+ cell resistance to HIV-1 infection [5]. Type 2 cytokine-secreting cells secrete IL-4 and IL-10. These two subsets play a central regulatory role in HIV-1 infection, and the balance between them may dictate the final immunological and clinical outcome [6,7]. Previous studies suggest that a type 2 cytokine environment, often found in HIV-1 disease, has an immunosuppressive anti-proliferative effect [8,9]. An atypical/anergic subset of the CD8+, CD28−, CD57+, CD38+ cells in HIV-1-infected patients may also substantiate the immunosuppressed state [10–12]. HAART treatment of chronic HIV-1 infection results in a gradual increase in non-specific CD4+ T cell proliferative responses, but increases in HIV-1-specific CD4+ T lymphocyte proliferation have not been observed [13–16].
We investigated the immunological response to IL-2 and/or granulocyte-macrophage colony-stimulating factor (GM-CSF) used as salvage therapy in conjunction with HAART in HIV-1-infected patients, presenting with complications such as treatment-resistant Mycobacterium avium complex (MAC) and little immune reconstitution despite virologically suppressive HAART. We performed flow cytometry, proliferation assays, cytokine production and message assessment by ELISPOT assays, bioassays and reverse transcriptase-polymerase chain reaction (RT-PCR), to assess alterations in cell-mediated immunity and the relationship with clinical outcome. Our findings suggest that the combination of HAART and immunotherapy reversed a type 2-like immunosuppressive state, decreasing IL-4 levels and inducing HIV-1 peptide-specific IFN-γ-secreting cells. Non-specific natural killer (NK) responses were also enhanced. HIV-1-specific proliferative responses, accompanied by IL-2 production and up-regulated expression of IL-2-specific mRNA, were noted only upon concomitant administration of IL-2 and GM-CSF. Further studies of such immunomodulators in severely immunocompromised patients are warranted before progressing to formal long-term randomized controlled trials.
PATIENTS AND METHODS
Patients
Three patients with advanced HIV-1 disease (CD4 counts < 100) had received HAART for > 12 months (plasma viral load below detectable levels; with modest effects on CD4 counts). Patient 3 was diagnosed with MAC which was unresponsive to conventional treatment, although partly responsive to liposomal amikacin. He had been on HAART for 14 months, with stavudine, lamivudine and indinavir, which had effectively suppressed plasma viral load to below detectable levels. However, the rise in CD4 count remained modest (nadir = 5, peak = 61 cells/μl). IL-2 (Chiron Therapeutics, Uxbridge, UK) was administered (5 million units b.d. subcutaneously for 5 days, in three cycles 6 weeks apart). During the third cycle in addition to IL-2, GM-CSF (Novartis, Schering-Plough, Camberley, UK) was included (subcutaneous injection 60 000 U/kg daily). Peripheral blood was taken for analysis immediately prior to the first IL-2 administration, and 3 weeks after completion of the first, second and third cycles. Patients 1 and 2 received only IL-2 in addition to HAART. The patients' informed consent and Ethics Committee approval were obtained for the studies described.
HLA typing
HLA haplotypes of patients and donors were assessed by PCR-SSP [17,18].
Plasma viral RNA assay
Viral load in patient plasma was measured at each time point of sample collection using the Amplicor HIV-1 Monitor assay (detection limit < 200 copies/ml; Roche Diagnostic System, Welwyn Garden City, UK).
Isolation of peripheral blood mononuclear cells and culture conditions
Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation and cultured in supplemented RPMI 1640 medium [19]. All cell lines were cultured in 10% fetal calf serum (FCS)/RPMI in 25-cm2 flasks (Greiner Laboritechnik, Dursley, UK) and incubated at 37°C.
Antibodies
The following murine MoAbs were used: anti-human CD3, CD4, CD8, CD16, CD19 (Ortho-Trio; Ortho-Clinical Diagnostics, Amersham, UK); anti-human CD45RA–FITC, CD45RO–FITC, CD57–PE and relevant isotype-matched controls (Sigma, Poole, UK); anti-human CD28–FITC, αβ TCR–FITC, γδ TCR–PE and relevant isotype-matched controls (Pharmingen Division, Becton Dickinson, Oxford UK); anti-human CD1a, CD40, CD80 and CD86 (Serotec, Oxford, UK); anti-human CD23 and HLA-DR (Dako, High Wycombe, UK); anti-human CD38 (Sigma) and anti-human CD95 (Pharmingen).
Flow cytometry
The Cytotron Absolute (Ortho) was used for flow cytometric analyses of total CD3+, CD4+, CD8+, CD16+ and CD19+ lymphocytes using whole blood. For further analysis of cell surface markers, PBMC were stained according to the manufacturer's instructions and where relevant, cells were further incubated with secondary antibody (FITC goat anti-mouse immunoglobulin; Dako). Detailed phenotypic evaluation was carried out on a Becton Dickinson FACScalibur and analysed with CellQuest software.
Proliferation assays
PBMC (105/well) were cultured with antigen, mitogen or cytokine in round-bottomed microtitre plates (Greiner), in 10% AB plasma/RPMI (200 μl; Sigma). Antigens and mitogens were used as shown in Table 1. On day 5, 100 μl of supernatant were collected and stored at −20°C for subsequent cytokine measurement. Each well was then pulsed with 1 μCi 3H-methyl thymidine (3H-TdR; Amersham Int., Aylesbury, UK) and 16 h later cells were harvested onto glassfibre filtermats (Wallac Oy, Turku, Finland). Proliferation as measured by 3H-TdR incorporation was evaluated by liquid scintillation spectroscopy using a 1205 Betaplate counter (Wallac) [19]. Results are expressed as the mean ct/min for triplicate cultures, with percentage error of the mean < 15%. Control wells, for calculation of background activity, contained PBMC only.
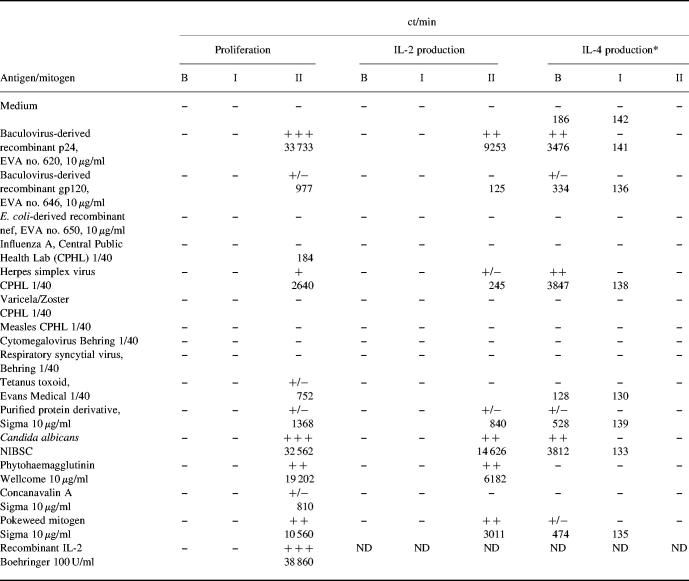
Table 1.
Proliferation and cytokine production in response to HIV-1 recombinant antigens, recall antigens, mitogens, and IL-2
B, Before IL-2 immunotherapy; I, after IL-2 immunotherapy; II, after IL-2 and granulocyte-macrophage colony-stimulating factor (GM-CSF) immunotherapy.
*Levels of IL-4 detected correspond to 1 U/ml of IL-4.
−, < 200 ct/min; +/−, 200–1500 ct/min; +, 1500–3000 ct/min; + +, 3000–20 000 ct/min; + + +, > 20 000 ct/min. Results are expressed as the mean ct/min for triplicate cultures, with percentage error of the mean < 15%.
Measurement of IL-2 and IL-4 production
For cytokine assays 50 μl of supernatants, from proliferative cultures, were transferred in two sets of 96-well round-bottomed plates as triplicates for measurement of IL-2 and IL-4 using indicator cell lines CTLL-2 (European Collection of Animal Cell Culture (ECACC), Salisbury, UK) and CT.h4S (a generous gift of W. Paul, Bethesda, MD) as described previously [19]. Briefly, CTLL-2 (103 cells/well) or CT.h4S (5 × 103 cells/well) were added in 50 μl to give a final 100 μl. After 24 h in culture, wells were pulsed with 3H-TdR and harvested as described above. Results are expressed as the mean ct/min for triplicate cultures, with percentage error of the mean < 15%.
ELISPOT assays
Detection of single-cell IFN-γ release was carried out according to the protocol described by Lalvani et al. [20]. Briefly, 2.5 × 105 cells/well were cultured with appropriate HLA-B35 restricted peptide (detailed in Fig. 3c) or phytohaemagglutinin (PHA) at final concentrations of 10 μg/ml in 96-well polyvinylidene difluoride-backed plates (Millipore, Watford, UK) coated with anti-IFN-γ MoAb (Mabtech, Stockholm, Sweden). Negative controls comprised cells cultured in the presence or absence of irrelevant peptide. Plates were incubated overnight at 37°C, and IFN-γ spot-forming cells (SFC) were detected according to the manufacturer's instructions (Mabtech).
Fig. 3.
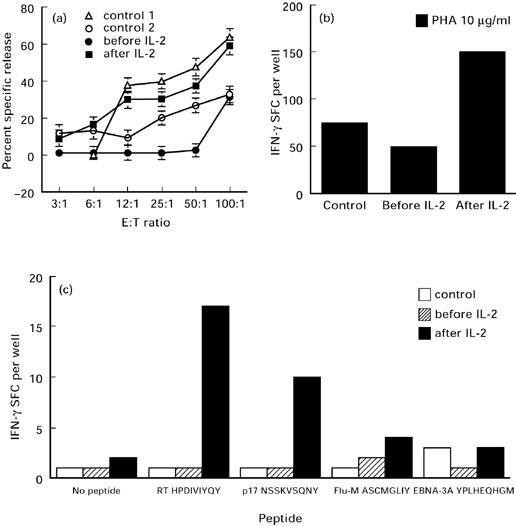
IL-2 increases natural killer (NK) activity and induces non-specific and HIV-1 peptide-specific IFN-γ-secreting cells. (a) Measurement of the NK-mediated cytotoxicity by patient's NK cells before (•) and after (▪) IL-2 immunotherapy, and by uninfected controls (open symbols); s.d. of the mean for each effector cell dilution was < 5%. (b) ELISPOT assay measuring IFN-γ release in response to phytohaemagglutinin (PHA) by peripheral blood mononuclear cells (PBMC) obtained before and after IL-2 immunotherapy. The data represent average values at each point and variation among duplicates was < 10%. (c) ELISPOT assays measuring IFN-γ release in response to HLA-B35-restricted peptides. Patient cells obtained before and after IL-2 immunotherapy, and HLA-B35 uninfected donor cells were stimulated with or without HLA-B35-restricted peptides: (i) HIV-RT: HPDIVIYQY; (ii) HIV-p17: NSSKVSQNY; (iii) Flu-M: ASCMGLIY; (iv) EBV-EBNA3A: YPLHEQHGM. Patient 3 HLA phenotype: A1/24, B35/61, Bw6 C4/1202 DR4/7 DRB4-53 DQB0601; uninfected donor HLA phenotype: A11/33 B7/35 Bw6 C4/1202 DR1/1303 DRB3-52 DRB4-53 DQ5/7.
NK assays
K562 target cells (107; ECACC) were labelled with 100 μCi 51Cr at 37°C for 1 h, washed, resuspended at 105/ml and 100 μl were added to each well of a microtitre plate. Effector cells (100 μl) (uninfected donor or patient PBMC) were added to give effector to target ratios ranging from 100:1 to 3:1. Each dilution was carried out in triplicate and two controls (spontaneous and total release) were included. Specific release of 51Cr from radiolabelled target cells was assessed as described previously [19].
RNA preparation and RT-PCR
PBMC, obtained after the IL-2 and GM-CSF cycle in patient 3, were cultured for 4 days with or without p24, Candida antigen (CAN) and IL-2, at final concentrations as described in Table 1. Every 24 h 106 cells were taken for RNA isolation. Total RNA was extracted using RNA STAT-60 (AMS Biotechnology, Witney, UK) and treated with RNase-free DNase I (Boehringer, Roche Diagnostics, Lewes, UK). The RNA sample was then preheated at 65°C for 10 min, placed on ice for a further 10 min, and first-strand cDNA synthesis was obtained using the Bulk First-Strand cDNA Synthesis kit (Pharmacia, St Albans, UK). After 1 h, the reaction was stopped by incubation at 90°C for 5 min and samples were cooled on ice. Amplification of IL-2, IL-4 and IL-10 gene-specific cDNA was performed using primers: IL-2 (upstream 5′ primer 5′ CATTGCACTAAGTCTTGCACTTGTCA 3′ and downstream 3′ 5′CGTTGATATTGCTGATTAAGTCCCTG 3′); IL-4 (5′ primer 5′ CGGCAACTTTGACCACGGACACAAGTGCGATA 3′ and 3′ primer 5′ ACGTACTCTGGTTGGCTTCCTTCACAG GACAG 3′); and IL-10 (5′ primer 5′ AAGCTGAGAACCAAG ACCCAGACATCAAGGCG 3′ and 3′ primer 5′ AGCTATCCC AGAGCCCCAGATCCGATTTTGG 3′) (all Clontech, Basingstoke, UK) following the standardized protocol from Clontech. Samples were submitted to 35 cycles of amplification with the following cycle profiles: denaturation at 94°C for 45 s, annealing at 60°C for 45 s, extension at 72°C for 2 min. After amplification, 15 μl of each PCR reaction were resolved on a 1.8% agarose gel (Gibco BRL, Life Technologies, Paisley, UK).
RESULTS
Accumulation of CD8+CD28− anergic subpopulations associated with lack of proliferation and IL-2 production in advanced HIV-1 disease
Analysis of lymphocyte subpopulations in all three patients with advanced HIV-1, on HAART before immunotherapy, revealed predominantly anergic CD8+CD28− cells with an increased CD57+ subset (Fig. 1). This anergy was reflected in the inability of cells to proliferate in response to all antigens tested (Fig. 2). Occasionally, weak proliferative responses to pokeweed mitogen (PWM) were seen. Lack of proliferation was associated with lack of IL-2 production as well as unresponsiveness to high doses of IL-2 (100 U/ml). However, low levels of IL-4 were detected in response to antigens to which the patients were known to have been exposed, such as herpes simplex virus (HSV) for patient 1, and HSV, Candida and p24 for patient 3. We further investigated in detail the in vivo influence of IL-2 on proliferative responses in all three patients and the influence of the combination of both IL-2 and GM-CSF on T cell subsets and immune responses in one patient.
Fig. 1.
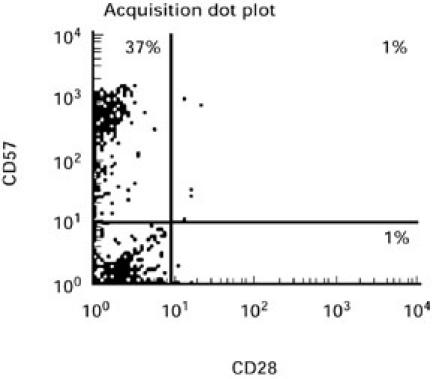
Two-colour flow cytometric analysis of the expression of CD28 and CD57 molecules on the predominant CD8+ lymphocytes (> 90% CD8+) reveals accumulation of the CD8+CD28−CD57+ anergic subset in advanced HIV-1 disease. Expression was assessed by direct immunofluorescence, using the FITC-conjugated anti-CD28 and PE-conjugated anti-CD57 MoAbs. A representative result is shown.
Fig. 2.
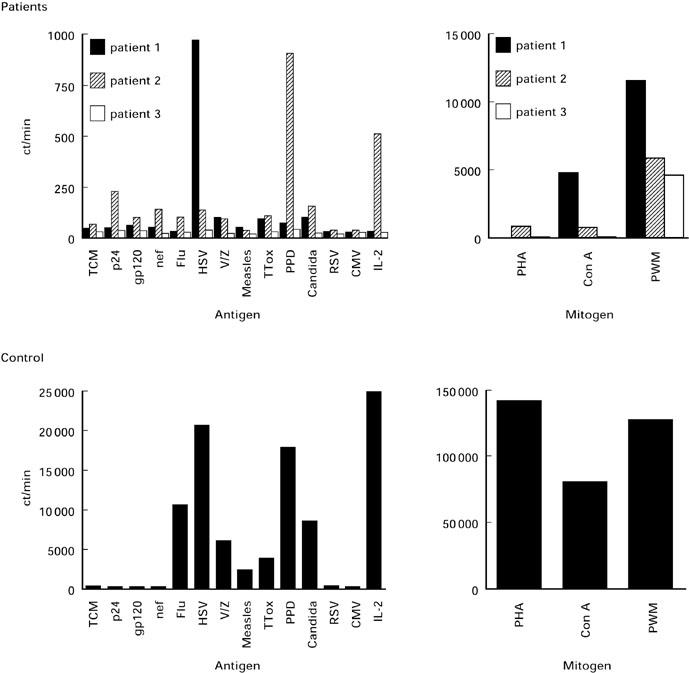
Absence of proliferative responses to recombinant HIV-1 antigens, recall antigens, mitogens and IL-2 in patients with advanced HIV-1 disease on highly active anti-retroviral therapy (HAART) (upper panel). Peripheral blood mononuclear cells (PBMC) were incubated with the indicated stimuli for 6 days and 3H-thymidine incorporation was measured. Proliferation of uninfected control cells is shown for comparison (results from a representative control are shown; lower panel).
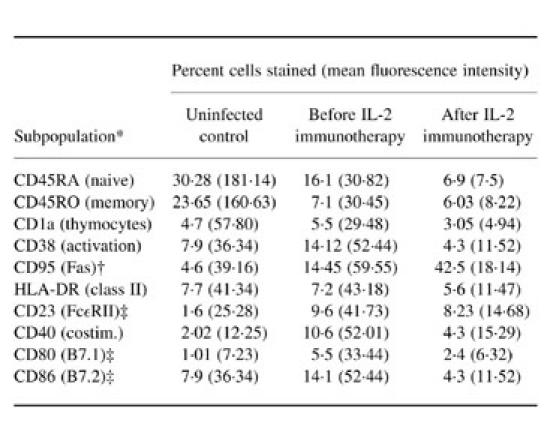
Immunophenotypic analysis of peripheral blood from patient 3 before and after IL-2 immunotherapy is shown in Tables 2 and 3. Lack of both proliferation and IL-2 production to HIV-1 recombinant antigens (p24 and gp120), mitogens and recall antigens was accompanied by the presence of measurable levels of IL-4 in tissue culture supernatant, approximately 1 U/ml, when cells were stimulated with p24, HSV and purified C. albicans antigens (Table 1). Up-regulated expression, in both percentage and mean fluorescence intensity, of IL-4-modulated markers CD23, CD40, CD80 and CD86 in comparison with control cells (Table 3), further substantiated the presence of IL-4 in the system.
Table 2.
Lymphocyte population and subpopulations by flow cytometry
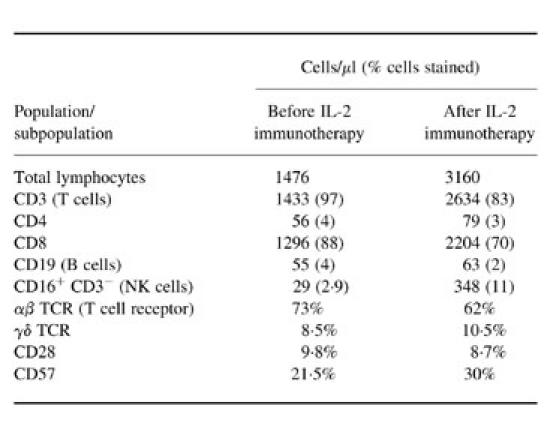
Table 3.
Single-colour flow cytometric analysis of naive/resting (CD45RA+) and memory/effector (CD45RO+) cell subpopulations, immature thymocytes, activation markers and IL-4-influenced/induced molecules
*Isotype-matched controls were < 2% (12.00).
†Need FasL for apoptosis.
‡Also expressed on activated T cells.
Representative of three replica experiments is shown.
Administration of IL-2 increased NK activity, induced HIV-1 peptide-specific IFN-γ-secreting CD8+ T cells which paralleled loss of IL-4
In all three patients after administrations of IL-2 there was no significant effect on CD4 counts and there was no improvement in proliferative responses to mitogens, recall or HIV-1 antigens. Three weeks after the first course of IL-2 in patient 3, flow cytometric analysis revealed a two-fold increase in total lymphocyte numbers, yet again barely detectable levels of CD4+ cells in peripheral blood (Table 2). Although overall numbers of CD3+ and CD8+ subsets almost doubled, their percentage of total lymphocytes decreased (Table 2). This decrease in the percentage of CD8+ subpopulation was associated with increase in the CD3−CD16+ NK cell subset (Table 2). This was accompanied by increased NK cell killing from 31% before IL-2 therapy to 59% after IL-2 therapy (Fig. 3a). Upon administration of IL-2 there was a decrease in the percentage of CD45RA+ whole PBMC subset and a further dramatic increase in CD95 expression (Table 3). Reduction in the αβ TCR subset was noted, whilst CD57 expression increased (Table 2). There were no significant changes in the expression of either γδ TCR or CD28+ subsets. There was, however, a significant decrease in CD38, CD23, CD40, CD80 and CD86 expression on total PBMC (Table 3). Despite the decrease of these molecules and loss of IL-4 in culture supernatants after IL-2 immunotherapy (Table 1), no apparent proliferation or IL-2 production were initially seen in response to mitogens, recall and HIV-1 recombinant antigens (Table 1). However, a three-fold increase in IFN-γ-secreting cells upon stimulation with PHA was seen (Fig. 3b). Measurement of HLA-B35-restricted HIV-1 peptide-specific IFN-γ-secreting cells revealed a significant increase in the activity of these cells following IL-2 immunotherapy (Fig. 3c). No IFN-γ-secreting cells were seen in negative cultures (cells only or irrelevant peptides) or by HLA-B35 uninfected control cells (low numbers of HLA-B35-restricted Epstein–Barr virus (EBV) peptide-specific IFN-γ SFC were seen).
Administration of IL-2 and GM-CSF restores proliferative responses to both HIV-1 and recall antigen, IL-2 production and responsiveness to IL-2
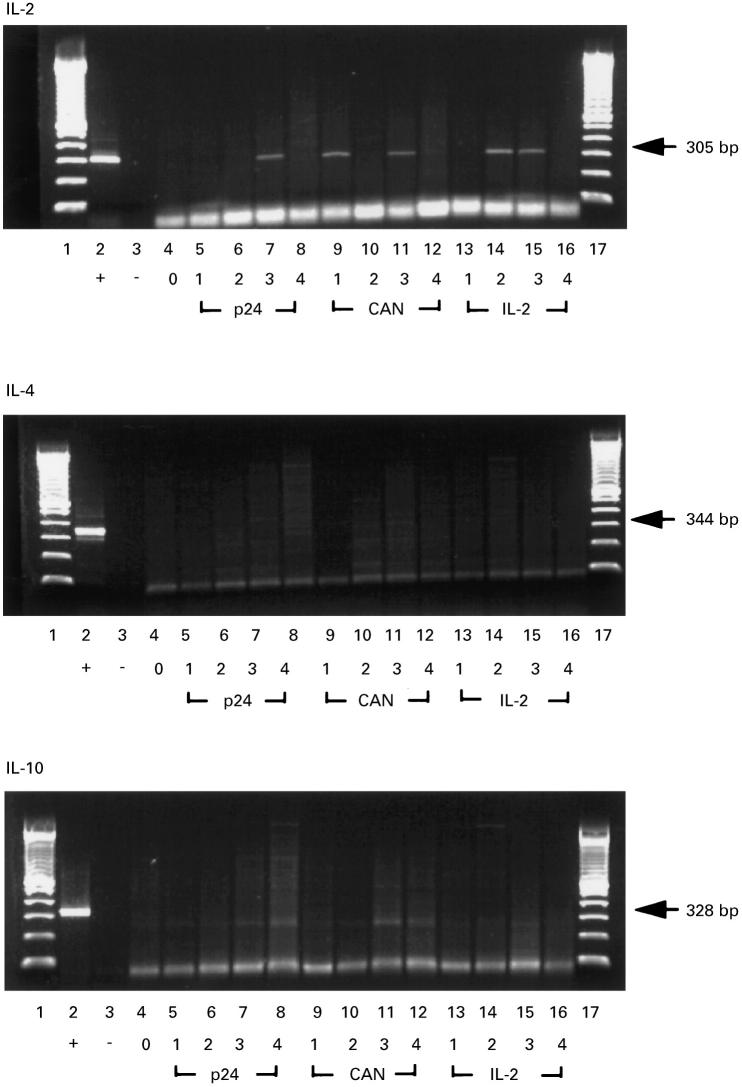
Following subsequent administrations of IL-2 and GM-CSF in patient 3, a partial restoration of proliferative responses to PHA and PWM was seen together with very strong proliferative responses to p24 and C. albicans (Table 1). All proliferative responses were associated with IL-2 production, whereas no IL-4 was detected in cultures (Table 1). Additionally, when PBMC were challenged with 100 U/ml of IL-2, a strong IL-2-induced response was seen (Table 1). Analyses of the kinetics of cytokine (IL-2, IL-4 and IL-10) mRNA expression by RT-PCR revealed up-regulated expression of IL-2 message at day 3 upon stimulation with p24 (lane 7, upper panel, Fig. 4) at days 1 and 3 upon stimulation with Candida antigen (lanes 9 and 11, upper panel, Fig. 4), and at days 2 and 3 in response to IL-2 (lanes 14 and 15, upper panel, Fig. 4). No IL-4- or IL-10-specific mRNA expression was detected in response to any of the three stimuli (middle and lower panel, respectively, Fig. 4).
Fig. 4.
Gel electrophoresis of reverse transcriptase-polymerase chain reaction (RT-PCR) products of IL-2, IL-4 and IL-10 mRNAs obtained from patient peripheral blood mononuclear cells (PBMC) after the IL-2 and granulocyte-macrophage colony-stimulating factor (GM-CSF) immunotherapy. Lanes 1 and 17, DNA ladder; lane 2, positive control; lane 3, negative control; lane 4, day 0; lanes 5–8, day 1–4 stimulation with recombinant p24; lanes 9–12, day 1–4 stimulation with Candida antigen; lanes 13–16, day 1–4 stimulation with IL-2.
Patients experienced mild fevers, rigors and night sweats during and immediately subsequent to immunotherapy. After three cycles of immunotherapy patient 3 experienced clinical improvement with increased weight, reduced fevers and night sweats, and cessation of his abdominal pain. Computed tomography scan of the abdomen demonstrated a reduction in para-aortic lymphadenopathy, and endoscopy showed a reduction in MAC load on duodenal biopsy. Blood cultures were negative for culture of MAC. Importantly, throughout the period of cytokine administration the patients' viral load remained below the detection limit.
DISCUSSION
IL-2 has been used clinically in short-term randomized comparisons using continuous i.v. therapy at varying doses and more recently in studies using anti-retroviral agents in combination with subcutaneous IL-2 administration [21,22]. These studies have shown a considerable expansion of the CD4+ subpopulation, mainly in those individuals with CD4 counts > 200 at the time therapy was initiated. In this study, we have described the impact of potent HAART with immunomodulators on the host immune response in patients with very advanced HIV-1 disease, one of them presenting with a severe opportunistic infection. In all three patients where HAART alone had a modest effect on CD4+ T cell counts, although viral loads were undetectable, we observed accumulation of anergic lymphocyte subsets which failed to proliferate in response to mitogen, recall or HIV-1 recombinant antigen. For patient 3, the patient's condition was complicated by MAC, which remained unresponsive to conventional treatment. Detailed immunovirological analyses revealed that before IL-2 therapy, 97% of lymphocytes were CD3+ (73% αβ and 8.5% γδ), with low levels of CD4+ cells (4%) and the majority (88%) being CD8+CD28− cells, of which 20% were CD57+. In culture, these cells appeared microscopically non-dividing but healthy, suggesting anergy. These observations are consistent with other reports, describing an atypical phenotype of CD8+CD28−CD57+ anergic cells in HIV-1-infected individuals [10–12]. Analysis of CD1a expression showed no presence of immature thymocytes. Increased levels of CD95 and CD38 expression were noted compared with controls. Surprisingly, upon administration of IL-2 there was a further increase in CD95 (Fas) expression. Increased expression of CD95 might indicate that cells have the potential to apoptose. Fas, however, needs to be cross-linked and the presence of FasL is required [23,24]. Increased Fas expression upon activation per se suggests that cells are activated but are not necessarily committed to apoptosis. No DNA fragmentation was seen (data not shown), but this was not surprising since the CD8+CD28− subpopulation has been shown to contain high levels of the anti-apoptotic Bcl-2, to have shortened telomeres and to have low clonogenic potential [12,25]. Additionally, increased levels of CD38, leading to the CD38–CD38L interaction, may provide rescue signals from programmed cell death [12,26]. More CD45RA+ (naive) cells were seen before IL-2 treatment, although lower levels of both naive and effector/memory (CD45RO+) were observed in comparison with those seen for the uninfected control. Reduction in the percentage of CD45RA+ subpopulation in PBMC upon IL-2 therapy suggests an altered pattern of migration, such as enhanced entry into lymph nodes.
The expansion of the CD4 compartment in all three patients was very limited after IL-2 immunotherapy. In patient 3, whilst both CD3+ and CD8+ cells increased numerically, their proportions were reduced by 14% and 18%, respectively, owing to the over 10-fold increase in the CD3−CD16+CD57+ NK cell numbers, reflected in an 8% rise in their proportion of the total lymphocyte population. This increase was reflected in the two-fold increase of NK cell-mediated cytotoxicity. Before and after the initial immunotherapy cells from all three patients failed to proliferate or produce IL-2 when challenged with HIV-1 recombinant antigens, mitogens or recall antigens. When pretherapy PBMC from patients 1 and 3 were stimulated with HSV; or with p24, HSV, or C. albicans, respectively, there was a lack of proliferation and IL-2 production. However secretion of IL-4 was detected in response to these specific antigens. Since patient 1 had recurrent genital HSV and patient 3 previously had oesophageal candidiasis and recurrent genital HSV, this IL-4 production is most likely to be antigen-specific. This was associated with up-regulated expression of CD23, CD40, CD80 and CD86, which are known to be induced by IL-4 and expressed by activated T cells [27–29]. Increased expression of these molecules before treatment may indicate the existence of an immunosuppressive microenvironment within these patients. Generation of the anti-p24, anti-HSV, and anti-Candida-specific responses within a microenvironment, rich in IL-4 and with T cells lacking costimulatory capacity, is most likely to lead to immunosuppression rather than activation and proliferation (IL-2 production). This concurs with the hypothesis that a type-2 cytokine environment may have a profound anti-proliferative effect [8]. Loss of IL-4 upon immunotherapy, mirrored by increase in IFN-γ release in response to mitogen as well as increases in both the HIV-1 peptide-specific IFN-γ-secreting cells and the NK cell-mediated cytotoxicity, suggests a shift from type-2 towards a type-1 response. IL-2 immunotherapy increased the number of CD8+ cells and CD3−CD16+CD57+ NK cells in the blood of patient 3, either by induction and/or clonal expansion or by changes in the migration pathways of these cells. This led to significantly higher levels of IFN-γ-producing, HIV-1 peptide-specific T cells (likely to be CD8+ cytotoxic T cells) in the blood. Because of the increased expression of CD95, these cells are probably the result of clonal expansion. However, they did not appear to express CD45RO, as there was no dramatic increase in the number of RO+ cells after IL-2 immunotherapy. This may be attributed to the fact that recently activated cells lose CD45RA and do not express RO until several days later [30]. The immunological changes observed are consistent with what is already known about the role of IL-2 in vitro, and from previous in vivo studies [31–33]. A considerable expansion of the NK cell subset was noted with a relative diminution of the type-2 IL-4-producing cells. Importantly, following IL-2 infusion more type-1-like cells producing IFN-γ were seen.
Since IL-2 immunotherapy did not result in major clinical improvements and in view of its inability to suppress symptomatic MAC infection, GM-CSF was co-administered with further IL-2. GM-CSF, produced by both type-1 and type-2 cytokine-secreting cells, is a cytokine with a broad spectrum of cell-differentiating and colony-stimulating activities [34,35]. The combination of GM-CSF and IL-2 injections appeared more effective in inducing both HIV-1-specific and non-specific proliferative responses, associated with IL-2 production and up-regulated IL-2-specific mRNA expression. In addition, restoration of responsiveness to IL-2 was seen, suggesting that up-regulation of IL-2/IL-2R interactions/signalling is essential to the ability of these cells to respond to antigenic stimuli. Moreover, neither IL-4- nor IL-10-specific mRNA expression was detected in response to p24, Candida or IL-2, indicating that administration of IL-2 and GM-CSF together does not induce the anti-proliferative type-2 responses. Of considerable importance was reappearance of anti-HIV-1 proliferative responses, which had not been seen before in advanced HIV-1 disease, raising the possibility that a combination of both IL-2 and GM-CSF may have a beneficial effect on the treatment of advanced HIV-1 disease. These considerable improvements in immunological function were associated with clinical improvement in the patients' ability to control MAC infection. Patients experienced mild side-effects during and immediately subsequent to immunotherapy. Utilization of cytokines to enhance and steer immune responses to HIV-1 antigens towards a desired phenotype has become an area of enormous interest [35]. Our data raise the possibility that, in late-stage HIV-1 disease, IL-2 and GM-CSF immunotherapy as a supplement to HAART may restore important immune responses, namely priming of CD4+ cells and subsequently CD8+ cell function as well as antigen presentation. These responses are considered to play an important role in remission of clinically persistent opportunistic disease and control of HIV-1 replication.
Acknowledgments
We thank the MRC AIDS Reagent Project (NIBSC) for the HIV recombinant reagents, R. Aspinall for helpful discussion and K. Begolli for computing assistance. We are also grateful to staff of the Clinical Laboratory, Department of Immunology, ICSM, Chelsea & Westminster Hospital for viral load testing. This work was supported by the Wellcome Trust (Grant number 050020), the EU BIOMED 2 (Grant number PL962055) and Crusaid & STAR Foundation.
REFERENCES
- 1.Gill J, Moyle G, Nelson M. Discontinuation of Mycobacterium avium complex prophylaxis in patients with a rise in CD4 count following highly active anti-retroviral therapy (HAART) AIDS. 1998;12:680. [PubMed] [Google Scholar]
- 2.Sepkowitz KA. Effect of HAART on natural history of AIDS-related opportunistic disorders. Lancet. 1998;351:228–30. doi: 10.1016/S0140-6736(05)78279-9. [DOI] [PubMed] [Google Scholar]
- 3.Race EM, Adelson-Mitty J, Kriegel GR, Barlam TF, Reimann KA, Letvin NL, Japour AJ. Focal mycobacterial lymphadenitis following initiation of protease-inhibitor therapy in patients with advanced HIV-1 disease. Lancet. 1998;351:252–5. doi: 10.1016/S0140-6736(97)04352-3. [DOI] [PubMed] [Google Scholar]
- 4.Carr A, Marriott D, Field A, Vasak E, Cooper DA. Treatment of HIV-1-associated microsporidiosis and cryptosporidiosis with combination antiretroviral therapy. Lancet. 1998;351:256–61. doi: 10.1016/S0140-6736(97)07529-6. [DOI] [PubMed] [Google Scholar]
- 5.Paxton WA, Martin SR, Tse D, et al. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposure. Nature Med. 1996;2:412–7. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 6.Clerici M, Shearer GM. A TH1 — TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–11. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 7.Clerici M, Shearer GM. The Th1-Th2 hypothesis of HIV infection: new insights. Immunol Today. 1994;15:575–81. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 8.Clerici M, Wynn TA, Berzofsky JA, Blatt SP, Hendrix CW, Sher A, Coffman RL, Shearer GM. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with human immunodeficiency virus. J Clin Invest. 1994;93:768–75. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, Walker BD. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–50. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 10.Borthwick NJ, Bofill M, Gombert WM, et al. Lymphocyte activation in HIV-1 infection. II. Functional defects of CD28− T cells. AIDS. 1994;8:431–41. doi: 10.1097/00002030-199404000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Kern F, Ode-Hakim S, Vogt K, Hoflich C, Reinke P, Volk HD. The enigma of CD57+CD28− T cell expansion—anergy or activation? Clin Exp Immunol. 1996;104:180–4. doi: 10.1046/j.1365-2249.1996.d01-635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg YJ, Anderson AO, Pabst R. HIV-induced decline in blood CD4/CD8 ratios: viral killing or altered lymphocyte trafficking? Immunol Today. 1998;19:10–17. doi: 10.1016/s0167-5699(97)01183-3. [DOI] [PubMed] [Google Scholar]
- 13.Kelleher AD, Carr A, Zaunders J, Cooper DA. Alterations in the immune response of human immunodeficiency virus (HIV)-infected subjects treated with an HIV-specific protease inhibitor, ritonavir. J Infect Dis. 1996;173:321–9. doi: 10.1093/infdis/173.2.321. [DOI] [PubMed] [Google Scholar]
- 14.Schnittman SM, Fox L. Preliminary evidence for partial restoration of immune function in HIV type 1 infection with potent antiretroviral therapies: clues from the Fourth Conference on Retroviruses and Opportunistic Diseases. AIDS Res Hum Retrovir. 1997;13:815–8. doi: 10.1089/aid.1997.13.815. [DOI] [PubMed] [Google Scholar]
- 15.Connors M, Kovacs JA, Krevat S, et al. HIV infection induces changes in CD4+ T-cell phenotype and depletions within the CD4+ T-cell repertoire that are not immediately restored by antiretroviral or immune-based therapies. Nature Med. 1997;3:533–40. doi: 10.1038/nm0597-533. [DOI] [PubMed] [Google Scholar]
- 16.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–6. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 17.Bidwell J. Advances in DNA-based HLA-typing methods. Immunol Today. 1994;15:303–7. doi: 10.1016/0167-5699(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 18.Bunce M, O'Neill CM, Barnardo MCNM, Krausa P, Browning MJ, Morris PJ, Welsh KI. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 & DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP) Tissue Antigens. 1995;46:355–67. doi: 10.1111/j.1399-0039.1995.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 19.Imami N, Larché M, Ritter MA. Inhibition of alloreactivity by mAb MR6: differential effects on IL-2- and IL-4-producing human T cells. Int Immunol. 1994;6:1575–84. doi: 10.1093/intimm/6.10.1575. [DOI] [PubMed] [Google Scholar]
- 20.Lalvani A, Brookes R, Hambelton S, Britton WJ, Hill AVS, McMichael AJ. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–65. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovacs JA, Vogel S, Albert JM, et al. Controlled trial of interleukin-2 infusions in patients infected with the human immunodeficiency virus. N Engl J Med. 1996;335:1350–6. doi: 10.1056/NEJM199610313351803. [DOI] [PubMed] [Google Scholar]
- 22.Davey RT, Jr, Chaitt DG, Piscitelli SC, et al. Subcutaneous administration of interleukin-2 in human immunodeficiency virus type 1-infected persons. J Infect Dis. 1997;175:781–9. doi: 10.1086/513971. [DOI] [PubMed] [Google Scholar]
- 23.Ju ST, Cui H, Panaka DJ, Ettinger R, Marshak-Rothstein A. Participation of target protein in apoptosis pathway induced by CD4+ Th1 and CD8+ cytotoxic cells. Proc Natl Acad Sci USA. 1994;91:4185–9. doi: 10.1073/pnas.91.10.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1 (Fas/CD95) Nature. 1995;373:438–41. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 25.Wolthers KC, Bea G, Wisman A, et al. T cell telomere length in HIV-1 infection: no evidence for increased CD4+ T cell turnover. Science. 1996;274:1543–7. doi: 10.1126/science.274.5292.1543. [DOI] [PubMed] [Google Scholar]
- 26.Funaro A, Spagnoli GC, Ausiello CM, Alessio M, Roggero S, Delia D, Zaccolo M, Malavasi F. Involvement of the multilineage CD38 molecule in a unique pathway of cell activation and proliferation. J Immunol. 1990;145:2390–6. [PubMed] [Google Scholar]
- 27.Bonnefoy J-Y, Plater-Zyberk C, Lecoanet-Henchoz S, Gauchat J-F, Aubry J-P, Graber P. A new role for CD23 in inflammation. Immunol Today. 1996;17:418–20. doi: 10.1016/0167-5699(96)10054-2. [DOI] [PubMed] [Google Scholar]
- 28.Katira A, Knox KA, Finney M, Michell RH, Wakelman M, Gordon J. Inhibition by glucocortcoid and staurosporine of IL-4-dependent CD23 production in B lymphocytes is reversed on engaging CD40. Clin Exp Immunol. 1993;92:347–52. doi: 10.1111/j.1365-2249.1993.tb03403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–58. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 30.Akbar AN, Terry L, Timms A, Beverly PCL, Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988;140:2171–8. [PubMed] [Google Scholar]
- 31.De Paoli P, Zanussi S, Simonelli C, et al. Effects of subcutaneous interleukin-2 therapy on CD4 subsets and in vitro cytokine production in HIV+ subjects. J Clin Invest. 1997;100:2737–43. doi: 10.1172/JCI119819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teppler H, Kaplan G, Smith KA, Montana AL, Meyn P, Cohn ZA. Prolonged immunostimulatory effect of low-dose polyethylene glycol interleukin-2 in patients with human immunodeficiency virus type 1 infection. J Exp Med. 1993;177:483–92. doi: 10.1084/jem.177.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farace F, Angevin E, Dietrich PY, Leboullaire C, Vanderplancke J, Escudier B, Triebel F. Low-dose IL-2 treatment: activation of discrete T- and NK-cell sub-populations in vivo. Int J Cancer. 1995;62:523–8. doi: 10.1002/ijc.2910620506. [DOI] [PubMed] [Google Scholar]
- 34.Baldwin GC. The biology of granulocyte-macrophage colony-stimulating factor: effects on hematopoietic and nonhematopoietic cells. Dev Biol. 1992;151:352–67. doi: 10.1016/0012-1606(92)90175-g. [DOI] [PubMed] [Google Scholar]
- 35.Ahlers JD, Dunlop N, Alling DW, Nara PL, Berzofsky JA. Cytokine-in-adjuvant steering of the immune response phenotype to HIV-1 vaccine constructs. J Immunol. 1997;158:3947–58. [PubMed] [Google Scholar]