Abstract
The Fas ligand (FasL)/Fas and the perforin-granzyme cytotoxic pathways presumably play a central role in the development of hepatocellular injury in viral hepatitis. To recognize the potential contribution of FasL and perforin-based cell killing in hepadnaviral infection, we adopted a cytotoxic assay using murine Fas+ P815 and human Fas− K562 cells as targets. Freshly isolated peripheral blood mononuclear cells (PBMC) from woodchucks with newly acquired woodchuck hepatitis virus (WHV) infection (n = 6), with chronic WHV hepatitis (n = 9), and from healthy animals (n = 11) were used as effector cells. We have found that woodchuck lymphoid cells kill cell targets via both the FasL/Fas and the perforin death pathways. The contribution of Fas-dependent cytolysis was ascertained in blocking experiments with anti-Fas antibody and by incubation of PBMC with cyclohexamide to prevent de novo synthesis of FasL. The involvement of the perforin pathway was confirmed by treatment of K562 cells with colchicine to inhibit the microtubule-dependent perforin release. Comparative analysis showed that peripheral lymphoid cells from acute WHV hepatitis, but not those from chronic WHV infection, are more cytotoxic and that this increase seems to be entirely due to activation of perforin-mediated killing. The data indicate that acute infection in woodchucks is associated with the augmented capacity of lymphoid cells to elicit perforin-dependent killing, but in chronic infection, independent of the severity of liver disease and duration of chronicity, these cells have the same or lower cytotoxic potential as PBMC from healthy controls. These findings suggest a role for non-specific cellular immunity, presumably natural killer (NK) cells, in the control of early WHV infection and in the progression of chronic hepatitis.
Keywords: viral hepatitis, woodchuck hepatitis virus, perforin, Fas, cytotoxicity
INTRODUCTION
The hepatitis B virus (HBV) is a non-cytopathic DNA virus that causes acute and chronic hepatitis and hepatocellular carcinoma. HBV is a member of the hepadnavirus family, which includes several mammalian and avian viruses, among them the woodchuck hepatitis virus (WHV) [1]. It is acknowledged that woodchucks (Marmota monax) infected with WHV reflect with a high degree of accuracy virological and immunopathological features encountered in HBV-infected patients [2].
Accumulating evidence implies that hepatocellular injury in hepatitis B is caused by the host immune responses, presumably by T cells specifically directed against viral epitopes exposed on the surface of infected liver cells [3]. Readily detectable HBV-specific polyclonal cytotoxic T lymphocytes (CTL) in the peripheral blood have been identified as a distinctive feature of acute infection [4]. In contrast, chronic hepatitis is accompanied by a weak or undetectable HBV-specific CTL response in the blood [5,6]. Activated immune effector cells use at least two independent mechanisms to kill targeted cells. These cytotoxic pathways are mediated by a Fas ligand (FasL)–Fas interaction and by perforin-granzyme release. In addition, it is assumed that cytokines, such as interferon-gamma (IFN-γ) and tumour necrosis factor-alpha (TNF-α), secreted by activated lymphoid cells cause cell damage and death [7].
Hepatocytes are Fas receptor-bearing cells and are highly sensitive to FasL-induced injury. Cross-linking of Fas molecules with an anti-Fas antibody has been shown to cause fatal hepatic failure in mice due to hepatocyte apoptosis [8]. In viral hepatitis, the involvement of the FasL–Fas system was postulated based on the presence of activated T cells, which display FasL, in the intrahepatic inflammatory infiltrates and on the unregulated expression of Fas receptor on hepatocytes during ongoing necroinflammation [9]. Demonstration that soluble Fas can prevent CTL-induced hepatitis in transgenic mice retaining HBV surface antigen (HBsAg) in hepatocytes supported a notion about a principal role of the FasL–Fas interaction in the development of viral hepatitis [10]. However, other experiments in HBV transgenic mice in the absence of hepatic HBsAg retention showed that both FasL and perforin-based cytotoxicity contribute to hepatocyte injury and that both these pathways must be operative to kill targeted cells [11]. The importance of the perforin pathway in T cell-mediated liver injury was also substantiated in perforin knockout mice, which fail to eliminate virus and are resistant to lymphocytic choriomeningitis virus-induced hepatitis [12]. Until now, the contribution of FasL and perforin-dependent killing had not been comparatively evaluated in acute and chronic phases of natural hepadnavirus infection.
In this study, we measured the cytolytic capacity of peripheral blood lymphomononuclear cells from woodchucks with acute and chronic WHV hepatitis and from healthy animals to investigate differences in the effector cell killing during hepadnaviral infection. To discriminate between FasL and perforin-based cytotoxicity and to assess the relative contribution of each of the mechanisms in acute and chronic WHV infections, we adopted an assay with heterologous FasL-sensitive and FasL-insensitive cells as targets and manipulated the testing conditions using agents that selectively block individual reactant molecules of each pathway. Our results show that the circulating woodchuck lymphomononuclear cells kill cells by both FasL- and perforin-dependent mechanisms. Furthermore, comparative analysis showed that acute hepatitis, but not chronic WHV infection, is uniformly associated with an enhanced ability of the circulating lymphoid cells to induce cell death, and that this increase is a consequence of activation of perforin but not the FasL-mediated pathway. These data suggest that an increased activity in the perforin effector system could be important in defence against early hepadnavirus infection, and that its decreased efficiency may contribute to the establishment and/or perpetuation of chronic infection.
MATERIALS AND METHODS
Animals and categories of WHV infection
The study group comprised in total 20 adult woodchucks (six males and 14 females) randomly selected from animals housed in the woodchuck colony maintained at the Faculty of Medicine, Memorial University of Newfoundland (St John's, Canada). Eleven of the animals were healthy and were investigated as controls (Table 1). Six of the initially healthy animals were subsequently inoculated with a WHV infectious pool [13,14] and analysed for serological indicators of WHV infection at weekly intervals during the preacute and acute phases of hepatitis. The remaining nine woodchucks were chronically infected with WHV and their sera typically tested monthly for WHV infection markers. Of these, five had chronic hepatitis which had developed after experimental injection with WHV and four others were brought to the colony as juveniles with serologically and histologically evident chronic infection acquired in the wild.
Table 1.
Details on woodchuck hepatitis virus (WHV) infection in 20 adult woodchucks at the time of acquisition of circulating lymphoid cells analysed in the cytotoxicity assays
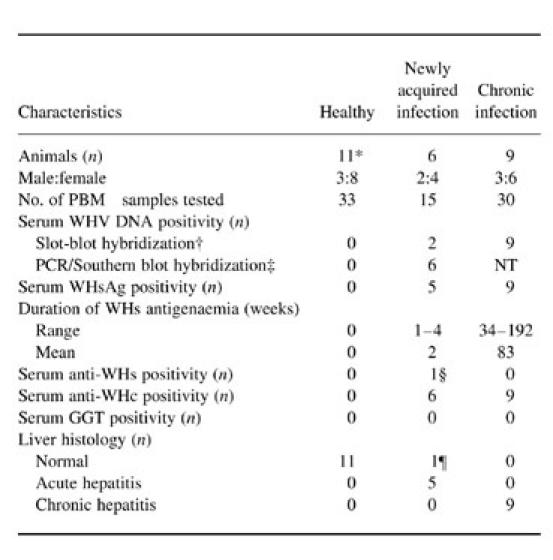
*Includes six animals that were subsequently inoculated with WHV and developed preacute or acute WHV infection.
†Approximate sensitivity 106–107 WHV genome copies/ml.
‡Amplified by nested polymerase chain reaction (PCR) with WHV core gene-specific primers and detected by Southern blot hybridization; approximate sensitivity 10–102 WHV genome copies/ml.
§One animal became anti-WHV surface (WHs)-reactive 5 weeks after inoculation with WHV following a 3-week period of WHsAg positivity (see Fig. 4a; 838/AH animal).
¶After WHV inoculation, one animal remained WHsAg non-reactive and had normal liver histology at the time of cytotoxicity assay despite the presence of WHV DNA and anti-WHV core (WHc) in the serum (see Fig. 4a; 821/preAH animal).
NT, Not tested.
Healthy woodchucks had never been exposed to WHV prior to the study. This was established based on negative results from testing of their sequential sera for WHV DNA by a nested polymerase chain reaction (PCR)/Southern blot hybridization assay [14,15]. These animals were also negative for antibody to WHV core antigen (anti-WHc), WHV surface antigen (WHsAg) and antibody to WHsAg (anti-WHs), as determined by immunoassays described in previous publications [14,16].
Onset of preacute WHV infection in experimentally inoculated animals was recognized when WHV DNA and anti-WHc first appeared in the circulation but serum remained WHsAg−. The beginning of the acute phase of WHV infection was considered when serum WHsAg was detected for the first time. Recovery from acute hepatitis was established when WHsAg permanently cleared from the circulation and anti-WHs appeared. The diagnosis of acute hepatitis was verified by histological examination of liver biopsies obtained by laparotomy 5–6 weeks after the first WHsAg appearance [13,17]. The diagnosis of chronic hepatitis was based on the persistent presence (> 6 months) of WHsAg in the circulation and on histological examination of liver biopsies obtained at 6–12-month intervals [13]. All animals had normal levels of γ-glutamyltransferase (GGT; normal range 0–2 U/l), as determined by the Vettest computerized assay system (Vettest S.A., Neuchatel, Switzerland). This excluded the likelihood that chronically infected woodchucks might have hepatocellular carcinoma.
Preparation of target cells
Murine mastocytoma P815 cells (ATCC no. TIB-64), which constitutively express Fas [18] and are susceptible to cross-species FasL- and perforin-mediated killing ([19]; data not shown), and human chronic myelogenous leukaemia K562 cells (ATCC no. CCL-243), which are Fas-negative [20] and are model cells for determination of perforin-induced cytotoxicity, were purchased from the American Type Culture Collection (ATCC; Rockville, MD). The cells were maintained in growth medium consisting of RPMI 1640 supplemented with 10% (v/v) fetal calf serum (FCS), 10 mm HEPES, 2 mml-glutamine, 2% (v/v) penicillin/streptomycin (all Gibco/BRL, Grand Island, NY) and 2 μmβ-mercaptoethanol (Sigma-Aldrich Canada, Oakville, Ontario, Canada). The cells were subcultured 24 h prior to cytotoxicity assay to allow for log-phase cell growth. To radiolabel the cells, approximately 5 × 106 cells were pelleted by centrifugation at 328 g for 10 min and incubated in minimal volume with 200 μCi Na251CrO4 (Amersham, Oakville, Ontario, Canada) at 37°C for 90 min. Labelled cells were washed four times in PBS pH 7.4 supplemented with 1% (v/v) FCS and resuspended in growth medium at a final concentration of 2 × 105 cells/ml. Labelled cells were used immediately for cytotoxicity assays.
Preparation of effector cells
Woodchuck peripheral blood mononuclear cells (PBMC) were isolated by density gradient separation as described previously [21]. Briefly, 10 ml of blood were collected from the femoral vein into EDTA-treated vacutainers (Becton Dickinson, Franklin Lakes, NJ), layered onto Ficoll–Paque (Pharmacia Biotech, Uppsala, Sweden), and fractionated for 30 min at 328 g in a GS-6R centrifuge (Beckman Instruments Inc., Palo Alto, CA). The buffy coat was collected and washed three times at 328 g for 10 min with PBS containing 10 mm EDTA. Viability of isolated PBMC was consistently > 90%, as evaluated by trypan blue exclusion. Cells were washed again with PBS containing 1% FCS and resuspended at a final concentration of 107 viable cells/ml in reaction medium which consisted of growth medium (described above) supplemented with 5 μg/ml phytohaemagglutinin (PHA; Murex Biotech Ltd, Dartford, UK). The cells were used immediately in cytotoxicity assays.
Cytotoxicity assay
The standard cytotoxicity assay was performed in 96-well round-bottomed plates (ICN Pharmaceuticals, Montreal, Quebec, Canada) with 200 μl of reaction medium (described above) per well. Effector (E) cells were added to duplicate wells to achieve three different E:T ratios, equivalent to 50:1, 25:1 and 12.5:1. Subsequently, 1 × 10451Cr-labelled target (T) P815 or K562 cells in 50 μl and an appropriate volume of growth medium were added to the wells to reach a final volume of 300 μl. The plates were incubated at 37°C in a humidified 5% CO2 incubator for 5 h. Following the incubation, 125-μl aliquots of cell free supernatant were transferred into 1-ml glass culture tubes and the radioactivity measured in a gamma counter. As a control, cells incubated in the absence of PHA were included in each assay. The maximum and spontaneous release were determined by incubation of 104 labelled target cells in 50 μl with 250 μl of 1 n HCl or 250 μl of reaction medium, respectively. The percentage specific lysis was calculated from means of duplicate evaluations as follows: 100 × (experimental release – spontaneous release)/(maximum release – spontaneous release). In all assays, the spontaneous 51Cr release was < 20% of the maximum release.
Determination of the effects of anti-Fas antibody and regulatory agents on cytotoxicity
To discriminate between FasL- and perforin-mediated killing of P815 cells caused by woodchuck PBMC, the hamster anti-mouse Fas Jo2 MoAb (purified IgG; PharMingen, Mississauga, Ontario, Canada) was used. It has been previously established that some cells, including P815, resist direct lysis by Jo2 MoAb despite the fact that the antibody specifically recognizes Fas receptor on these cells and blocks FasL interaction with Fas (data not shown; [22]). Thus, prior to the cytotoxicity assay, approximately 3 × 10651Cr-labelled P815 cells in a minimal volume were incubated with 5 μg of Jo2 MoAb or an unrelated hamster antibody (control) at 37°C in a humidified atmosphere of 5% CO2 for 30 min. The cells were resuspended in growth medium at a final concentration of 2 × 105 cells/ml, and then the standard cytotoxicity assay was performed as described above.
To determine the effect of inhibition of de novo FasL synthesis on the PBMC cytotoxic activity, 0.177 mm cyclohexamide (CHX; CalBiochem-NovaBiochem, La Jolla, CA) was added to test wells with E:T ratio of 50:1 at the start of the standard 5-h cytotoxicity assay. In parallel experiments, the effect of a microtubule polymerization inhibitor, colchicine (Sigma-Aldrich) on the perforin-mediated killing of Fas-deficient K562 cells was tested. In these assays, reaction mixtures containing effector and target cells at 50:1 ratio were supplemented with colchicine at a final concentration of 1 mm and incubated for 5 h at 37°C. In all assays, the results were compared with the data from assays done under identical conditions in the absence of the test agents.
RESULTS
Woodchuck PBMC kill both Fas-positive and Fas-negative target cells
Initial experiments were aimed at determining whether heterologous cells expressing Fas or being Fas-negative could serve as woodchuck lymphoid cell targets and whether they could provide a means to differentiate between FasL- and perforin-based cell killing caused by the woodchuck cells. For this purpose, murine P815 cells susceptible to cross-species FasL- and perforin-mediated killing were used as the principal target cells. In addition, to ascertain the accuracy in discriminating perforin- from FasL-mediated cytolysis, Fas-deficient human K562 cells, model cells for determining perforin-dependent toxicity, were used. Employing this two-target cell system, PBMC from healthy woodchucks lysed both P815 and K562 cells in the presence of PHA in a manner strictly dependent on the effector cell concentration. Thus, a linear decrease in the level of killing of both target cells was observed as the effector cell number decreased in the assay (Fig. 1). The above cytolysis did not occur in the absence of PHA (data not shown), indicating that lectin-mediated triggering of effector cells is required to facilitate the target cell killing.
Fig. 1.
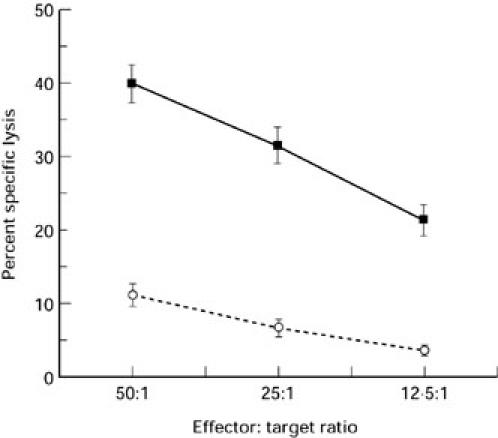
Cytotoxicity of normal woodchuck peripheral blood mononuclear cells (PBMC) towards Fas-competent and Fas-deficient target cells. PBMC from 11 healthy, woodchuck hepatitis virus (WHV)-negative woodchucks were incubated with 51Cr-labelled Fas expressing P815 cells (solid line) or Fas-deficient K562 cells (dashed line) at indicated E:T ratios. Data from evaluations with 30 and 19 individual PBMC samples were used to determine P815 and K562 cell killing, respectively. The results are expressed as percentage specific lysis ± s.e.m.
At all three E:T ratios examined, Fas-deficient K562 cells were killed with an efficiency of about 25% of the killing of an equivalent number of P815 cells (Fig. 1), suggesting that the woodchuck PBMC lyse targeted cells more efficiently via the FasL–Fas pathway. Preincubation of P815 cells with Jo2 MoAb reduced P815 cell lysis by an average of 61% (see Fig. 2a), supporting the conclusion that the woodchuck cells eliminated P815 cells to a larger extent via the FasL-mediated mechanism. A similar level of inhibition of P815 cell lysis (by approximately 72%) was seen in the presence of CHX, an inhibitor of de novo FasL synthesis (see Fig. 2a). This last finding provided additional evidence that the observed cytolysis resulted mainly from activation of the FasL–Fas pathway.
Fig. 2.
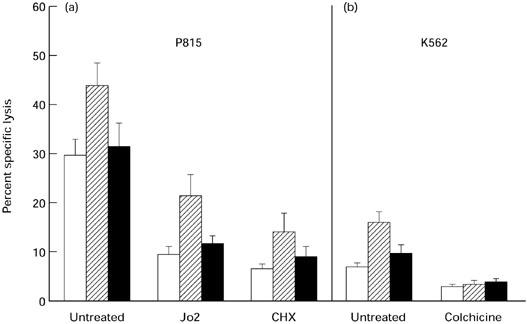
Non-Fas-mediated killing of P815 and K562 target cells by peripheral blood mononuclear cells (PBMC) from woodchucks with newly acquired and chronic woodchuck hepatitis virus (WHV) infections and from healthy controls. PBMC from WHV-negative, healthy animals (10 individual PBMC samples; □) and from woodchucks with recently induced WHV infection (14 PBMC samples; hatched bars) or advanced chronic WHV hepatitis (11 PBMC samples; ▪) were incubated with 51Cr-labelled P815 or K562 cells. (a) Fas-positive P815 cells were used intact (untreated), preincubated with anti-Fas Jo2 MoAb or tested in the presence of the protein synthesis inhibitor cyclohexamide (CHX). (b) The Fas-negative K562 cells were used intact (untreated) or tested in the presence of the perforin release inhibitor colchicine. Results are presented at an E:T ratio of 50:1 from five separate experiments and are expressed as a mean percentage of the specific lysis of P815 or K562 cells ± s.e.m.
Acute but not chronic WHV hepatitis is associated with the increased PBMC killing activity
To establish whether the cytolytic potential of woodchuck effector cells varies in different stages of WHV infection, circulating lymphoid cells collected from animals in the pre- or acute phases of WHV infection and during advanced chronic infection were analysed in a 5-h 51Cr-release assay with P815 cells. Figure 3 shows that the PBMC from animals with newly acquired infection displayed approximately 50% greater capacity to kill P815 cells than PBMC from healthy animals at all three E:T ratios. In contrast, peripheral lymphoid cells from woodchucks with chronic hepatitis lysed P815 targets at substantially lower rates than those found for animals with the recently acquired infection. In chronic hepatitis, the rates of PBMC-mediated killing were the same or, at 50:1 and 25:1 E:T ratios, even lower than those detected for PBMC from healthy animals (Fig. 3). Of note is the fact that all PBMC samples from chronically infected woodchucks, independent of the severity of hepatitis and the duration of chronicity, uniformly displayed a low cytolytic activity comparable to that of PBMC from WHV-naive animals. Although most PBMC samples from woodchucks with recently acquired preacute or acute WHV infection displayed markedly elevated killing of P815 targets, there were also some which induced cell death at levels similar to those typically found for PBMC from healthy woodchucks (see Fig. 4).
Fig. 3.
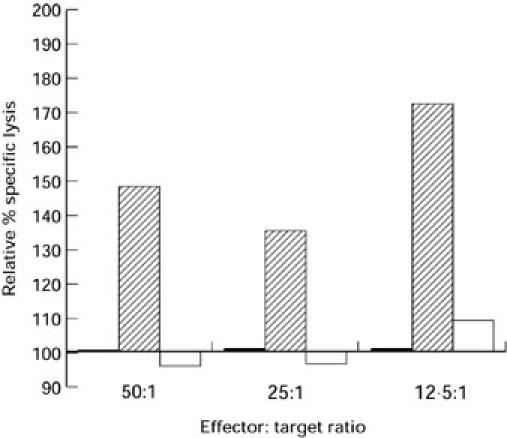
P815 cell killing by peripheral blood mononuclear cells (PBMC) from woodchucks with newly acquired and chronic woodchuck hepatitis virus (WHV) infections. Circulating lymphomononuclear cells isolated from animals with newly induced preacute or acute WHV infection (14 PBMC samples; hatched bars) or with chronic hepatitis (11 PBMC samples; □) and from healthy woodchucks (10 individual PBMC samples; ▪) were incubated with 51Cr-labelled P815 cells at indicated E:T ratios. Results for each E:T ratio are expressed as a percentage of the specific lysis induced by PBMC derived from healthy animals and tested at the same E:T ratio (taken as 100%).
Fig. 4.
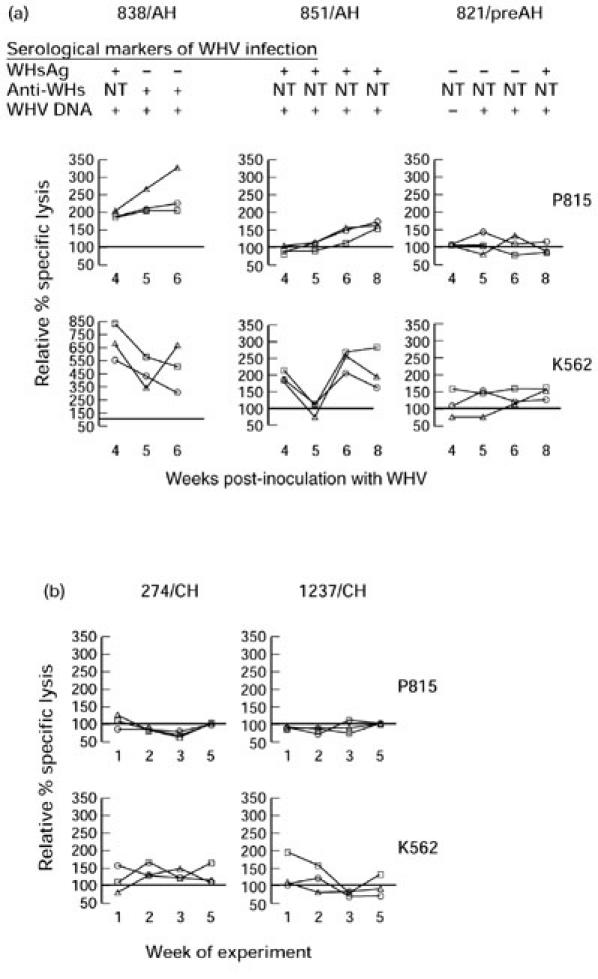
Profiles of peripheral blood mononuclear cell (PBMC)-induced cytotoxicity toward P815 or K562 target cells determined in individual woodchucks during newly acquired and advanced chronic woodchuck hepatitis virus (WHV) infections. Sequential PBMC samples collected from three woodchucks between weeks 4 and 8 after WHV inoculation (a) and serial PBMC samples from two WHV surface antigen (WHsAg)+ animals with chronic WHV hepatitis (b) were tested in parallel at given time points for cytotoxicity against Fas-positive P815 or Fas-negative K562 cells at E:T ratios of 50:1 (○), 25:1 (□) and 12.5:1 (Δ). Points represent the means of duplicate evaluations and are shown as a percentage of the cytotoxicity exhibited by PBMC from healthy animals against a given cell target and tested in the same assay. The results on serum WHsAg and anti-WHs reactivities and WHV DNA detection are presented for animals with newly acquired WHV infection in (a). All animals chronically infected with WHV (b) were WHsAg- and WHV DNA-positive at all time points tested.
The increased PBMC cytotoxicity in acute WHV infection is perforin- but not FasL-mediated
To uncover the mechanism of the augmented cell killing by lymphoid cells from acutely infected animals, P815 cells preincubated with anti-Fas Jo2 MoAb were used as targets. In a parallel experiment, woodchuck PBMC with inhibited de novo expression of FasL by CHX were incubated with P815 cells. As illustrated in Fig. 2a, anti-Fas antibody decreased killing of P815 targets by 52%, whereas CHX treatment of the same effector PBMC reduced killing of unaltered P815 cells by 68%. Thus, neither of these antagonistic treatments blocked the enhanced cytolysis caused by PBMC from acutely infected animals, indicating that the FasL–Fas interaction probably did not contribute to the increased killing. This was supported by the data obtained after subtracting values of the killing of P815 cells preincubated with Jo2 MoAb (Fig. 2a; columns depicted as Jo2) from the values of the killing of untreated P815 cells (Fig. 2a; columns marked as untreated) by PBMC from different groups of the animals studied. The final values were almost identical both for healthy animals (20.3%) and for woodchucks with recently acquired (22.6%) and chronic (19.8%) WHV infections. This suggests that Fas-mediated cytotoxicity participated to the same extent in the cytolysis caused by PBMC from either WHV-naive or infected animals, and therefore that the enhanced cell killing in newly acquired infection was a consequence of the non-Fas-mediated pathway.
Circulating lymphoid cells from woodchucks with newly acquired WHV infection killed Fas-negative K562 cells with approximately twice the efficiency of PBMC from healthy or chronically infected animals (Fig. 2b). The perforin-release inhibitor, colchicine, equalized the target cell killing between healthy woodchucks and those acutely and chronically infected with WHV. This finding provided strong evidence that the increased killing by PBMC from animals with recently acquired infection was non-Fas-mediated but probably perforin-related.
Profiles of Fas- and perforin-mediated killing in newly acquired and chronic WHV infections
To investigate dynamics of the PBMC-mediated cell killing and the contribution of Fas- and perforin-based cytotoxicity during newly induced and advanced chronic WHV infections, serial PBMC samples collected from three recently infected animals and two chronic WHV carriers were assayed using P815 and K562 cells as targets. Two of the acutely infected animals (838/AH and 851/AH; Fig. 4a), which were WHsAg-reactive at 4 weeks post-WHV inoculation (wpi), showed high and continuous increase in the cytotoxicity toward P815 cells at all three E:T ratios when sequential PBMC samples were investigated. One of these woodchucks (838/AH) cleared WHsAg and seroconverted to anti-WHs at 5 wpi, whereas the second (851/AH) remained WHsAg-reactive during follow up. Both animals showed histological features of acute hepatitis in liver tissue samples obtained immediately after completion of the cytotoxicity assays. In contrast, the killing exhibited by PBMC from 821/preAH animal, which was WHsAg− until 8 wpi, fluctuated at levels comparable to that exhibited by PBMC from healthy controls tested in parallel (Fig. 4a). This animal showed normal liver morphology at the time of the cytotoxicity assays despite the presence of WHV DNA and anti-WHc in the serum, and was classified as being in the preacute phase of WHV hepatitis (Table 1). PBMC from woodchucks with chronic hepatitis (274/CH and 1237/CH) killed P815 cells at levels greatly below those found for woodchucks with acute infection, and these levels were close to or slightly below those for healthy animals (Fig. 4b).
The level of non-Fas-mediated killing was determined in the same serial PBMC samples using K562 cell targets. In 838/AH animal, the lysis of these target cells decreased as the time from the WHV inoculation progressed and the anti-WHs developed. Nevertheless, despite the decrease in non-Fas-dependent killing over time, the level of cytolysis was evidently elevated (3–8.5-fold) over the cytotoxicity displayed by PBMC from healthy and chronically infected animals at all time points and E:T ratios tested (Fig. 4b). Killing exhibited by PBMC from 851/AH woodchuck was approximately two-fold greater at all E:T ratios than that for healthy controls and animals with chronic hepatitis, except 5 wpi (Fig. 4a). In contrast to 838/AH and 851/AH, 821/preAH animal demonstrated a slightly elevated (up to 1.5-fold) K562 cell killing, and this level remained relatively stable over the 5-week test period.
The Fas-independent cytolysis exhibited by serial PBMC samples collected during the 5-week investigation period from chronically infected animals was at the same level or moderately augmented in comparison with PBMC from healthy woodchucks (Fig. 4b). In contrast to acutely infected animals, the level of the K562 cell killing in chronic hepatitis showed no or minimal fluctuations.
DISCUSSION
The present study demonstrates that circulating woodchuck lymphoid cells are capable of direct lysis of heterologous target cells through activation of both FasL/Fas and perforin death pathways, that this cytopathic effect requires intimate effector to target cell contact, and that acute WHV hepatitis is selectively associated with the enhanced capacity of lymphoid cells to induce cell death via the perforin-dependent mechanism. The employed two-cell target cytotoxicity assay system, which is based on the cross-species productive interaction between woodchuck lymphoid cell FasL and Fas receptor on murine P815 target cells and on the cross-species perforin-mediated cell killing, showed that Fas-dependent cytotoxicity could be the main pathway by which woodchuck PBMC induce cell death. The accuracy of this determination was ascertained using Jo2 MoAb, non-cytolytic for P815 cells, which blocks cell surface Fas antigen, and by treatments with CHX and colchicine to inhibit FasL expression and microtubule-dependent perforin release, respectively. In addition, the obtained results demonstrate that close cross-species compatibility exists in the FasL/Fas and perforin-granzyme systems between woodchuck, mouse and human, as has been previously shown between human and mouse [19,23,24]. In the case of FasL, this was further supported by sequence analysis of a 508-bp fragment that included complete sequences of exons 2 and 3 and flanking fragments of exons 1 and 4 of the woodchuck FasL (Hodgson & Michalak; GeneBank accession number AF152368). This analysis showed 89% and 86% homology with the human and mouse FasL nucleotide sequences, respectively (data not shown).
Although woodchuck peripheral lymphoid cells killed virus uninfected cells mainly through the FasL–Fas pathway, the increased cytotoxicity found for animals with recently acquired WHV infection was entirely mediated by perforin release. This indicates that perforin-dependent killing is selectively activated in the early phase of WHV infection. In this respect, considerable evidence has been accumulated to conclude that perforin-dependent cytotoxicity is the principle mechanism by which natural killer (NK) cells eliminate targeted cells [12,25], that the peak of NK cell cytolytic activity and proliferation usually occurs shortly after viral invasion (reviewed in [26]), and that NK cells are an important element of the natural resistance to many viruses (reviewed in [26,27]). Considering our finding in the context of the data previously reported by others, it is likely that the increased perforin-mediated killing in the early stage of WHV infection was a consequence of NK cell activation, although at this stage we do not have reliable tools to separate woodchuck NK cells from other circulating effector cells. Nevertheless, the augmented killing of K562 cells by PBMC from acutely infected animals corroborates the previous observations on the increased cytotoxicity toward K562 cells by PBMC from patients with acute hepatitis B, which has been interpreted as indicative of enhanced NK cell cytotoxicity [28,29]. The ability of the host to mount a strong cytotoxic NK cell response very early in the course of hepadnavirus infection could play a decisive role in controlling virus spread and limiting progression of the disease. The observed co-occurrence of the initially very strong PBMC cytotoxic activity followed by a swift seroconversion to anti-WHs and recovery in one of the woodchucks with acute hepatitis in our study (838/AH; Fig. 4a) could be interpreted in support of this possibility.
The main advantage of our experimental approach is the ability to dissect the contribution of the FasL/Fas system from the perforin-dependent killing induced by the same effector cells and to assess simultaneously the cytopathic effect caused by PBMC derived from different stages of viral hepatitis and from healthy animals. By parallel examination of the effector cells from acute and chronic infections using the two-cell cytotoxicity assay system supplemented with selective inhibition of reactant molecules of both the FasL–Fas and perforin pathways, we have circumvented concerns raised by other authors that the target cell itself may have the last word in selecting its mode of execution [11]. In consequence, this allowed for an unbiased demonstration that chronic WHV hepatitis is accompanied by cytotoxic activity in the peripheral blood equal to or lower than in healthy controls, and that there is no relation between the level of PBMC-induced cell killing and the severity of hepatitis or the duration of chronic WHV infection. This finding appears to be comparable to the reported reduced cytotoxicity of circulating NK cells in patients with chronic active and persistent hepatitis B [30,31], although elevated levels of this cell activity have also been observed in an aggressive form of chronic hepatitis B [31]. Our study also implies that, in contrast to acute infection, the levels of non-specific cell killing remain relatively stable in chronic infection, as was shown by analysis of serial PBMC samples collected during the 5-week examination period of two of the chronically infected animals.
Of note, in the above context, is the earlier observation that HBsAg decreases, in a dose-dependent manner, NK cell cytotoxicity in vitro by interfering with their binding to target cells [32,33]. If the continuous persistence of large quantities of circulating HBsAg and WHsAg (which is typical for chronically infected humans and woodchucks, respectively) in fact suppresses NK cells, it would be reasonable to expect relatively normal or decreased function of these cells reflected in the suppressed PBMC cytotoxic activity. The same could also be true for intrahepatic NK cells. It has been well documented that chronic WHV hepatitis is accompanied by an extensive, irreversible accumulation of WHsAg in the outer membranes of infected hepatocytes, as opposed to acute hepatitis (reviewed in [2]). It is possible that the incorporation of the saturable quantities of virus envelope material into hepatocyte surface, co-existing with the large amounts of virus envelope antigen in serum, acts as a negative modulator for in situ NK cell cytotoxicity in chronic WHV infection. This might be an important element of virus strategy devised to protect infected cells against immunocytolysis, and could be in agreement with our recent observation of the impaired expression of the woodchuck MHC class I molecules on hepatocytes in chronic WHV infection (Michalak et al., manuscript in preparation), whose depletion is known to up-regulate local NK cell activity (reviewed in [34]). Our present data suggest that virus non-specific immunity may contribute to the induction and perpetuation of chronic liver disease in hepadnaviral infection. The detected difference in the efficiency of the perforin-mediated cell killing between acute and chronic WHV infections provides a basis for further investigations of the pathogenic role of this form of cytotoxicity in HBV-infected humans and in animal models of hepatitis B.
Acknowledgments
We thank Colleen L. Trelegan, Norma D. Churchill, and Maureen Gallant for expert assistance. This work was supported by an operating grant MT-14818 from the Medical Research Council of Canada (to T.I.M.) and a grant 66-01120 from the National Health Research Development Program (to M.D.G.). P.D.H. is a recipient of a Memorial University Fellowship.
REFERENCES
- 1.Summers J. Three recently described animal virus models for human hepatitis B virus. Hepatology. 1981;1:179–83. doi: 10.1002/hep.1840010215. [DOI] [PubMed] [Google Scholar]
- 2.Michalak TI. The woodchuck animal model of hepatitis B. Viral Hepatitis Rev. 1998;4:139–65. [Google Scholar]
- 3.Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Ann Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 4.Rehermann B, Fowler P, Sidney J, et al. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J Exp Med. 1995;181:1047–58. doi: 10.1084/jem.181.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrari C, Penna A, Bertoletti A, et al. Cellular and immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J Immunol. 1990;145:3442–9. [PubMed] [Google Scholar]
- 6.Rehermann B, Lau D, Hoofnagle JH, Chisari FV. Cytotoxic T lymphocyte responsiveness after resolution of chronic hepatitis B virus infection. J Clin Invest. 1996;97:1655–65. doi: 10.1172/JCI118592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ando K, Moriyama T, Guidotti LG, et al. Mechanisms of class I restricted immunopathology: a transgenic mouse model of fulminant hepatitis. J Exp Med. 1993;178:1541–54. doi: 10.1084/jem.178.5.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogasawara J, Watanabe-Fukunaga R, Adachi M, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–9. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 9.Galle PR, Hofmann WJ, Walczak H, et al. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med. 1995;182:1223–30. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kondo T, Suda T, Fukuyama H, Adachi M, Nagata S. Essential roles of Fas ligand in development of hepatitis. Nat Med. 1997;3:409–13. doi: 10.1038/nm0497-409. [DOI] [PubMed] [Google Scholar]
- 11.Nakamoto Y, Guidotti LG, Pasquetto V, Schreiber RD, Chisari FV. Differential target cell sensitivity to CTL-activated death pathways in hepatitis B virus transgenic mice. J Immunol. 1997;158:5692–7. [PubMed] [Google Scholar]
- 12.Kagi D, Lederman B, Burki K, et al. Cytotoxicity mediated by T-cells and natural killer cells is greatly impaired in perforin deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 13.Michalak TI, Lin B. Molecular species of hepadnavirus core and envelope polypeptides in hepatocyte plasma membrane of woodchucks with acute and chronic viral hepatitis. Hepatology. 1994;20:275–86. [PubMed] [Google Scholar]
- 14.Michalak TI, Pardoe IU, Coffin CS, et al. Occult lifelong persistence of infectious hepadnavirus and residual liver inflammation in woodchucks convalescent from acute viral hepatitis. Hepatology. 1999;29:928–38. doi: 10.1002/hep.510290329. [DOI] [PubMed] [Google Scholar]
- 15.Pardoe IU, Michalak TI. Detections of hepatitis B and woodchuck hepatitis viral DNA in plasma and mononuclear cells from heparinized blood by polymerase chain reaction. J Virol Methods. 1995;51:277–88. doi: 10.1016/0166-0934(94)00116-x. [DOI] [PubMed] [Google Scholar]
- 16.Michalak TI, Snyder RL, Churchill ND. Characterization of the incorporation of woodchuck hepatitis virus surface antigen into hepatocyte plasma membrane in woodchuck hepatitis and in the virus-induced hepatocellular carcinoma. Hepatology. 1989;10:44–55. doi: 10.1002/hep.1840100111. [DOI] [PubMed] [Google Scholar]
- 17.Michalak TI, Lin B, Churchill ND, Dzwonkowski P, Desousa JRB. Hepadna virus nucleocapsid and surface antigens and the antigen-specific antibodies associated with hepatocyte plasma membranes in experimental woodchuck acute hepatitis. Lab Invest. 1990;62:680–9. [PubMed] [Google Scholar]
- 18.De Leon M, Jackson KM, Cavanaugh JR, Mbangkollo D, Verret CR. Arrest of the cell-cycle reduces susceptibility of target-cells to perforin mediated lysis. J Cell Biochem. 1998;69:425–35. [PubMed] [Google Scholar]
- 19.Takahashi T, Tanaka M, Inazawa J, Abe T, Suda T, Nagata S. Human Fas ligand: gene structure, chromosomal location and specific specificity. Int Immunol. 1994;6:1567–74. doi: 10.1093/intimm/6.10.1567. [DOI] [PubMed] [Google Scholar]
- 20.Montel AH, Bochan MR, Hobbs JA, Lynch DH, Brahmi Z. Fas involvement in cytotoxicity mediated by human NK cells. Cell Immunol. 1995;166:236–46. doi: 10.1006/cimm.1995.9974. [DOI] [PubMed] [Google Scholar]
- 21.Michalak TI, Churchill ND, Codner D, Drover S, Marchall WH. Identification of woodchuck class I MHC antigens using monoclonal antibodies. Tissue Antigens. 1995;45:333–42. doi: 10.1111/j.1399-0039.1995.tb02463.x. [DOI] [PubMed] [Google Scholar]
- 22.Kuwano K, Arai S. Involvement of two distinct killing mechanisms in bystander target cell lysis induced by a cytotoxic T lymphocyte clone. Cell Immunol. 1996;169:288–93. doi: 10.1006/cimm.1996.0120. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka M, Suda T, Takahashi T, Nagata S. Expression of the functional soluble form of human Fas ligand in activated lymphocytes. EMBO. 1995;14:1129–35. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smyth MJ, Sutton VR, Kershaw MH, Trapani JA. Xenospecific cytotoxic T lymphocytes use perforin and Fas mediated pathways. Transplantation. 1996;62:1529–32. doi: 10.1097/00007890-199611270-00030. [DOI] [PubMed] [Google Scholar]
- 25.Sayers TJ, Brooks AD, Lee J-K, et al. Molecular mechanisms of immune-mediated lysis of murine renal cancer: differential contributions of perforin-dependent versus Fas-mediated pathways in lysis by NK and T cells. J Immunol. 1998;161:3957–65. [PubMed] [Google Scholar]
- 26.See DM, Khemka P, Sahl L, Bui T, Tilles JG. The role of natural killer cells in viral infection. Scand J Immunol. 1997;46:217–24. doi: 10.1046/j.1365-3083.1997.d01-121.x. [DOI] [PubMed] [Google Scholar]
- 27.Smyth MJ, Trapani JA. The relative role of lymphocyte granule exocytosis versus death receptor-mediated cytotoxicity in viral pathophysiology. J Virol. 1998;72:1–9. doi: 10.1128/jvi.72.1.1-9.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chemello L, Bortolotti MF, Schiavon E, et al. Natural killer activity in patients with acute viral hepatitis. Clin Exp Immunol. 1986;64:59–64. [PMC free article] [PubMed] [Google Scholar]
- 29.Echevarria S, Casafont F, Miera M, et al. Interleukin-2 and natural killer activity in acute type B hepatitis. Hepato-Gastroenterol. 1991;38:307–10. [PubMed] [Google Scholar]
- 30.Actis GC, Ponzzetto A, D'Urso N, et al. Chronic active hepatitis B. Interferon-activated natural killer-like cells against a hepatoma cell line transfected with the hepatitis B virus nucleic acid. Liver. 1991;11:106–13. doi: 10.1111/j.1600-0676.1991.tb00500.x. [DOI] [PubMed] [Google Scholar]
- 31.Ono K, Yamanaga Y, Yamamoto K, Koga S-I, Nishimura J, Nawata H. Natural killing activities in chronic liver diseases and hepatocellular carcinoma. J Clin Immunol. 1996;16:41–45. doi: 10.1007/BF01540971. [DOI] [PubMed] [Google Scholar]
- 32.De Martino M, Rossi ME, Muccioli AT, Resti M, Vierucci A. Interference of hepatitis B surface antigen with natural killer cell function. Clin Exp Immunol. 1985;61:90–95. [PMC free article] [PubMed] [Google Scholar]
- 33.Azzari C, Rossi ME, Resti M, et al. VIP restores natural killer cell activity depressed by hepatitis B surface antigen. Viral Immunol. 1992;5:195–200. doi: 10.1089/vim.1992.5.195. [DOI] [PubMed] [Google Scholar]
- 34.Brutkiewicz RR, Welsh RM. Major histocompatibility complex class I antigens and the control of viral infections by natural killer cells. J Virol. 1995;69:3967–71. doi: 10.1128/jvi.69.7.3967-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]


