Abstract
Methotrexate (MTX) is an effective immunosuppressive agent in various chronic inflammatory diseases such as rheumatoid arthritis (RA). However, its mechanisms of action are only partially understood. In this study, we assessed the effects of MTX on the differentiation of peripheral blood (PB) CD4+CD45RA ‘naive’ and CD4+CD45RO ‘memory’ T cells from healthy controls and patients with RA. Accordingly, purified T cells were primed and restimulated in vitro via the T cell receptor (TCR) in the presence of IL-2 to generate effector T cells secreting large amounts of Th1 and Th2 cytokines. We observed that low doses of MTX strongly suppress TNF and to a lesser extent interferon-gamma (IFN-γ) production by T cells from both healthy donors and RA patients when present during T cell priming via the TCR. Similar data were obtained for TCR-primed synovial fluid mononuclear cells in RA. In contrast, production of IL-4 by TCR-primed CD45RA T cells was significantly increased upon MTX treatment. Interestingly, MTX did not enhance IL-4 production when present during restimulation of effector CD45RO T cells, although it still suppressed TNF production. The results indicate that MTX effects depend on the stage of T cell activation and identify TNF production by TCR-primed T lymphocytes as a target for low-dose MTX treatment in RA. These findings could explain the delayed clinical effects of MTX and may contribute to its potent anti-inflammatory and immunoregulatory properties.
Keywords: methotrexate, immunosuppression, tumour necrosis factor
INTRODUCTION
Low-dose methotrexate (MTX) has proved efficacious in suppressing the inflammatory polyarthritis in patients with rheumatoid arthritis (RA) [1–3]. In this disease, large quantities of proinflammatory cytokines such as IL-1β, IL-6 and TNF-α are found in the synovial fluid and synovial membrane [4,5]. These proinflammatory cytokines are produced by activated macrophages or T cells and strongly contribute to synovial cell proliferation and cartilage destruction in RA [4].
Although MTX has become a first line immunosuppressive agent for patients with RA, its mechanism of action is only partially understood. It is a folic acid antagonist that competitively inhibits dihydrofolate reductase [6–9]. In addition, MTX suppresses B cell and macrophage function, inhibits neovascularization, induces CD95-independent T cell apoptosis and clonal deletion of T cells and interferes with neutrophil activity and cell adherence [10–18]. Furthermore, it suppresses IL-1 and IL-8 production by stimulated peripheral blood mononuclear cells (PBMC) and inhibits the binding of IL-1β to its receptor on peripheral blood cells [19]. Interestingly, most of the data on cytokine modulation by MTX have been obtained using short-term in vitro cell culture experiments. These data may thus only partially account for the in vivo effects of MTX on cytokine production in RA, since MTX shows a well-known delayed clinical response. However, recent data suggest that treatment with MTX has specific effects on cytokine production in RA, since decreased IL-6 and interferon-gamma (IFN-γ) production by PBMC was observed in MTX-treated RA patients [20,21]. In addition, some studies also suggested a reduction of TNF serum levels or soluble TNF receptor levels in MTX-treated patients with RA [9,22].
Since a more profound understanding of the immunological and biochemical mechanisms of action of MTX may result in the design of new therapeutic strategies for RA, we focused in the present study on the effects of MTX on cytokine production by human T cells and synovial mononuclear cells. In particular, we addressed effects of MTX on Th1 (IFN-γ, TNF) and Th2 (IL-4) cytokine production by T cells. In these studies, we used a recently described system for T cell priming and restimulation via the T cell receptor (TCR) [23], since repeated TCR stimulation probably occurs in RA as a result of persistent infectious agents and/or antigens in the body over prolonged periods of time [4]. We demonstrate that low doses of MTX strongly suppress TNF production by TCR-primed T cells. These data may at least partially explain MTX-mediated mechanisms of immunosuppression in patients with RA.
PATIENTS AND METHODS
Isolation of peripheral blood CD14+ monocytes and CD19+ B lymphocytes
Human PBMC from healthy volunteers (age 25–45 years) were isolated using Ficoll–Hypaque gradients. To isolate CD14+ monocytes, PBMC were further purified using CD14 MoAbs attached to immunomagnetic beads and MACS technique according to the protocol provided by the manufacturer (Miltenyl Corp., München, Germany). CD19+ B lymphocytes were isolated from PBMC using specific immunomagnetic beads followed by treatment with Detachabead as recommended by the manufacturer (Dynal, Oslo, Norway). Reanalysis of sorted populations by FACS revealed a purity of > 92%. Cells were finally resuspended in complete RPMI media and cultured as specified below.
Isolation of primary CD4+ T lymphocytes from peripheral blood
PBMC from healthy volunteers were isolated using Ficoll–Hypaque gradients as described above. PBMC were further purified using CD4 MoAbs attached to immunomagnetic beads according to the protocol provided by the manufacturer, followed by treatment with Detachabead (obtained from Dynal). CD4+ T cells were further separated by negative selection techniques using CD45RO or CD45RA MoAbs (Pharmingen, San Diego, CA) and immunomagnetic beads (Dynal). Reanalysis of sorted populations by FACS revealed a purity of > 96%.
Cell culture and stimulation conditions
Cell cultures of lymphocytes and monocytes were performed in complete medium consisting of RPMI 1640 supplemented with 3 mml-glutamine, 10 mm HEPES buffer, 100 U/ml each of penicillin and streptomycin (Whittaker, Bethesda, MD), 0.05 mm 2-mercaptoethanol (2-ME; Sigma Chemical Co., St Louis, MO) and 10% heat-inactivated fetal calf serum (FCS) (cell density 106 cells/ml). Endotoxin levels in cell culture were determined by Seromed Biochrom (Berlin, Germany) using a chromogene assay and were below 0.01 EU/ml (12 EU = 1 ng).
In an initial series of experiments, B cells and monocytes were activated with 10 μg/ml bacterial lipopolysaccharide (LPS) and Staphylococcus aureus Cowan's antigen (SAC; 0.001% w/v) for 48 h. In additional experiments, monocytes were cultured in complete RPMI media with 10 μg/ml LPS, SAC (0.001% w/v) and 10 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) for 7 days as specified in Results. Supernatants were taken and analysed for cytokine concentration by specific ELISA, as described below.
Anti-CD3 antibody OKT3 was obtained as murine ascites produced by the hybridoma cell line OKT3 (ATCC, Rockville, MD) and affinity-purified using protein G columns (Pierce, Rockville, IL). For T cell stimulation purified anti-CD3 antibodies were coated at 200 ng/ml and recombinant IL-2 (R&D Systems, Heidelberg, Germany) was used at a final concentration of 40 U/ml. To induce cytokine production in an initial series of studies, T cells were stimulated with anti-CD3 for 2 days. Supernatants were taken after 2 days and analysed for cytokine concentration by specific ELISA. In additional experiments, purified T cells were primed via the TCR [23]. For T cell priming T cells were activated by plate-bound anti-CD3 antibody and recombinant IL-2 for 9 days in the presence or absence of indicated amounts of MTX (Sigma, Munich, Germany). For restimulation of primed CD4+ T cells, cells were restimulated in fresh medium with anti-CD3 plus IL-2 in the presence or absence of MTX for 48 h. Supernatants were finally analysed by ELISA as described below.
ELISA analysis
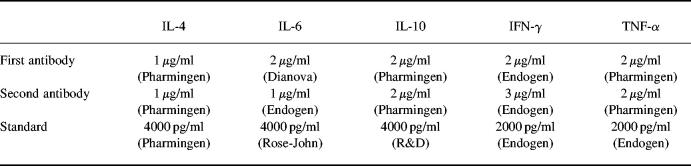
To measure cytokine production, 1 × 106 CD4+ T cells, B cells or macrophages were activated with indicated stimuli and cultured in culture medium at 37°C in a humidified atmosphere containing 5% CO2 in the presence or absence of indicated concentrations of methotrexate. After 48 h, culture supernatants were removed and assayed for cytokine concentration. Cytokine concentrations were determined by specific ELISA as previously described [24]. Antibodies used for ELISA are shown in Table 1.
Table 1.
Recombinant cytokines and primary/secondary antibodies used for ELISA
Isolation of synovial fluid mononuclear cells and PBMC from patients with RA
Four patients (two male and two female; mean age 48 years) who fulfilled the American College of Rheumatology 1987 criteria for RA [25] were included. All patients had active RA. RA activity was defined by the presence of at least three of the following criteria: morning stiffness > 45 min, number of swollen joints > 5, number of tender joints > 8, erythrocyte sedimentation rate (ESR) > 27 mm/h. PBMC from all patients with RA were isolated as described above. Furthermore, synovial fluid from three of the above patients with active RA was obtained by puncture of the affected knee joint after informed consent. Synovial fluid mononuclear cells (SFMC) were finally isolated by Ficoll–Hypaque gradients as specified above. In addition, PBMC were isolated from three patients with non-RA arthritis: a 34-year-old woman with acute Shigella-induced reactive arthritis and two female patients with psoriatic arthritis. Cell cultures were performed as described above.
Immunocytochemistry
Immunocytochemistry was performed on cytospins from cultured CD4+ T lymphocytes. In addition, Jurkat T cells stimulated with phorbol myristate acetate (PMA; 50 ng/ml) plus ionomycin (1 μg/ml) served as positive control. Briefly, cells were fixed in 4% paraformaldehyde–PBS or methanol and washed in 0.01 m PBS. Cytospins were then pretreated with 10% of serum (corresponding to the secondary antibody) in PBS–0.1% Triton-X and incubated overnight at 4°C with the primary antibody (10 μg/ml monoclonal anti-human TNF or IL-4; Pharmingen) in PBS–0.1% bovine serum albumin (BSA)–0.1% Triton-X. Samples without primary antibody served as negative control. The following day, samples were rinsed in PBS and incubated with a biotinylated secondary IgG antibody (1:100; Vector, Burlingame, CA) for 1 h at room temperature followed by incubation with streptavidin-conjugated Cy2 (Dianova, Hamburg, Germany) (1:500) for 2 h at room temperature. Samples were rinsed with PBS and subjected to a second cycle of staining using anti-CD4 as primary antibody (rat anti-mouse CD4; Pharmingen) and streptavidin-conjugated Cy3 as chromogen. Slides were mounted with mounting medium for fluorescence (Vector) and analysed with a Zeiss Microscope. Ten high-power fields (HPF) were finally counted in three samples per condition. No staining was detected in samples without primary antibody.
Statistical analysis
Tests for significance of differences were made by Student's t-test using the program StatWorks.
RESULTS
MTX does not affect production of various immunoregulatory cytokines by purified human CD19+ B lymphocytes and CD14+ monocytes after 2 days
In an initial series of studies, we analysed whether treatment with MTX induced changes in cytokine production by B lymphocytes and monocytes from the peripheral blood (PB) of healthy donors after 2 days. Accordingly, PB CD19+ B cells and CD14+ monocytes were isolated using positive selection techniques and immunomagnetic beads (see Patients and Methods). The cells were stimulated with LPS plus SAC in the presence or absence of MTX and culture supernatants were analysed for cytokine content by specific ELISA. It was found that MTX treatment did not significantly change IL-6 and IL-10 cytokine production by purified B lymphocytes after 2 days (Table 2a). Furthermore, IL-6, TNF and IL-10 production by purified CD14+ monocytes after 2 days was also not affected by MTX treatment (Table 2b,c). Finally, MTX did not suppress TNF production by monocytes that were cultured in the presence of LPS, SAC and GM-CSF for 7 days (Table 2d). These data indicate that MTX has no striking effects on the production of various immunoregulatory cytokines by PB B lymphocytes and monocytes under our experimental conditions.
Table 2.
a,b Methotrexate (MTX)-induced effects on cytokine production by purified CD19+ B lymphocytes (a) and CD14+ monocytes (b) from the peripheral blood of healthy donors
a. B cells
b. Monocytes
c. Monocytes
d. Monocytes
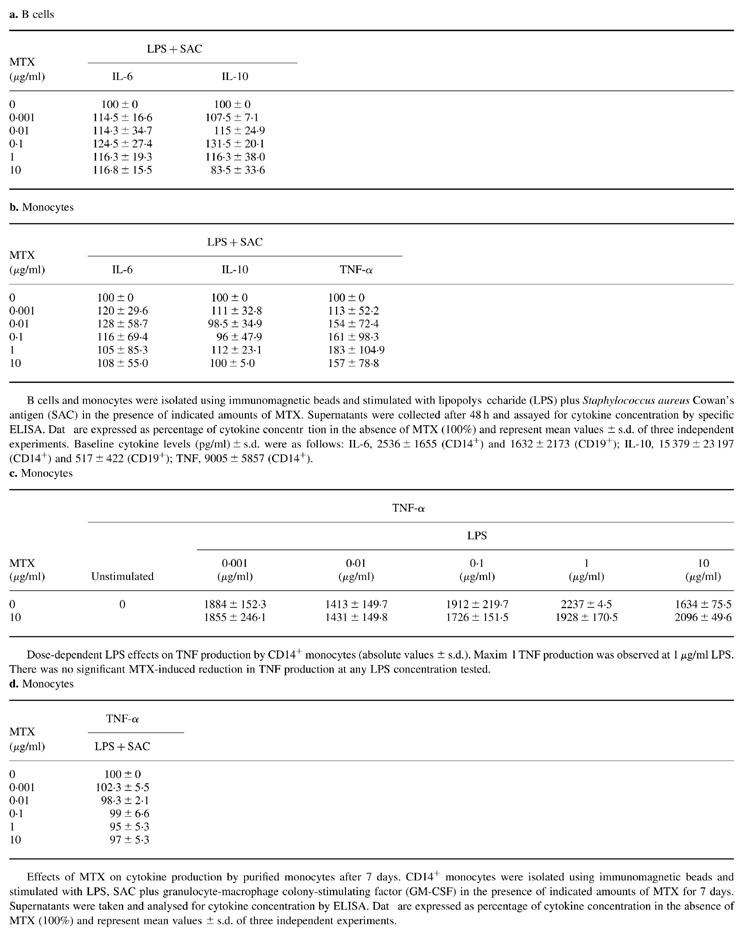
B cells and monocytes were isolated using immunomagnetic beads and stimulated with lipopolysaccharide (LPS) plus Staphylococcus aureus Cowan's antigen (SAC) in the presence of indicated amounts of MTX. Supernatants were collected after 48 h and assayed for cytokine concentration by specific ELISA. Data are expressed as percentage of cytokine concentration in the absence of MTX (100%) and represent mean values ± s.d. of three independent experiments. Baseline cytokine levels (pg/ml) ± s.d. were as follows: IL-6, 2536 ± 1655 (CD14+) and 1632 ± 2173 (CD19+); IL-10, 15 379 ± 23 197 (CD14+) and 517 ± 422 (CD19+); TNF, 9005 ± 5857 (CD14+)
Dose-dependent LPS effects on TNF production by CD14+ monocytes (absolute values ± s.d.). Maximal TNF production was observed at 1 μg/ml LPS. There was no significant MTX-induced reduction in TNF production at any LPS concentration tested.
Effects of MTX on cytokine production by purified monocytes after 7 days. CD14+ monocytes were isolated using immunomagnetic beads and stimulated with LPS, SAC plus granulocyte-macrophage colony-stimulating factor (GM-CSF) in the presence of indicated amounts of MTX for 7 days. Supernatants were taken and analysed for cytokine concentration by ELISA. Data are expressed as percentage of cytokine concentration in the absence of MTX (100%) and represent mean values ± s.d. of three independent experiments.
MTX suppresses TNF and IFN-γ production by human CD4+ CD45RA and CD45RO T lymphocytes after 2 days only at high concentrations
In further studies, we addressed MTX-induced changes in cytokine production by purified CD4+ T cell subsets. Accordingly, we separated CD45RA ‘naive’ and CD45RO ‘memory’ T cell subsets from blood samples of healthy donors using immunomagnetic beads and obtained > 96% pure populations, as assessed by FACS analysis. The T cells were then co-incubated with MTX and cytokine production was assessed after 2 days by specific ELISA. As shown in Fig. 1b, MTX caused significantly reduced production of TNF and IFN-γ by CD45RO T cells only at concentrations of 1–10 μg/ml that are outside the therapeutic serum levels obtained after administration of 10 mg MTX/week [9,26]. In contrast, IL-4 production by CD45RO T cells was virtually unaffected. Furthermore, 1–10 μg/ml MTX led to significantly reduced production of TNF and IL-4 by CD45RA T cells, whereas IFN-γ production was not affected (Fig. 1a). These data thus show that treatment of T cells with MTX for 2 days causes specific changes in cytokine production that are dependent on the T cell subset. These changes occurred only at high concentrations of MTX that appear not to be relevant for the treatment of RA patients.
Fig. 1.
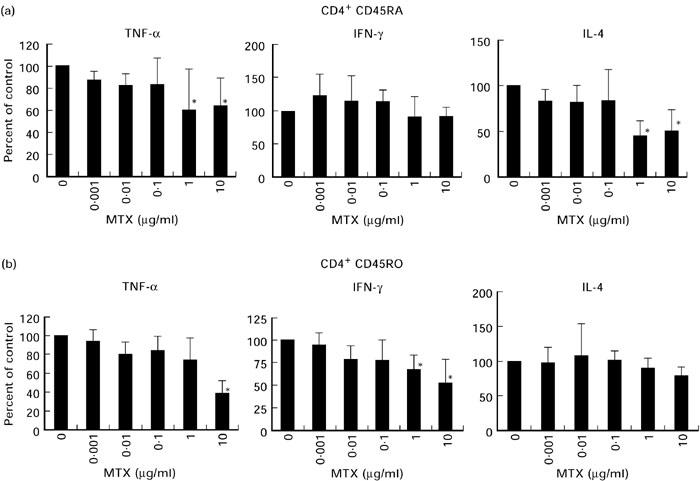
Effects of methotrexate (MTX) on cytokine production by purified CD45RA (a) and CD45RO (b) T cells from the peripheral blood (PB) of healthy donors after 2 days. PB CD45RA and CD45RO T lymphocytes were isolated using immunomagnetic beads and stimulated with anti-CD3 in the presence of indicated amounts of MTX. Supernatants were collected after 48 h and assayed for cytokine concentration by specific ELISA. Data are expressed as percentage of cytokine concentration in the absence of MTX (100%) and represent mean values ± s.d. of three independent experiments. Significant changes compared with the MTX-free culture condition: *P < 0.05.
Low doses of MTX strongly suppress TNF production by TCR-primed CD45RA and CD45RO T lymphocytes
In further studies on the effects of MTX, we used a recently described system for priming and restimulation of CD4+ T cell subsets via the TCR to induce development of cytokine-producing effector T cells [23]. In this experimental system, T cells were primed via the TCR/CD3 complex in the presence of recombinant IL-2 for 9 days. After this period, cells were restimulated with anti-CD3/IL-2 for an additional 2 days. Indicated amounts of MTX were added to the T cell cultures during priming or restimulation (see Patients and Methods).
MTX strongly affected the priming of CD45RA and CD45RO T cells with respect to the resulting pattern of cytokine production (Fig. 2). When present during priming, MTX (0.1–10 μg/ml) caused significantly reduced production of IFN-γ by both CD45RA and CD45RO T cells. Furthermore, TNF production by CD45RA and CD45RO T cells was completely abrogated at these dosages. Even dosages as low as 0.001 μg/ml that are below the therapeutic serum levels in MTX-treated RA patients [9,26] caused a significant reduction in TNF production by both CD45RA and CD45RO T cells. In contrast to these observations, IL-4 production by CD45RA T cells (but not by CD45RO cells) was significantly increased upon low dose MTX treatment (0.001–0.1 μg/ml). These data on changes in TNF and IL-4 production by TCR-primed T cells upon MTX treatment were further supported by quantitative immunofluorescence studies. In these experiments, we quantified the percentage of TNF- and IL-4-expressing T cells by immunofluorescence analysis (see Patients and Methods). It was found that low doses of MTX cause a significant increase in the percentage of IL-4-expressing CD4+ T cells, whereas the percentage of TNF-expressing CD4+ T cells was significantly reduced when MTX was present during T cell priming via the TCR/CD3 complex (Table 3).
Fig. 2.
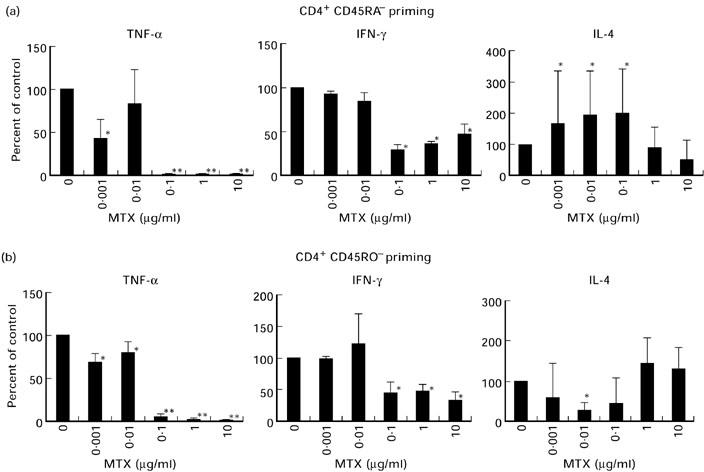
Effects of methotrexate (MTX) during priming on pro- and anti-inflammatory cytokine production by T cell receptor (TCR)-primed CD45RA (a) and CD45RO (b) T lymphocytes. CD4+ T cells were primed via the TCR/CD3 complex in the presence of IL-2 for 9 days in the presence of indicated amounts of MTX. Cells were then restimulated with anti-CD3 plus IL-2 in the absence of MTX for an additional 2 days. Cytokine concentrations in cell-free supernatants were then determined by specific ELISA. Data are expressed as percentage of cytokine concentration in the absence of MTX (100%) and represent mean values ± s.d. of three independent experiments. Significant changes compared with the MTX-free culture condition: *P < 0.05; **P < 0.01.
Table 3.
Quantification of TNF and IL-4-positive CD4+ T cells by immunofluorescence
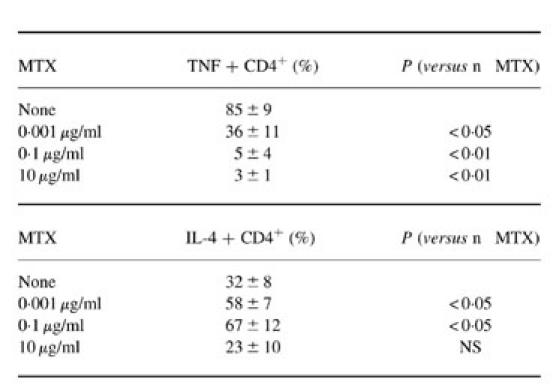
T cells were primed via the T cell receptor (TCR)/CD3 complex in the presence of methotrexate (MTX) for 9 days. Cytospins were made and analysed by immunofluorescence for the presence of T cells expressing CD4 plus TNF or CD4 plus IL-4.
When MTX was present during restimulation of primed effector T cells, the effects on TNF cytokine production occurred at lower dosages, although the overall decrease in TNF production was less pronounced (Fig. 3). Furthermore, IFN-γ production by CD45RA and CD45RO T cells was significantly reduced at all dosages tested (0.001–0.1 μg/ml). In comparison with the priming experiments above, IL-4 production by CD45RA T cells was increased to a lesser extent upon MTX treatment.
Fig. 3.
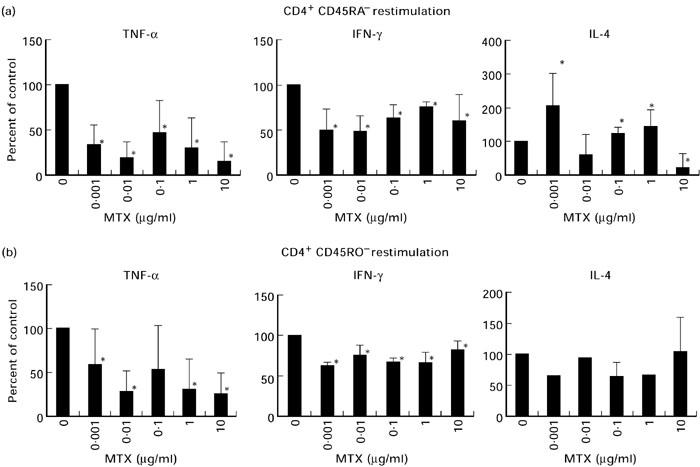
Effects of methotrexate (MTX) during restimulation on pro- and anti-inflammatory cytokine production by T cell receptor (TCR)-primed CD45RA and CD45RO T lymphocytes. CD4+ T cells were primed via the TCR/CD3 complex in the presence of IL-2 for 9 days in the absence of MTX. Cells were then restimulated with anti-CD3 plus IL-2 in the presence of MTX for an additional 2 days. Cytokine concentrations in cell-free supernatants were then determined by specific ELISA. Data are expressed as percentage of cytokine concentration in the absence of MTX (100%) and represent mean values ± s.d. of three independent experiments. Significant changes compared with the MTX-free culture condition: *P < 0.05.
MTX down-regulates TNF production by TCR-primed blood T lymphocytes and synovial mononuclear cells in RA
In a final series of studies, we assessed whether MTX had similar effects on IL-4 and TNF production by PB T cells and SFMC in patients with RA. In an initial series of studies, we obtained PB T cells from three patients with RA and three patients with non-RA arthritis and assessed cytokine production in the presence of MTX after 2 days (Fig. 4a). Consistent with the above observations on healthy blood donors, it was found that low doses of MTX did not significantly modulate TNF cytokine production by PB T cells after 2 days. Similar data were obtained for SFMC in RA patients (Fig. 4a). In further studies, the effects of MTX were then assessed on cytokine production by PB T cells and SFMC that were primed and restimulated via the TCR in the presence of IL-2. It was observed that the presence of MTX during priming of PB T cells and SFMC from RA patients caused an abrogation of TNF production, while IL-4 production was increased at low dosages of MTX (0.001–0.1 μg/ml). Interestingly, MTX-induced changes in cytokine production by SFMC in RA patients occurred at lower dosages compared with PB T cells.
Fig. 4.
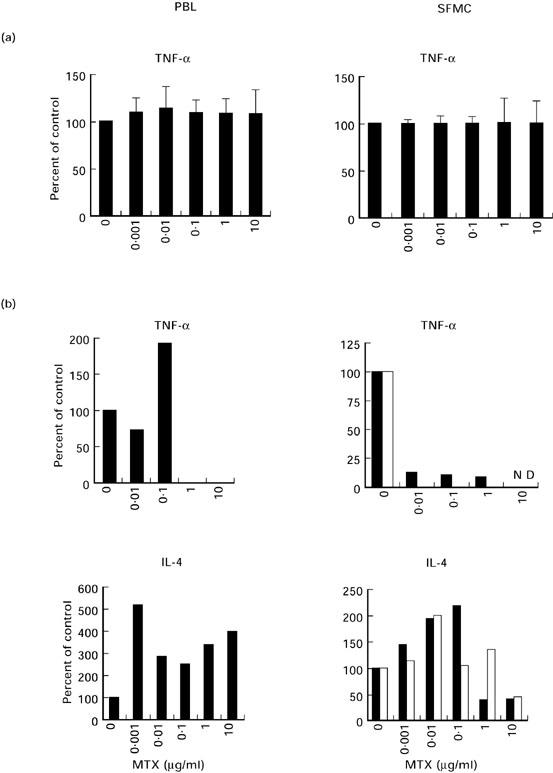
(a) Effects of methotrexate (MTX) on cytokine production by peripheral blood (PB) T lymphocytes and synovial fluid mononuclear cells (SFMC) in three patients with RA and three patients with non-RA arthritis after 2 days. PB T lymphocytes and SFMC were isolated from six patients and stimulated with anti-CD3 in the presence of indicated amounts of MTX. Supernatants were collected after 48 h and assayed for cytokine concentration by specific ELISA. Data are expressed as percentage of cytokine concentration in the absence of MTX (100%) and represent mean values ± s.d. of six independent experiments. (b) Effects of MTX during priming on pro- and anti-inflammatory cytokine production by T cell receptor (TCR)-primed PB T cells and SFMC in RA. T cells and SFMC were primed via the TCR/CD3 complex in the presence of IL-2 for 9 days in the presence of indicated amounts of MTX. Cells were then restimulated with anti-CD3 plus IL-2 in the absence of MTX for an additional 2 days. Cytokine concentrations in cell-free supernatants were then determined by specific ELISA. Data are expressed as percentage of cytokine concentration in the absence of MTX (100%). One representative experiment using PB T cells and SFMC from two RA patients is shown. Baseline TNF levels (pg/ml): PBL patient 1, 306; SFMC patient 1, 1506; PBL patient 2, 149; SFMC patient 2, 1541; baseline IL-4 levels (pg/ml): PBL patient 1, 21; SFMC patient 1, 180; PBL patient 2: 45; SFMC patient 2, 120. ▪, Patient 1; □, patient 2.
DISCUSSION
In the present study, we demonstrate a time-dependent and cell type-specific modulation of cytokine production by MTX. In initial studies, we demonstrated that MTX had no significant effect on cytokine (IL-6, IL-10, TNF) production by PB CD19+ B cells and CD14+ monocytes. This finding may be explained by the fact that MTX has no detectable effects on cytokine production by these cells or, alternatively, by the requirement for specific cell culture conditions for MTX effects that are not present under our experimental conditions. Furthermore, it was observed that only high doses of MTX caused suppression of TNF production by cultured T cells after 2 days, whereas a significant suppression of TNF production by TCR-primed T cells was found after low dose MTX treatment. Finally, we observed that MTX effects on TNF cytokine production were also observed in PB T cells and SFMC of patients with RA. Taken together with the increased TNF levels in patients with RA, these data suggest that suppression of TNF production by TCR-activated T cells could be an important target mechanism for MTX effects that may contribute to the potent anti-inflammatory and immunoregulatory properties of this agent.
TNF is a pleiotropic cytokine that plays a key regulatory role in antibacterial host defence, endotoxic shock, contact hypersensitivity, chronic intestinal inflammation and experimental arthritis [27–29]. Furthermore, various studies have demonstrated the pivotal role of TNF in patients with RA [4,30]. In this disease, TNF is produced by many cells in the inflamed rheumatoid synovium, including macrophages and lymphocytes [4,5]. The importance of TNF in patients with RA is further supported by recent studies showing that regulation of TNF levels by several different strategies including chimeric (mouse/human) anti-TNF MoAb cA2 antibodies, humanized anti-TNF MoAbs (CDP571) and recombinant TNF receptor (p75)-Fc fusion proteins (TNFR(p75)-Fc fusion protein) may be a novel promising approach for treatment of this disease [31–34].
Based on the functional role of TNF in RA and on previous studies in mice suggesting that TCR-dependent activation of cytokine production is a key target of MTX [35], the present study focused on MTX-induced changes in cytokine production by TCR-activated human CD4+CD45RA naive and CD4+CD45RO memory T cells [23]. Interestingly, it was found that low doses of MTX significantly suppressed TNF production by TCR-primed and restimulated CD45RA and CD45RO T cells. These data on MTX effects on TNF production by human T cells are in agreement with some previous observations in animals: in experimental adjuvant arthritis, MTX treatment caused decreased TNF concentrations in the synovial fluid and reduced TNF production ex vivo [36,37]. Furthermore, we recently described that low doses of MTX strongly suppress TCR- and IL-15-dependent TNF production by T cells in healthy and arthritic mice [35]. However, MTX-induced suppression of TNF production in this experimental system occurred already after 2 days [35]. In contrast to these observations in mice, it was found here that treatment with low doses of MTX did not significantly reduce TNF production by T cells after 2 days, but potently suppressed TNF production by TCR-primed T cells. These data suggest that low doses of MTX may require prolonged periods of time to achieve effects on cytokine production by T cells in response to antigenic TCR-dependent stimulation, and are in agreement with the delayed clinical response upon MTX treatment.
Low doses of MTX significantly reduced production of the proinflammatory cytokines TNF and IFN-γ by PB CD45RA and CD45RO T cells when present during priming. The effects of MTX on the latter cells could be clinically relevant, since an enrichment of memory T cells (CD4 CD45RO CD27−) has been found in the blood of some RA patients and in all synovial tissue and fluid samples in RA [38,39]. This expansion of memory cells in RA probably reflects a disordered T cell differentiation in the presence of repeated antigenic stimulation via the TCR that may play a role in perpetuating synovial inflammation [38,39]. Furthermore, the above data suggest that MTX may be very efficient in blocking activation of naive CD45RA T cells in response to a primary antigenic stimulus. In any case, the suppression of TNF and IFN-γ production by CD45RA/RO T cells could be important for clinical effects of MTX, since increased production of these cytokines and a shift towards a Th1 T cell phenotype in PB and synovial CD4+ T cells have been described in patients with RA [40–44]. In support of this hypothesis, recent data showed suppression of Th1 cytokine mRNA levels in PBMC from MTX-treated patients [21].
Interestingly, MTX-induced effects on TNF cytokine production by synovial T cells in RA occurred at lower dosages and appeared to be more pronounced compared with PB T cells. This finding could be relevant for the clinical effects of MTX, since synovial memory T cells provide an ongoing important proinflammatory stimulus within the synovium of rheumatic joints that probably contributes to the perpetuation of the disease [45]. Recent studies in mice, however, indicated that MTX-induced suppression of TNF production is more striking compared with that of IFN-γ [35]. This hypothesis was supported by studies showing that MTX effects on IFN-γ production were augmented in TNF transgenic Tg197 mice but abrogated in TNF knockout animals. However, this MTX-mediated inhibition of IFN-γ production by T cells could also at least partially contribute to the recently described synergistic effects of anti-TNF antibodies and MTX in clinical trials in RA [46]. In addition, synergistic effects could be due to suppression of human anti-mouse antibody responses by MTX that may reduce the immunogenic potential of the cA2 anti-TNF antibody [46].
In contrast to these data on IFN-γ and TNF, production of the anti-inflammatory cytokine IL-4 by TCR-primed CD45RA T cells was significantly increased upon low dose MTX treatment (0.001–0.1 μg/ml), suggesting specific induction of this cytokine by this agent. IL-4 inhibits activation of monocytes/macrophages and synovial cells, suppresses IFN-γ production by Th1 T lymphocytes and induces Th2 T cell differentiation [47]. Since recent data suggest that synovial memory CD4+ T cells in RA produce exclusively IFN-γ but no IL-4 [45], the induction of IL-4 could be important for suppression of synovial Th1 effector T cells by MTX. Furthermore, MTX could be capable of suppressing activity of synovial macrophages in RA via IL-4 and induce the development of Th2 T cells from CD45RA naive T cells in response to new antigenic stimuli.
The observed effects of MTX on cytokine production occurred at low concentrations of MTX that are well within the physiologic serum levels in RA patients given 10 mg MTX/week [9,26]. These data on MTX serum levels may even underestimate the effects of this drug in vivo, since MTX concentrations in the synovium and bone of RA patients are known to be roughly 10-fold higher compared with plasma levels [48]. Thus, low dose treatment with MTX in RA appears to be capable of suppressing local TNF and IFN-γ production by TCR-primed T cells in the rheumatic joint, while favouring the generation of T cells producing the anti-inflammatory cytokine IL-4. The observed characteristic dose- and time-dependent modulation of cytokine profiles by MTX argues against a general inhibitory effect on mRNA/protein synthesis, and suggests additional specific effects of MTX on cytokine production by T cells. This specific modulation of cytokine production by TCR-primed CD45RA/CD45RO T cells may contribute to the well-known immunosuppressive properties of MTX in RA. In contrast to these data on T cells, MTX did not affect cytokine production by monocytes and B cells in our experimental system. This may indicate that cytokine production by T cells rather than B cells or monocytes is the major target of MTX. Alternatively, one may speculate that MTX effects on cytokine production by B cells and monocytes require cell–cell interactions or antigen-specific conditions that were not present in our experimental system.
Taken together, the data reported here provide direct evidence for a regulatory effect of MTX on TNF production by TCR-primed CD45RA/CD45RO T cells in humans. This finding may at least partially contribute to MTX-mediated mechanisms of immunosuppression in patients with RA.
Acknowledgments
The research of M.F.N. was supported by grants from the Innovationsstiftung Rheinland-Pfalz, the Deutsche Forschungsgemeinschaft (Ne 490/1-1, Ne 490/2-1) and the Gerhard Hess programme of the Deutsche Forschungsgemeinschaft (Ne 490/3-1). The authors gratefully acknowledge Dr E. Schmitt (Department of Immunology, University of Mainz) for critical reading of the manuscript. In addition, the authors would like to thank Mrs B. Bartsch for excellent technical assistance. This study contains data from the MD thesis of K.H.
REFERENCES
- 1.Weinblatt ME, Kaplen H, Germain BF, et al. Methotrexate in rheumatoid arthritis: a five year prospective multicenter study. Arthritis Rheum. 1994;37:1492–8. doi: 10.1002/art.1780371013. [DOI] [PubMed] [Google Scholar]
- 2.Kremer JM, Phelps CT. Long-term prospective study of the use of methotrexate in the treatment of rheumatoid arthritis: update after a mean of 90 months. Arthritis Rheum. 1992;35:138–45. doi: 10.1002/art.1780350203. [DOI] [PubMed] [Google Scholar]
- 3.Weinblatt ME, Coblyn JS, Fox DA, Fraser PA, Holdsworth DE, Glass DN, Trentham DE. Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med. 1985;312:88–92. doi: 10.1056/NEJM198503283121303. [DOI] [PubMed] [Google Scholar]
- 4.Kavanaugh AF, Lipsky PE. Rheumatoid arthritis. In: Rich RR, editor. Clinical immunology. 1996. pp. 1093–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Firestein GS, Alvaro-Garcia JM, Maki R. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol. 1990;144:3347–53. [PubMed] [Google Scholar]
- 6.Baggott JE, Morgan SL, Koopman WJ. The effect of methotrexate and 7-hydroxymethotrexate on rat adjuvant arthritis and on urinary aminoimidazole carboxamide excretion. Arthritis Rheum. 1998;41:1407–10. doi: 10.1002/1529-0131(199808)41:8<1407::AID-ART9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 7.Kremer JM. Possible mechanisms of action of methotrexate in patients with rheumatoid arthritis. Br J Rheumatol. 1995;34(Suppl. 2):26–29. [PubMed] [Google Scholar]
- 8.Moreland LW, Heck LW, Jr, Koopman WJ. Biologic agents for treating rheumatoid arthritis: concepts and progress. Arthritis Rheum. 1997;40:397–409. doi: 10.1002/art.1780400302. [DOI] [PubMed] [Google Scholar]
- 9.Cronstein BN. Molecular therapeutics: methotrexate and its mechanism of action. Arthritis Rheum. 1996;39:1951–60. doi: 10.1002/art.1780391203. [DOI] [PubMed] [Google Scholar]
- 10.Segal R, Mozes E, Yaron M, Tartakovsky B. The effects of methotrexate on the production and activity of interleukin-1. Arthritis Rheum. 1989;32:370–7. doi: 10.1002/anr.1780320403. [DOI] [PubMed] [Google Scholar]
- 11.Hu SK, Mitcho YL, Oronsky AL, Kerwar SS. Studies on the effect of methotrexate on macrophage function. J Rheumatol. 1988;15:206–9. [PubMed] [Google Scholar]
- 12.Meyer FA, Yaron I, Mashiah V, Yaron M. Methotrexate inhibits proliferation but not interleukin 1 stimulated secretory activities of cultured human synovial fibroblasts. J Rheumatol. 1993;20:238–42. [PubMed] [Google Scholar]
- 13.Conolly KM, Stecher VJ, Danis E, Pruden DJ, LaBrie T. Alteration of interleukin-1 production and the acute phase response following medication of adjuvant arthritic rats with cyclosporin-A or methotrexate. Int J Immunopharmacol. 1988;10:717–28. doi: 10.1016/0192-0561(88)90025-2. [DOI] [PubMed] [Google Scholar]
- 14.Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102:322–8. doi: 10.1172/JCI2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cronstein BN, Eberle MA, Gruber HE, Levin RI. Methotrexate inhibits neutrophil function by stimulating adenosine release from connective tissue cells. Proc Natl Acad Sci USA. 1991;88:2441–5. doi: 10.1073/pnas.88.6.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cronstein BN, Naime D, Ostad E. The antiinflammatory mechanisms of methotrexate: increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest. 1993;92:2675–82. doi: 10.1172/JCI116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirata S, Matsubara T, Saura R, Tateishi H, Hirohata K. Inhibition of in vitro vascular endothelial cell proliferation and in vivo neovascularization by low-dose methotrexate. Arthritis Rheum. 1989;32:1065–73. doi: 10.1002/anr.1780320903. [DOI] [PubMed] [Google Scholar]
- 18.Sperling RI, Coblyn JS, Larkin JK, Benincaso AI, Austen KF, Weinblatt ME. Inhibition of leukotriene B4 synthesis in neutrophils from patients with rheumatoid arthritis by a single oral dose of methotrexate. Arthritis Rheum. 1990;33:1149–55. doi: 10.1002/art.1780330815. [DOI] [PubMed] [Google Scholar]
- 19.Brody M, Bohm I, Bauer R. Mechanism of action of methotrexate: experimental evidence that methotrexate blocks the binding of interleukin 1-beta to the interleukin 1 receptor on target cells. Eur J Clin Chem Clin Biochem. 1993;31:667–74. doi: 10.1515/cclm.1993.31.10.667. [DOI] [PubMed] [Google Scholar]
- 20.Crilly A, McInnes IB, McDonald AG, Watson J, Capell HA, Madhok R. Interleukin-6 (IL-6) and soluble IL-2 receptor levels in patients with rheumatoid arthritis treated with low dose oral methotrexate. J Rheumatol. 1995;22:224–6. [PubMed] [Google Scholar]
- 21.Constantin A, Loubet P, Lambert N, Yassine B, Abbal M, Mazieres B, Preval C, Cantagrel A. Antiinflammatory and immunoregulatory action of methotrexate in the treatment of rheumatoid arthritis. Arthritis Rheum. 1998;41:48–57. doi: 10.1002/1529-0131(199801)41:1<48::AID-ART7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 22.Seitz M, Loetscher P, Dewald B, Towbin H, Rordorf C, Gallati H, Baggiolini M, Gerber NJ. Methotrexate action in rheumatoid arthritis: stimulation of cytokine inhibitor and inhibition of chemokine production by peripheral blood mononuclear cells. Br J Rheumatol. 1995;34:602–9. doi: 10.1093/rheumatology/34.7.602. [DOI] [PubMed] [Google Scholar]
- 23.Brinkmann V, Kristofic C. Regulation by corticosteroids of Th1 and Th2 cytokine production in human CD4+ effector T cells generated from CD45RO- and CD45RO+ subsets. J Immunol. 1995;155:3322–8. [PubMed] [Google Scholar]
- 24.Neurath MF, Pettersson S, Meyer zum Büschenfelde KH, Strober W. Local administration of antisense oligonucleotides to the p65 subunit of NF-kappaB abrogates experimental colitis in mice. Nature Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- 25.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries FJ, Cooper NS. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 26.Sinnett MJ, Groff GD, Raddatz DA, Franck WA, Bertino J. Methotrexate pharmacokinetics in patients with rheumatoid arthritis. J Rheumatol. 1989;16:745–8. [PubMed] [Google Scholar]
- 27.Beutler B, Cerami A. The biology of cachectin/TNF—a primary mediator of the host response. Ann Rev Immunol. 1989;7:625–55. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- 28.Strober W, Kelsall BL, Fuss I, Marth T, Ludviksson B, Ehrhardt R, Neurath MF. Reciprocal IFN-γ and TGF-β responses regulate the occurrence of mucosal inflammation. Immunol Today. 1997;18:61–64. doi: 10.1016/s0167-5699(97)01000-1. [DOI] [PubMed] [Google Scholar]
- 29.Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, Kollias G. Transgenic mice expressing human tumour necrosis: a predictive genetic model of arthritis. EMBO J. 1991;10:4025–31. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brennan FM, Maini RN, Feldmann M. TNF-alpha: a pivotal role in rheumatoid arthritis. Br J Rheumatol. 1993;31:293–8. doi: 10.1093/rheumatology/31.5.293. [DOI] [PubMed] [Google Scholar]
- 31.Moreland LW, Baumgartner SW, Schiff MH, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor -Fc fusion protein. N Engl J Med. 1997;337:141–7. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- 32.Rankin ECC, Choy ESH, Kassimos D, et al. The therapeutic effects of an engineered human anti-tumour necrosis factor alpha antibody (CDP571) in rheumatoid arthritis. Br J Rheumatol. 1995;34:334–42. doi: 10.1093/rheumatology/34.4.334. [DOI] [PubMed] [Google Scholar]
- 33.Elliott MJ, Maini RN, Feldmann M, et al. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheum. 1993;36:1681–90. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- 34.Elliott MJ, Maini RN, Feldmann M, et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumor necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994;344:1105–10. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- 35.Neurath MF, Hildner K, Becker C, et al. Methotrexate specifically modulates cytokine production by T cells and macrophages in collagen-induced arthritis. A mechanism for methotrexate-mediated immunosuppression. Clin Exp Immunol. 1998;115:42–55. doi: 10.1046/j.1365-2249.1999.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Welles WL, Silkworth J, Oronsky AL, Kerwar SS, Galivan J. Studies on the effect of low dose methotrexate on rat adjuvant arthritis. J Rheumatol. 1985;12:904–6. [PubMed] [Google Scholar]
- 37.Williams AS, Camilleri JP, Topley N, Williams BD. Prostaglandin and tumor necrosis factor secretion by peritoneal macrophages isolated from normal and arthritic rats treated with liposomal methotrexate. J Pharmacol Toxicol Methods. 1994;32:53–58. doi: 10.1016/1056-8719(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 38.Kohem CL, Brezinschek RI, Wisbey H, Tortorella C, Lipsky PE, Oppenheimer-Marks N. Enrichment of differentiated CD45RBdim, CD27-memory T cells in the peripheral blood, synovial fluid, and synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:844–54. doi: 10.1002/art.1780390518. [DOI] [PubMed] [Google Scholar]
- 39.Thomas R, McIlraith M, Davis LS, Lipsky PE. Rheumatoid synovium is enriched in CD45RBdim mature memory T cells that are potent helpers for B cell differentiation. Arthritis Rheum. 1992;35:1455–65. doi: 10.1002/art.1780351209. [DOI] [PubMed] [Google Scholar]
- 40.Neighbour PA, Grayzel AI. Interferon production in vitro by leukocytes from patients with systemic lupus erythematosus and rheumatoid arthritis. Clin Exp Immunol. 1981;45:576–82. [PMC free article] [PubMed] [Google Scholar]
- 41.Quayle AJ, Chomarat P, Miossee P, Kjeldsen-Kragh J, Forre O, Natvig JB. Rheumatoid inflammatory T-cell clones express mostly Th1 but also Th2 and mixed (Th0 like) cytokine patterns. Scand J Immunol. 1993;38:75–82. doi: 10.1111/j.1365-3083.1993.tb01696.x. [DOI] [PubMed] [Google Scholar]
- 42.Miossec P, van den Berg W. Th1/Th2 cytokine balance in arthritis. Arthritis Rheum. 1997;40:2105–15. doi: 10.1002/art.1780401203. [DOI] [PubMed] [Google Scholar]
- 43.Barrera BR, Haagsma CJ, Boerbooms AM, Van Riel PL, Borm GF, Van dePutte LB, Van der Meer JW. Effect of methotrexate alone or in combination with sulphasalazine on the production and circulating concentrations of cytokines and their antagonists. Longitudinal evaluation in patients with rheumatoid arthritis. Br J Rheumatol. 1995;34:747–55. doi: 10.1093/rheumatology/34.8.747. [DOI] [PubMed] [Google Scholar]
- 44.Dolhain RJEM, van der Heiden AN, ter Haar NT, Breedveld FC, Miltenburg AMM. Shift toward T lymphocytes with a T helper 1 cytokine-secretion profile in the joints of patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:1961–9. doi: 10.1002/art.1780391204. [DOI] [PubMed] [Google Scholar]
- 45.Davis LS, Schulze-Koops H, Lipsky PE. Rheumatoid synovial memory T cells have been primed in vivo to be interferon-gamma producers. Arthritis Rheum. 1997;40(Suppl. 9):S119. [Google Scholar]
- 46.Maini RN, Breedveld FC, Kalden JR, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41:1552–63. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 47.Mosmann T, Sad R. The expanding universe of T-cell subsets: TH1, TH2, and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 48.Bologna C, Edno L, Anaya JM, et al. Methotrexate concentrations in synovial membrane and trabecular and cortical bone in rheumatoid arthritis patients. Arthritis Rheum. 1994;37:1770–3. doi: 10.1002/art.1780371210. [DOI] [PubMed] [Google Scholar]