Abstract
We studied the effect of a Chinese medicinal herb, Acanthopanax gracilistylus, extract (AGE), on human lymphocytes in vitro. AGE markedly suppressed the proliferative responses of human peripheral blood lymphocytes stimulated with mitogens concanavalin A (Con A) and Staphylococcus aureus Cowan I (SAC). Both T cell and B cell activities—production of interferon-gamma and immunoglobulin—were suppressed by AGE. The mechanism of AGE-induced suppression of lymphocytes is to arrest the cell cycle at the G0/G1 stage without a direct cytotoxic effect. AGE also suppressed the alloantigen-specific cytotoxic T lymphocyte response. However, natural killer cell activity was less sensitive to the suppressive activity of AGE. In contrast, AGE markedly enhanced monocyte function to produce cytokines. These activities of AGE were associated with a 60-kD protein which was sensitive to treatment with pronase E, but not with NaIO4. These results suggest that AGE has an immunomodulating activity on human lymphocytes and its properties could be clinically applied in the treatment of several diseases such as autoimmune and allergic diseases.
Keywords: Chinese medicinal herb, T cell, B cell, monocyte, natural killer cell
INTRODUCTION
For a long time, Chinese medicinal herbs (CMH) have been traditionally used to treat several diseases such as autoimmune diseases and cancers in China [1]. Animal experiments and modern clinical trails have shown that many of these herbs have immunomodulating activities [2]. However, the mechanism of their effectiveness and the chemical nature of CMH have not been fully analysed. In a previous paper we studied the effect of several CMH on immune responses using human and mouse lymphocytes in vitro [3,4]. Some CMH such as Oldenlandia diffusa and Cinamomum cassia presl exhibit potentiating activity of lymphocyte functions. However, Acanthopanax gracilistylus extract (AGE) had a suppressive activity on lymphocytes. AG belongs to the Acanthopanax genus and has been used to treat patients with rheumatism. It has been reported that AG has an anti-inflammatory effect [5]. However, little is known about its mechanism and chemical nature. In this study we examined the effect of AGE on human lymphocytes and their chemical nature in vitro.
MATERIALS AND METHODS
Preparation of AGE
Dried barks of AG were purchased from the Chinese Herbal Medicine Co. (Shijiazhung, Heibei, China). One gram of AG was steeped in 100 ml of distilled water at room temperature overnight, then boiled for 60 min. The extract was filtrated through a filter paper (Whatman type 42; Whatman International Ltd, Maidstone, UK) to remove insoluble materials and used for experiments as a crude sample after Millipore (Milex-GP, 0.22 μm pore size; Nippon Millipore Ltd, Tokyo, Japan) filtration.
Preparation of peripheral blood lymphocytes and in vitro culture
Peripheral blood lymphocytes (PBL) were isolated from the peripheral blood of healthy volunteers by density gradient centrifugation with a lymphoprep (Nycomed Pharm As, Oslo, Norway) at 400 g for 30 min. PBL (2 × 105) were cultured with several concentrations of AGE, 10 μg/ml concanavalin A (Con A; EY Labs, San Mateo, CA), 0.01% Staphylococcus aureus Cowan I (SAC; Funakoshi Yakuhin Co., Tokyo, Japan) in 0.2 ml of RPMI 1640 (Nissui Seiyaku Co., Yokohama, Japan) medium containing 10% fetal calf serum (FCS; Gibco, Grand Island, NY) in wells of flat-bottomed microtitre culture plates (Falcon no. 3072; Becton Dickinson Co., Lincoln Park, NJ) at 37°C for 3 days in 5% CO2 and 95% air. The cells were labelled with 0.5 μCi of tritiated thymidine (3H-TdR; specific activity 6.0 Ci/mmol; Amersham Plc, Aylesbury, UK) for the last 15 h and were harvested with the aid of a semiautomated cell harvester (Abe Kagaku Co., Chiba, Japan) [6]. The amount of radioactivity incorporated into the DNA in the cells was measured with a liquid scintillation counter (Aloka Co., Tokyo, Japan). The results are expressed as the mean ct/min of 3H-TdR incorporated by the PBL with standard error (s.e.m.) in triplicate cultures.
B cell- and T cell-enriched fractions were prepared by a rosette formation method. PBL (107) were incubated with 109 sheep erythrocytes at 37°C for 15 min and at 4°C for 2 h and fractionated by density gradient centrifugation on a lymphoprep at 400 g for 30 min [7]. The sheep erythrocyte rosette-positive fraction (T cells) contained > 90% CD3+ cells and < 5% CD20+ cells. The sheep erythrocyte rosette-negative fraction (B cells) contained > 90% CD20+ cells and < 5% CD3+ cells by flow cytometric analysis using fluorescent antibodies.
Production and assay of interferon-gamma
PBL (106/ml) were cultured with 10 μg/ml Con A in the presence or absence of AGE in RPMI–10% FCS medium in culture dishes (Falcon no. 3002) at 37°C for 24 h and the culture supernatants were harvested. The amount of interferon-gamma (IFN-γ) in the culture supernatants was assayed by ELISA. The wells of flat-bottomed microtitre plates (Sumitomo Bakelite Co., Tokyo, Japan) were coated with anti-IFN-γ capture antibody (Pharmingen, Becton Dickinson Co., San Diego, CA), and the culture supernatant from PBL was added to the plate. After washing, biotin-labelled detection antibody, streptoavidin-conjugated alkaline phosphatase (Pharmingen) and p-nitrophenyl phosphate (Zymed Labs Inc., San Francisco, CA) as a substrate were added. The optical density (OD) at 490 nm was measured using Immunoreader (Japan Intermed Co., Tokyo, Japan). The results were expressed as the pg of IFN-γ produced by 106 PBL and s.e.m. in triplicate cultures compared with recombinant human IFN-γ (Pharmingen) as the standard.
Production and assay of immunoglobulin
PBL (5 × 105/ml) were cultured with 0.01% SAC in the presence or absence of AGE for 5 days. The culture supernatants were harvested, and total IgG in the culture supernatants was assayed by ELISA by the same method as for the detection of IFN-γ [8] using sheep anti-human IgG antibody, peroxidase-conjugated sheep anti-human IgG antibody (The Binding Site, Birmingham, UK) and o-phenylenediamine dihydrochloride (5.5 × 10−4m; Wako Junyaku Co., Kyoto, Japan) in 0.01% H2O2 solution as a substrate. Purified human IgG was used as the standard. The results are expressed as the mean μg of IgG produced by 5 × 105 PBL with s.e.m. in triplicate cultures.
Cell cycle analysis
PBL (106) were cultured with 10 μg/ml Con A in the presence or absence of AGE in RPMI–10% FCS medium in culture dishes (Falcon no. 3002) at 37°C for 3 days. Cells were harvested and washed with PBS pH 7.4, then fixed with 99.5% ethanol at 4°C for 2 h. Cells were treated with 0.25 mg/ml of RNase A (Sigma Chemical Co., St Louis, MO) at 37°C for 1 h. After washing, cells were stained with 500 μg/ml propidium iodide (PI; Sigma) at room temperature for 10 min. Analysis was performed on a flow cytometer (Coulter Co., Hialeah, FL) [9]. The percentage of cells in each stage of the cell cycle was determined using a Cellfit analysis program on the staining profile of viable cells.
Induction and assay system of cytotoxic T lymphocytes
To induce CTL, PBL (2.5 × 106) were cultured with a mitomycin C (50 μg/ml; Kyowahakko Kogyo Co., Tokyo, Japan)-treated adult T cell leukaemia cell line, MT-2 (105), in 24-well culture plates (Falcon no. 3047) in 2 ml of RPMI–10% FCS medium at 37°C for 5 days in 5% CO2and 95% air [10]. The cytotoxic T lymphocyte (CTL) activity was detected by culturing in vitro sensitized PBL (105) and 51Cr-sodium chromate (1.2 Ci/mg; New England Nuclear, Boston, MA)-labelled MT-2 cells (104) in wells of round-bottomed microtitre culture plates (Falcon no. 3077). After effector-to-target cell interaction at 37°C for 5 h, the supernatant in each well was harvested and the radioactivity in the supernatant was counted with a gamma counter (Aloka Co.) [11]. The percentage of specific lysis was calculated from the formula: % specific lysis = [(experimental release − control release)/(maximum release − control release)] × 100, where maximum release was obtained by incubating 51Cr-labelled MT-2 cells in the presence of 1% Nonidet P-40 (Sigma), and control release was obtained by incubating MT-2 cells with unsensitized PBL. The results were expressed as the means and s.e.m. of the specific lysis of target cells in triplicate cultures.
Assay system of natural killer cell activity
The assay system of natural killer (NK) cell activity was the same as the assay system of CTL activity, except for the use of PBL without 5 days culture as effector cells, and U937 cells as target cells.
Production and assay of tumour necrosis factor
PBL (106/ml) were cultured in RPMI–10% FCS medium in culture dishes (Falcon no. 3002) at 37°C for 2 h. Non-adherent cells were discarded by gentle agitation and dish-adherent cells were obtained by scraping with a rubber policeman. Adherent cells (5 × 105/ml) were stimulated with 10 μg/ml lipopolysaccharide (LPS) from Escherichia coli (Sigma) in the presence or absence of AGE at 37°C for 18 h and the culture supernatants were harvested. The activity of tumour necrosis factor (TNF) in the culture supernatants was assayed according to the method of Ruff & Gifford [12]. L929 cells (104) were cultured in RPMI–10% FCS medium in wells of flat-bottomed microtitre culture plates (Falcon no. 3072) for 20 h. The culture medium was then replaced with TNF samples and the cells were further cultured for 20 h in the presence of 1 μg/ml actinomycin D (Sigma). After culture, the cells were stained with 0.2% crystal violet (Sigma) in 20% methanol, were solubilized by 1% SDS (Sigma) and the OD at 490 nm was measured by Immunoreader. The results were expressed as the units of TNF produced by 5 × 105 monocytes and s.e.m. in triplicate cultures, compared with recombinant human TNF-α (Pharmingen) as the standard.
Fractionation of AGE by gel filtration
Crude AGE was applied to a Sephacryl S-200 column (1.9 × 45 cm; Pharmacia Fine Chemicals, Uppsala, Sweden) equilibrated and eluted with PBS. Aliquots of 2 ml were collected. The sugar and protein content in each fraction was measured according to the phenol-sulphuric acid method [13] and OD at 280 nm, respectively. The activity of each fraction was assayed by the inhibition of PBL proliferation. The active fractions were combined and further purified in a Sephadex G-100 column (Pharmacia) and the resulting active fractions were combined and used as the purified component.
SDS–PAGE and elution of samples from SDS–PAGE
Crude and fractionated samples of AGE were separated with SDS–PAGE [14] using 10% polyacrylamide (Funakoshi Yakuhin Co.). The gel was stained with coomassie brilliant blue (CBB; Sigma).
The protein was eluted from the gel slices using an Electroeluter (BioRad Labs, Hercules, CA) at 10 mA/glass tube for 6 h. The eluted protein was recovered and dialysed against PBS.
Treatment with pronase E and NaIO4
Crude and fraction samples of AGE were incubated with 0.4 mg of pronase E (Serva Feinbiochemica GmbH & Co., Heidelberg, Germany) at 30°C in 4 ml of 0.1 m Tris–HCl buffer (pH 8.0) containing 50 mm CaCl2. After 36 h incubation, 0.2 mg of pronase E was supplemented and the incubation was continued for another 36 h. The reaction mixture was then heated at 100°C for 10 min to inactivate the pronase E and dialysed against PBS.
For NaIO4 treatment, crude and fraction samples of AGE were incubated in 100 μl of 0.1 m NaIO4(Sigma) at 25°C for 4 h. Then 250 μl of 20% ethylene glycol (Sigma) were added [15] and the samples were dialysed against PBS.
Statistical analysis
All experiments were repeated at least three times and some representative results are shown in the tables and figures. Statistical analysis was performed by Student's t-test. A confidence level of < 0.05 was considered significant [16].
RESULTS
Inhibitory effect of AGE on PBL proliferative responses
At first we studied the effect of AGE on the proliferative responses of PBL. As shown in Table 1, AGE markedly inhibited the proliferative responses of PBL stimulated with Con A and SAC. Figure 1 shows the dose–response curve of the inhibitory effect of AGE on Con A and SAC responses. AGE dose-dependently inhibited both Con A (Fig. 1a) and SAC (Fig. 1b) responses. The responses of sheep erythrocyte rosette-positive cells (T cells) and sheep erythrocyte rosette-negative cells (B cells) were similarly inhibited by AGE (Fig. 2), suggesting that AGE directly affects both T and B cells.
Table 1.
Effect of Acanthopanax gracilistylus extract (AGE) on the proliferative response of peripheral blood lymphocytes† (PBL)
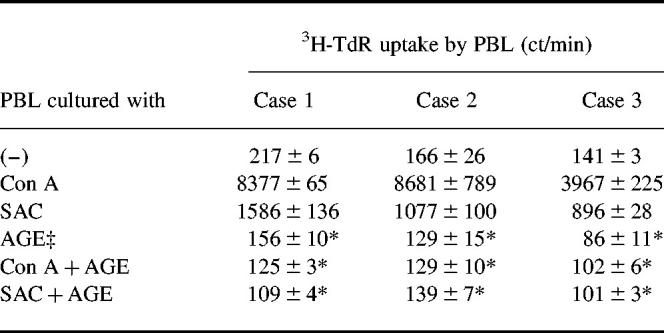
†PBL (2 × 105) were cultured with 10 μg/ml concanavalin A (Con A), 0.01% Staphylococcus aureus Cowan I (SAC) or 10 μg/ml AGE for 3 days, labelled with 3H-TdR for the last 15 h and harvested. Then the 3H-TdR uptake by PBL was counted. Results are expressed as mean ct/min of 3H-TdR uptake with s.e.m. in triplicate cultures.
‡AGE (1 g dried weight) was extracted in 100 ml of boiling water for 1 h. The extract contained 1780 μg/ml protein determined by the Lowry method.
*Significantly inhibited.
Fig. 1.
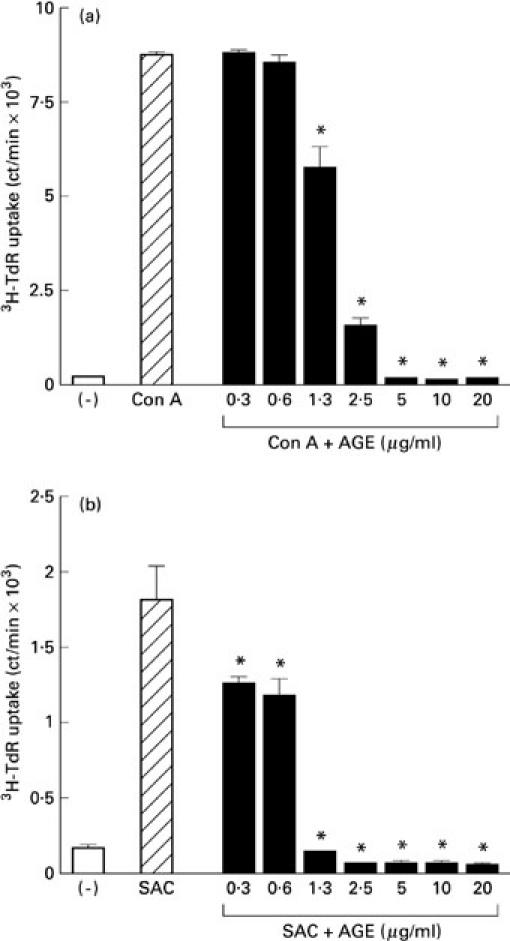
Dose–response curve of Acanthopanax gracilistylus extract (AGE). Peripheral blood lymphocytes (PBL; 2 × 105/well) were cultured with concanavalin A (Con A) (a) or Staphylococcus aureus Cowan I (SAC) (b) in the presence of several concentrations of AGE at 37°C for 3 days. Results are expressed as mean ct/min of 3H-TdR uptake with s.e.m. in triplicate cultures.*Significantly inhibited.
Fig. 2.
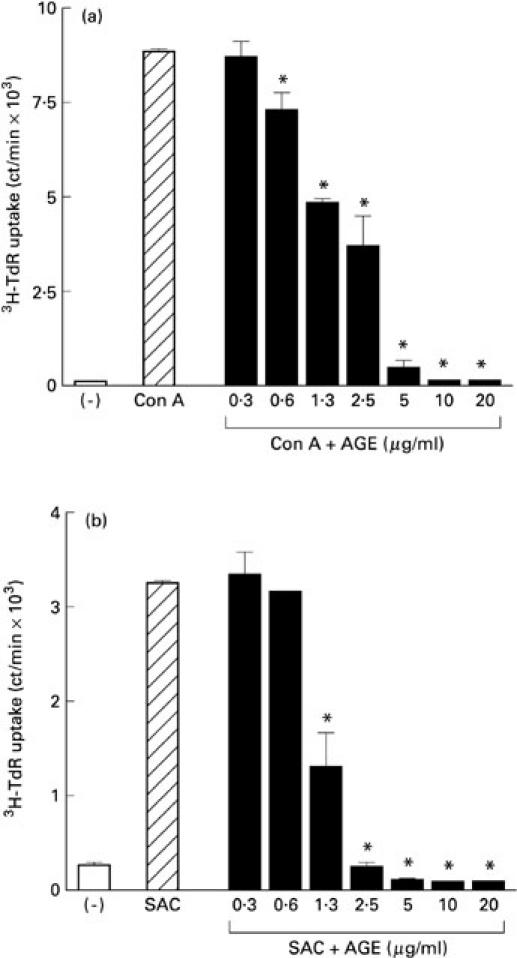
Inhibitory effect of Acanthopanax gracilistylus extract (AGE) on B cell- and T cell-enriched fractions. Peripheral blood lymphocytes (PBL) were fractionated with sheep erythrocytes. Sheep erythrocyte rosette-positive cells (T cells) and sheep erythrocyte rosette-negative cells (B cells) were cultured with concanavalin A (Con A) (a) or Staphylococcus aureus Cowan I (SAC) (b) in the presence of AGE at 37°C for 3 days. Results are expressed as mean ct/min of 3H-TdR uptake with s.e.m. in triplicate cultures. *Significantly inhibited.
AGE inhibited IFN-γ and IgG production by PBL
We next studied the effect of AGE on IFN-γ and IgG production by PBL as a representative function of T cells and B cells, respectively. AGE suppressed both IFN-γ production in Con A-stimulated PBL (Fig. 3a) and IgG production in SAC-stimulated PBL (Fig. 3b) in a dose-dependent manner.
Fig. 3.

Inhibitory effect of Acanthopanax gracilistylus extract (AGE) on IFN-γ and IgG production by peripheral blood lymphocytes (PBL). PBL (1 × 106) were cultured with concanavalin A (Con A) in the presence of several concentrations of AGE for 24 h. The amount of IFN-γ in the culture supernatants was assayed by ELISA. Results are expressed as mean pg of IFN-γ produced by 106 PBL with s.e.m. in triplicate cultures (a). PBL (5 × 105) were cultured with Staphylococcus aureus Cowan I (SAC) in the presence of several concentrations of AGE for 5 days. The amount of IgG in the culture supernatants was examined by ELISA. Results are expressed as mean μg of IgG produced by 5 × 105 PBL with s.e.m. in triplicate cultures (b). *Significantly inhibited.
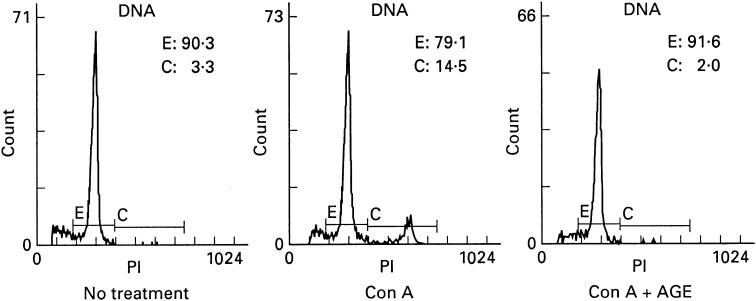
Effect of AGE on the cell cycle
To study the mechanism of AGE-induced suppression of PBL responses, we examined the cell cycle by flow cytometry. PBL were stimulated with Con A in the presence or absence of AGE for 72 h, washed, stained with PI and the cell cycle was analysed. In control cells without stimulation, 3% of cells were in the S or G2 phase and 90% of cells were in the G0/G1 phase of the cell cycle (Fig. 4). Con A stimulation induced 15% of cells to move to the S or G2 phase and the cells in the G0/G1 phase decreased to 80%. After AGE treatment, the cell cycle remained at the level of the control cells and necrotic and apoptotic cells did not increase. Furthermore, the viability of AGE-treated PBL was not different from that of non-treated PBL (data not shown). These results suggest that AGE inhibits cell cycle progression, but does not induce cell death.
Fig. 4.
Effect of Acanthopanax gracilistylus extract (AGE) on the cell cycle. Peripheral blood lymphocytes (PBL; 2 × 106) were stimulated with concanavalin A (Con A) in the presence or absence of AGE for 3 days, stained with propidium iodide (PI) and analysed using a flow cytometer. The results are expressed as the profile of PI+ cells and the percentage of each cell cycle. E, G0/G1; C, S/G2.
AGE inhibited CTL response, but not NK cell activity
Alloantigen-specific CTL was induced by culturing PBL with MT-2 cells for 5 days and the activity was assayed with 51Cr-labelled MT-2 cells. AGE suppressed the induction of CTL in a dose-dependent manner (Fig. 5a).
Fig. 5.
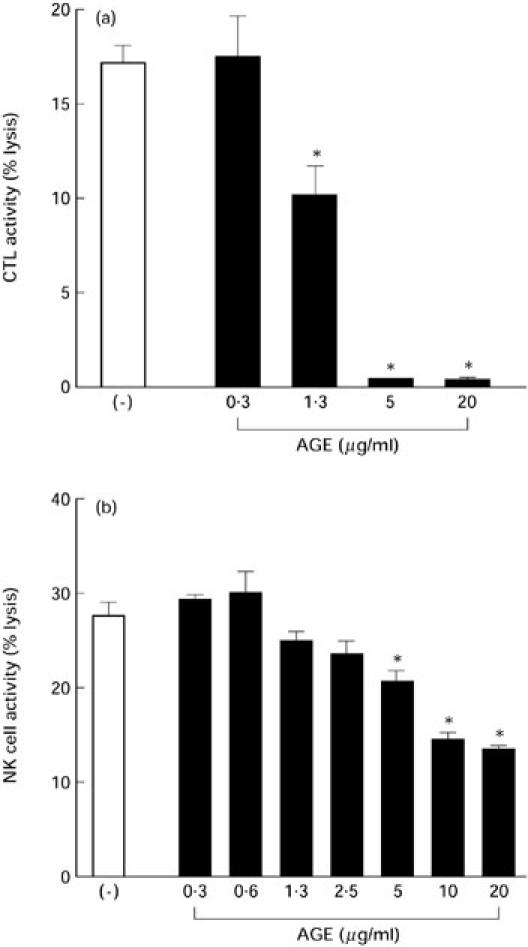
Effect of Acanthopanax gracilistylus extract (AGE) on cytotoxic T lymphocytes (CTL) and natural killer (NK) cell activity. Peripheral blood lymphocytes (PBL; 2.5 × 106) were cultured with mitomycin C-treated MT-2 cells (105) in the presence of several concentrations of AGE at 37°C for 5 days to induce CTL. Cytotoxic activity was detected by 51Cr-labelled MT-2 cells as the target cells. The results are expressed as mean and s.e.m. of the percentage of specific lysis of the target cells in triplicate cultures (a). PBL (5 × 105) were precultured with various concentrations of AGE for 24 h, then cultured with 51Cr-labelled U937 cells as the target for 5 h. The results are expressed as the mean percentage of specific lysis of U937 cells and s.e.m. in triplicate cultures (b). *Significantly suppressed.
The effect of AGE on NK cell activity was determined by incubating PBL with AGE for 24 h, and the activity was assayed using 51Cr-labelled U937 cells as target. NK cell activity was less sensitive to AGE (Fig. 5b).
AGE enhanced cytokine production by monocytes
We next examined the effect of AGE on monocyte function by studying cytokine production. As shown in Fig. 6, AGE markedly stimulated monocytes to produce TNF in a dose-dependent manner. AGE also enhanced LPS-induced TNF production. Other cytokine production by monocytes such as IL-1α and IL-6 was also induced by AGE (data not shown).
Fig. 6.
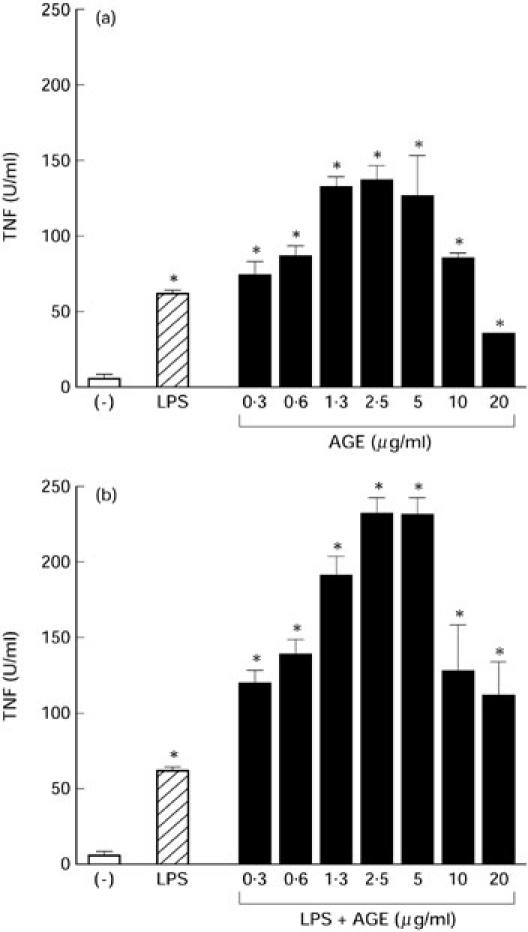
Effect of Acanthopanax gracilistylus extract (AGE) on tumour necrosis factor (TNF) production by monocytes. Peripheral blood lymphocytes (PBL; 1 × 106) were cultured at 37°C for 2 h. Non-adherent cells were discarded. Adherent cells (5 × 105) were cultured with 10 μg/ml lipopolysaccharide (LPS) in the presence or absence of AGE for 24 h. TNF activity in the culture supernatants was assayed using L929 cells. The results are expressed as units of TNF produced by 5 × 105 monocytes and s.e.m. in triplicate cultures. *Significantly enhanced.
Active components of AGE are proteins
We studied the chemical nature of the active component of AGE. At first, AGE was chromatographed on a Sephacryl S-200 column. The activity of each fraction was assayed by the inhibition of PBL proliferative responses (Fig. 7a). The active fractions were combined, lyophilized, dialysed and rechromatographed on a Sephadex G-100 column (Fig. 7b). As illustrated in Fig. 7, the active component was eluted as a single peak whose molecular weight was about 50 kD.
Fig. 7.

Fractionation of active components of Acanthopanax gracilistylus extract (AGE). AGE was chromatographed on a Sephacryl S-200 column (a). The active fractions were combined and rechromatographed on a Sephadex G-100 column (b). The protein concentration was detected by optical density (OD) at 280 nm (□). The glucose concentration was measured using the phenol-sulphuric acid method (Δ). The activity of each fraction was determined by 3H-TdR uptake (○) of peripheral blood lymphocytes (PBL). The column size was determined by eluting marker proteins: void (blue dextran), bovine serum albumin (68 kD) and Cytochrome C (12 kD).
Next, we treated crude and fraction samples of AGE with pronase E and NaIO4. As shown in Fig. 8, treatment with pronase E significantly abrogated its activity, but treatment with NaIO4 did not, suggesting that the active components of AGE are proteins but not sugars.
Fig. 8.
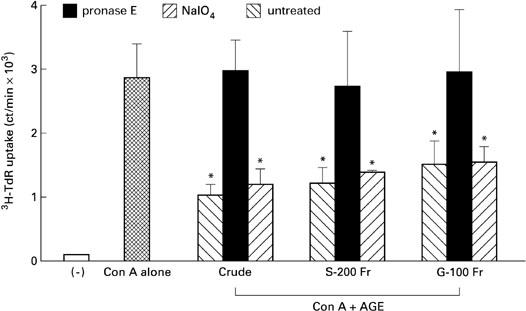
Effect of pronase E or NaIO4 treatment on Acanthopanax gracilistylus extract (AGE). Crude, S-200 and G-100 fractions of AGE were treated with pronase E, NaIO4 or untreated and their activity was assayed by 3H-TdR uptake of peripheral blood lymphocytes (PBL) stimulated with concanavalin A (Con A). *Significantly suppressed.
We also purified the active component of AGE by SDS–PAGE. Crude AGE showed two major bands of approx. 50–70 kD mol. wt. The column-purified fraction showed one band at approx. 60 kD mol. wt. The eluted fraction of this band (F2) showed inhibitory activity on the proliferative response of PBL (Fig. 9).
Fig. 9.
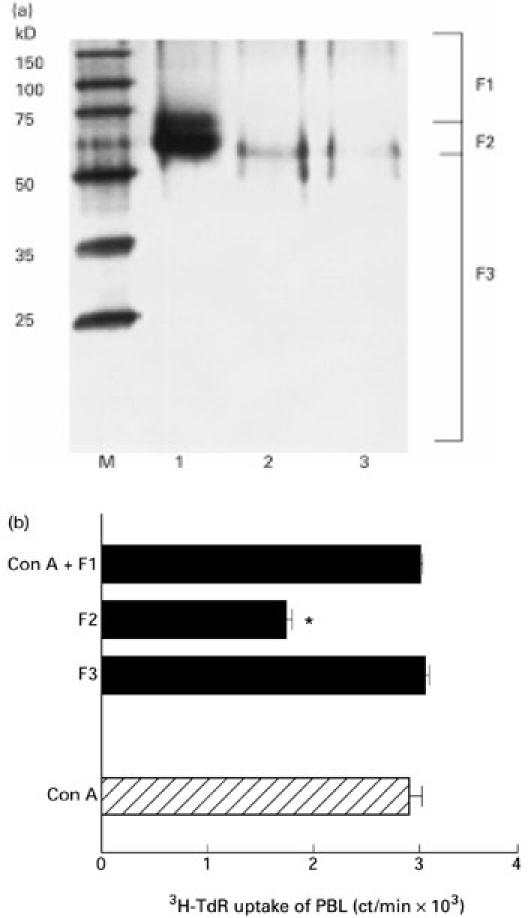
Purification of Acanthopanax gracilistylus extract (AGE) with SDS–PAGE. Crude, S-200 and G-100 fractions of AGE were separated with SDS–PAGE and stained with coomassie brilliant blue (CBB) (a). Lane 1, crude AGE; lane 2, S-200 Fr; lane 3, G-100 Fr; M, molecular weight marker. SDS–PAGE was cut out into three fractions and the proteins in them were eluted. The activity of the elutions was assayed by the 3H-TdR uptake of peripheral blood lymphocytes (b). *Significantly suppressed.
DISCUSSION
CMH have been traditionally used to prevent and treat numerous types of diseases in China, especially autoimmune diseases and cancers [1]. AG has been used to treat patients with rheumatism and is reported to have an anti-inflammatory effects [5]. However, its mechanism of action and chemical nature have not been fully analysed. In this study, we examined the effect of AGE on human lymphocytes in vitro.
AGE markedly inhibited the proliferative responses of PBL stimulated with mitogens, such as Con A and SAC, in a concentration-dependent manner. AGE not only inhibited the proliferative responses of T cells and B cells, but also their functions of producing IFN-γ and immunoglobulin. The suppressed B cell responses with AGE were not due to the suppressed T cell responses, because the proliferative responses and immunoglobulin production by purified B cells and cell lines maintained in vitro were similarly suppressed by AGE as well as PBL (data not shown). The mechanism of AGE-induced immunosuppression is to arrest the cell cycle at the G0/G1 stage without a direct cytotoxic effect on lymphocytes. AGE also inhibited the induction of alloantigen-specific CTL, but NK cell activity was less sensitive to AGE. In contrast, AGE stimulated monocytes to produce cytokines such as TNF, IL-1 and IL-6. The enhancing effect of cytokine production with AGE was not due to a cytotoxic effect on monocytes, which can release intracellular cytokines, because there was no significant difference between the viability of AGE-treated and untreated monocytes. The suppressed T cell responses with AGE were not due to non-specific monocyte activation, which can produce a large amount of non-specific suppressive factors [17], because the proliferative responses and IFN-γ production by purified T cells and cell lines maintained in vitro were also suppressed by AGE as well as PBL (data not shown). Thus, AGE is not a non-specific immunosuppressant, but its activity has cellular selectivity. The mechanisms of the different susceptibilities to AGE between T cells, B cells and monocytes are under investigation.
Several proteins or polysaccharides originating from higher plants have immunopotentiating and anti-tumour activities as biological response modifiers [18–21]. Acanthopanax senticosus, as well as its polysaccharide fraction, enhanced the phagocytic activity of macrophages and inhibited the growth of transplanted tumours in mice [2,22,23]. We also studied the effect of AGE on the growth of tumour cells in vitro. AGE inhibited the growth of tumour cells in a dose-dependent manner. Furthermore, we found that monocytes treated with AGE had a growth-inhibitory effect on tumour cells. We think that monocytes/macrophages are activated by AGE and work suppressively on tumour cell growth (data not shown).
Next, we studied the chemical nature of AGE. We purified AGE by gel filtration chromatography. The active component of AGE was eluted as a single peak by sequential filtration using Sephacryl S-200 and Sephadex G-100 columns. All activities to suppress T and B cell functions, and to enhance monocyte functions, resided in this fraction. The activity is sensitive to treatment with pronase E, but not to NaIO4, suggesting that the active component of AGE is a protein, but not a sugar. We further purified AGE using SDS–PAGE. The active component of AGE was fractionated around 60 kD by SDS–PAGE.
We did not extensively examine the molecular mechanism of AGE-induced immunosuppression in this study.
The next step to be elucidated in this series of studies is to clarify the nature of the binding molecules for AGE on the cells and intracellular events.
As a preliminary experiment, we observed that AGE had a regulatory effect on retinoblastoma (Rb) protein and cyclin-dependent kinase (Cdk), which regulate the cell cycle (data not shown).
Although the study was performed only with an in vitro assay system, evidence that AGE has a selective immunomodulating activity on human lymphocytes provides a rational basis for the efficacy of this medicinal herb. For example, AGE may be applied to the treatment of autoimmune and allergic diseases as an immunosuppressive drug and to the treatment of cancers as a cell cycle-regulating drug. Further purification of the active components and examination of their effects in vivo seem to be required for clinical applications.
REFERENCES
- 1.Li XY. Immunomodulating Chinese herbal medicines. Mem Inst Cruz. 1991;86:159–64. doi: 10.1590/s0074-02761991000600036. [DOI] [PubMed] [Google Scholar]
- 2.Shen ML, Zhai SK, Chen HL, Luo YD, Tu GR, Ou DW. Immunopharmacological effect of polysaccharides from Acanthopanax senticosus on experimental animals. Int J Immunopharm. 1992;13:549–54. doi: 10.1016/0192-0561(91)90075-i. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida Y, -Wang MQ, Liu JN, Shan BE, Yamashita U. Immunomodulating activity of Chinese medicinal herbs and Oldenlandia diffusa in particular. Int J Immunopharm. 1997;19:359–70. doi: 10.1016/s0192-0561(97)00076-3. [DOI] [PubMed] [Google Scholar]
- 4.Shan BE, Yoshida Y, Sugiura T, Yamashita U. Stimulating activity of Chinese medicinal herbs on human lymphocytes in vitro. Int J Immunopharm. 1999;21:149–59. doi: 10.1016/s0192-0561(98)00074-5. [DOI] [PubMed] [Google Scholar]
- 5.Tang X, Ma Y, Li P. Separation and identification of the anti-inflammatory diterpene from the root cortices of Acanthopanax gracilistylus. Chung Kuo Chung Yao Tsa Chih. 1995;20:231–53. [PubMed] [Google Scholar]
- 6.Harrison MR, Thurmond G, Thomas GM. A simple and versatile harvesting device for processing radioactive label incorporated into and/or released from cells in microculture. J Immunol Methods. 1974;4:11–20. doi: 10.1016/0022-1759(74)90027-1. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka Y, Shirakawa F, Ota T, Suzuki H, Eto S, Yamashita U. Mechanism of spontaneous activation of B cells in patients with systemic lupus erythematosus. Analysis with anti-class II antibody. J Immunol. 1988;140:761–7. [PubMed] [Google Scholar]
- 8.Segawa K, Ono K, Oka S, Jyo T, Kuroiwa A, Yamashita U. B cell mitogenic activity of sea squirt antigen. Inter Arch Aller Immunol. 1994;104:270–6. doi: 10.1159/000236676. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan MH, Daniel C, Schindler U, Grusby MJ. Stat proteins control lymphocyte proliferation by regulating p27kip1 expression. Mol Cell Biol. 1998;18:1996–2003. doi: 10.1128/mcb.18.4.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamashita U, Tanaka Y. Suppressive activity of interleukin 4 on the induction of antigen-specific cytotoxic T cells in humans. Jpn J Can Res. 1991;82:585–92. doi: 10.1111/j.1349-7006.1991.tb01890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerottini JC, Engers HP, MacDonald HR, Brunner KT. Generation of cytotoxic T lymphocytes in vitro. I. Response of normal and immune mouse spleen cells in mixed leukocyte cultures. J Exp Med. 1974;140:703–25. doi: 10.1084/jem.140.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruff RM, Gifford EG. Purification and physicochemical characterization of rabbit tumor necrosis factor. J Immunol. 1980;12:1671–7. [PubMed] [Google Scholar]
- 13.Dubis M, Gillis KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1962;28:350–6. [Google Scholar]
- 14.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Oka S, Shigeta S, Ono K, Jyo T. An epitope residing in carbohydrate chains of sea squirt antigen termed Gi-rep. J Allergy Clin Immunol. 1987;80:57–63. doi: 10.1016/s0091-6749(87)80191-4. [DOI] [PubMed] [Google Scholar]
- 16.Zar JH. Englewood Cliffs, NJ: Prentice Hall Inc.; 1974. Biostatistical analysis. [Google Scholar]
- 17.Yamashita U, Jiang HJ, Inoue H, Mutoh Y, Furukawa T. Immune functions of Toxocara canis-infected mice. Jpn J Parasitol. 1993;42:211–9. [Google Scholar]
- 18.Chu DT, Wong WL, Mavligit GM. Immunotherapy with Chinese medicinal herbs. Immune restoration of local xenogeneic graft-versus-host reaction in cancer patients by fractionated Astragalus membranaceus in vitro. J Clin Lab Immunol. 1993;25:119–23. [PubMed] [Google Scholar]
- 19.Franz G. Polysaccharides in pharmacy: current applications and future concepts. Plant Med. 1989;55:493–7. doi: 10.1055/s-2006-962078. [DOI] [PubMed] [Google Scholar]
- 20.Lan ZF, Cheng GQ, Wang FL, Xi SF. Effects of Rodix hedysari polysaccharide on immunological function and transplanted tumor in mice. Chin Pharmacol Acta. 1987;8:257–77. [PubMed] [Google Scholar]
- 21.Shimura K, Ito H. Screening of host-mediated antitumor polysaccharides by crossed immunoelectrophoresis using fresh human serum. Jpn J Pharmacol. 1983;33:403–8. doi: 10.1254/jjp.33.403. [DOI] [PubMed] [Google Scholar]
- 22.Fang JN, Proksch A, Wagner H. Immunologically active polysaccharides of Acanthopanax senticosus. Phytochemistry. 1985;24:2619–22. [Google Scholar]
- 23.Xie SS. Immunoregulatory effect of polysaccharide of Acanthopanax senticosus (PAS). Immunological mechanism of PAS against cancer. Chung Hua Chung Liu Tsa Chih. 1989;11:338–40. [PubMed] [Google Scholar]