Abstract
Keratinocyte cytotoxicity is an important component of the immunopathology of photosensitive lupus erythematosus, and antibody-dependent cell-mediated cytotoxicity (ADCC) has been shown to be an important mechanism by which autoantibodies, especially those specific for SS-A/Ro, can induce keratinocyte damage in models of photosensitive lupus. We provide further evidence that keratinocytes from patients with photosensitive lupus show significantly greater ultraviolet radiation (UVR)-induced cytotoxicity, and that ADCC of these targets is especially enhanced by autologous patient's serum or by anti-SS-A/Ro+ sera. Keratinocytes from normal uninvolved skin of 29 patients with cutaneous lupus erythematosus (LE) were grown in cell culture and tested as targets in cytotoxicity experiments in vitro. Cultured keratinocytes from patients with systemic lupus erythematosus (SLE) and subacute cutaneous lupus erythematosus (SCLE) showed significantly greater cytotoxicity following UVR treatment than did keratinocytes from normal adult controls or from neonatal foreskins (P < 0.01). The same cultures also showed greater UVR-induced binding of IgG from fractionated anti-SS-A/Ro+ preparations. ADCC experiments were also performed using keratinocytes cultured from patients with SLE, SCLE, discoid lupus erythematosus (DLE), and normal controls. When keratinocytes were incubated in autologous serum plus a standard mononuclear cell effector population, the percentage of ADCC observed was significantly greater in cultures containing keratinocytes and sera from the SLE and SCLE patients (P < 0.001). When cultured keratinocytes were added to different IgG antibody probes, plus standard mononuclear effector populations, greater ADCC was seen using the anti-SS-A/Ro probe and keratinocytes from patients with SLE or SCLE. With normal human neonatal keratinocyte targets, the anti-SS-A/Ro probe induced greater ADCC than that seen with anti-ssDNA or normal human serum. We have shown that keratinocytes from patients with some forms of lupus erythematosus (SLE and SCLE) show greater cytotoxicity in vitro when irradiated with UVR, and greater susceptibility to ADCC whether the antibody source is their own serum or an anti-SS-A/Ro probe.
Keywords: lupus erythematosus, skin, photosensitivity, ultraviolet light, SS-A/Ro
INTRODUCTION
Photosensitivity is one of the major symptoms of systemic lupus erythematosus (SLE), neonatal LE (NLE) and subacute cutaneous LE (SCLE). An exacerbation or aggravation of LE-characteristic skin lesions may be induced by ultraviolet light (UVL) exposure in photosensitive patients. Although the major action spectrum for LE has been recognized as the UVB range (wavelength 280–320 nm) [1, 2], modern phototesting protocols have shown that both UVB and UVA, or combinations of both, induce lupus skin lesions in different patients [3]. However, photosensitivity in lupus patients can be variable, even in similar patient populations, ranging from 0% to 91% of discoid LE (DLE), 27% to 90% of SCLE and 6% to 61% of SLE patients based on the patients' history and/or light sensitivity test reactions [3, 4].
The mechanisms underlying photosensitive cutaneous LE have been investigated from several aspects including direct autoantibody effects, UVR sensitivity, immunoglobulin deposits at the dermoepidermal junction, the effects of anti-UV-DNA antibodies and cellular immunity [5]. Anti-SS-A/Ro and anti-SS-B/La antibodies in the sera are strongly associated with cutaneous LE, especially SCLE and NLE [6, 7]. Therefore, studies on the pathogenesis of photosensitive cutaneous LE have focused on the close association of antibodies against the SS-A/Ro antigen with the development of clinical symptoms [7, 8]. The epidermal keratinocyte, especially the basal cell, is the major target of immunological damage in photosensitive lupus. Interestingly, these antibodies have been reported to bind to UVB-irradiated human keratinocytes in vitro and in vivo [9–13]. The binding of these antibodies on the keratinocytes is dependent on UVB dose and glycosylation, but independent of the cell cycle [10]. In addition, we have previously shown that the binding of anti-SS-A/Ro antibodies to the surface of epidermal cells is an important inducer of antibody-dependent keratinocyte damage in photosensitive cutaneous LE [14]. These experiments are mostly performed by using cultured keratinocytes from normal human foreskins. Therefore, it is of special interest whether keratinocytes cultured from cutaneous LE patients are UVB-induced photosensitive, and whether anti-SS-A/Ro antibody-dependent keratinocyte damage was induced by UVB irradiation. In order to address this issue, we performed extensive in vitro cytotoxicity and antibody-dependent cell-mediated cytotoxicity (ADCC) assays using keratinocytes cultured from patients with cutaneous LE.
PATIENTS AND METHODS
Culture of keratinocytes
Suction-blister epidermal roofs were obtained from 29 cutaneous LE patients and from 13 age- and gender-matched normal healthy controls. All subjects were Japanese. The epidermal roofs from the cutaneous LE patients were taken from non-lesional inner forearm and other lesional sites. The specimens from the normal controls were taken from the inner forearm. Keratinocytes from the roofs were cultured in serum-free keratinocyte growth medium (Clonetics, San Diego, CA) as described in our previous report [15]. Cutaneous LE patients included SLE, SCLE and DLE patients. Primary cultures were expanded in the first passage and the second passage keratinocytes were then cultured on Lab-Tek chamber slides (Miles, Naperville, IL), or culture dishes. A part of the first-passage keratinocytes were trypsinized into suspension and frozen in aliquots for ADCC tests [16]. Cultured human neonatal foreskin keratinocytes were purchased from Clonetics. These cells were also cultured in the same manner.
Serum probes
Serum probes were obtained from patients with collagen diseases at Hamamatsu University Hospital and Kyoto University Hospital. The specificity was determined by immunofluorescence staining, double immunodiffusion and immunoblotting [11, 17].
Pooled specific anti-SS-A/Ro sera were collected from four patients with cutaneous LE, and recognized mainly a 60-kD protein which was confirmed with the ELISA method (Medical and Biological Laboratory, Nagoya, Japan) [18]. The serum samples for specific anti-SS-A/Ro probes were finally fractionated by protein A Sepharose column chromatography (Pharmacia Fine Chemicals, Uppsala, Sweden) [19], and were absorbed with calf thymus DNA (Sigma, St Louis, MO) as previously described [20] in order to exclude the possibility that the anti-DNA antibodies had an effect on the cell surface binding assay and ADCC assay [21]. Native bovine Ro (Immunovision, Springdale, AK) was used for the absorption test according to the method of Zhang & Reichlin [22]. Pooled specific anti-single-stranded (ss)DNA sera were collected from five patients with SLE or localized scleroderma. Pooled normal human sera (NHS) were also processed in the same manner. These patients or the normal subjects did not include the keratinocyte donors.
Autologous sera were also obtained from these patients and normal controls. The antibodies from these sera were screened for antibody specificity in the routine manner and fractionated by protein A Sepharose for assays. The sera were kept at −70°C until use.
UV irradiation
Before irradiation, the culture medium in the Lab-Tek chamber slides (Miles) or culture dishes was replaced with warmed PBS (30°C) free of any photoactive compounds. At least one dish of cells was not exposed to UVR, but was handled in the same fashion as the irradiated cells. The remaining cells were exposed to 50 or 100 mJ/cm2 of UVB. The light source and other details were the same as previously described [23, 24]. The cells were incubated in a humidified incubator for 24 h at 37°C in a 5% CO2 atmosphere, and cell viability was then determined. Each test was performed in duplicate. The surface binding assay and cytotoxicity assays were then performed after changes of the medium.
Surface binding assay
A fluorescence-activated cell sorter (FACS) technique was developed that allowed identification of cell surface antibody binding but limited subsequent internalization [11, 17]. The second-passage cells were cultured in serum-free keratinocyte growth medium (Clonetics) on 60-mm plastic dishes for 5–6 days. When the cells had grown to 70–80% confluence, they were treated with UVB light. The cells were harvested 24 h later and stained for FACS as previously described [10]. Briefly, after washing twice with PBS, cell suspensions were incubated with fractionated serum probes at 4°C for 1 h. The dilution of the fractionated serum probes was 1:100 of the original sera. After washing twice with PBS, cells were post-fixed in cold 2% paraformaldehyde solution at 4°C for 30 s. As a next step, cells were incubated with diluted FITC-conjugated rabbit F(ab)′2 anti-human IgG (Dako, Santa Barbara, CA) (1:100 in dilution) at 4°C for 4 h. After the final washing with PBS, these cells were analysed. In each experiment, negative controls included non-irradiated cells incubated with the same fractionated serum probes plus the second FITC-conjugated antibody and also irradiated cells incubated with fractionated NHS probes and the second FITC-conjugated antibody. Each test was performed in duplicate.
Confocal microscopic assessment of the localization was also analysed by a BioRad MRC600 (Cambridge, MA) equipped with an argon ion laser exciting maximally at 488 nm and 514 nm, and was controlled by CoMoS software [25, 26]. Cell surface binding of IgG was demonstrated by confocal microscopy [26], confirming many previous reports [5,9–13].
ADCC assay
This assay was carried out according to the method of Norris et al. [27], with modifications [14]. Briefly, sera from patients or normal controls served as antibody sources, and were heat-inactivated for 1 h at 56°C. IgG-fractionated specific serum probes were used in most of the experiments. Two sets of fractionated serum dilution were prepared in each test. The first dilution was equal to the 1:20 of the original unfractionated serum. The second dilution of patients' sera was adjusted to the mean level of serum IgG levels from normal controls (1:20 in dilution), in which the final dilution of patients' sera varied from 25-fold to 62-fold.
As the ADCC effectors, peripheral mononuclear cells (MNC) from normal volunteers were separated by Ficoll–Hypaque (Pharmacia). After washing twice with PBS, the diluted test sera (50 ml) and the separated MNC (5 × 106) were added to each chamber of a culture plate containing keratinocytes irradiated with 100 mJ/cm2 of UVB light. The cells were then incubated in a humidified incubator for 16 h at 37°C in a 5% CO2 atmosphere. After 16 h, an acridine orange/ethidium bromide (AO/EB) cytotoxicity assay was used for the determination of keratinocyte damage [27]. Cells were counted using a fluorescence microscope in a ‘blind’ fashion by three researchers (F.F., I.T., H.W.) without prior knowledge of specimen identification. At least 2000 cells were counted in each experiment, and each test was performed in triplicate.
Each ADCC assay contained multiple controls to evaluate the effects of different combinations of target (T), antibodies (A) and mononuclear effectors (E) on cytotoxicity. Each assay included at least three combinations: (i) TAE, (ii) TE, and (iii) TA. The combination of TA produced cytotoxicity of < 1% with unirradiated keratinocytes. The following formulae were used: ADCC = TAE − TE. ADCC of irradiated targets = (TUVRAE − TUVRE) − (TAE − TE). TUVR are targets irradiated with UVB 24 h before the experiment was performed, and T are keratinocytes which were sham-irradiated.
Cell viability
The AO/EB cytotoxicity assay was used to assess cell viability [27].
Statistical analysis
Student's t-test and paired t-test for paired samples were used for statistical analysis, and P < 0.05 was considered significant.
RESULTS
Keratinocyte cultures from lupus erythematosus donors
Second passage keratinocytes from non-lesional sites were successfully cultured in 18 out of 31 patients, but no cells from the lesional sites could be cultured. These 18 patients included seven SLE (seven females, aged 28–41 years), four SCLE (three females and one male, aged 21–35 years) and seven DLE (four females and three males, aged 28–46 years) patients. In the normal controls, 11 (eight females and three males, aged 20–42 years) of the 13 donors produced successful serial cultures of keratinocytes.
Susceptibility to UVB-induced cytotoxicity in keratinocytes cultured from lupus erythematosus patients
The cytotoxicity of cultured keratinocytes was significantly higher in cells from LE patients and normal adult controls than from neonatal foreskins when the viability was determined 24 h after UVB (100 mJ/cm2) irradiation (Table 1); this was compatible with our previous report [14]. Among the cells cultured from patients and adult controls, cells from SLE patients were the highest in cytotoxicity (P < 0.001 versus cells from adult controls), and the cytotoxicity in cells from SCLE and DLE was significantly higher than that from adult controls (0.001 < P < 0.01). No correlation was present between the percentage cytotoxicity and the presence or absence of in vivo photosensitivity. Irradiation of 50 mJ/cm2 also resulted in similar findings.
Table 1.
Cultured keratinocytes from lupus patients show significant increases in cytotoxicity following UVR treatment
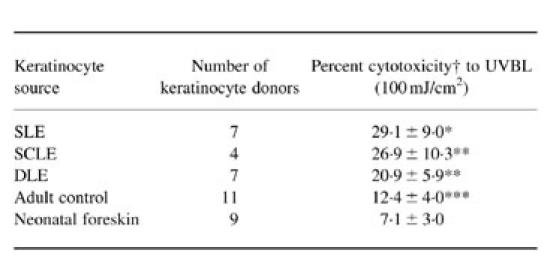
The irradiated cells were incubated in a humidified incubator for 24 h at 37°C in a 5% CO2 atmosphere, and cell viability was then determined by the acridine orange/ethidium bromide (AO/EB) test.†Each cell strain from each donor was tested at least three times, and the values were summed and expressed as mean ± 1 s.d.Percent cytotoxicity = TUVR − T, where TUVR is the cytotoxicity in irradiated keratinocytes and T is cytotoxicity in sham-irradiated keratinocytes.*P < 0.001 (versus cells from adult controls), P < 0.001 (versus cells from neonatal foreskin); **P < 0.001 (versus cells from neonatal foreskin), P < 0.01 (versus cells from adult controls); ***P < 0.01 (versus cells from neonatal foreskin).
UVB induces elevated binding of fractionated anti-SS-A/Ro serum probes to the surface of cultured keratinocytes from lupus erythematosus
The UVB-induced binding of IgG-fractionated antibodies from standard serum probes containing specific anti-SS-A/Ro antibodies to the surface of keratinocytes was significantly higher in LE patients than neonatal foreskin controls (Table 2). The results in cells from neonatal foreskins were compatible with those from our previous report [10]. In these experiments to measure accurately cell surface IgG binding, UVB-irradiated cells were washed with PBS before and after incubation with antibody probes. Under these conditions detached, non-viable cells were removed, and then cell surface antibody binding was measured on viable attached cells. Less than 2% of cells were dead, as determined just after the surface binding assay, in all of the experimental groups.
Table 2.
Keratinocytes from lupus patients show enhanced binding of IgG from anti-SS-A/Ro+ sera following UV radiation
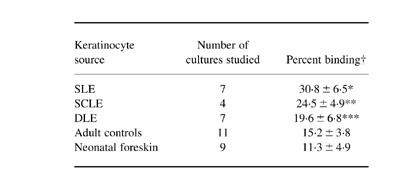
†Each cell strain from each donor was tested at least three times, and the values were summed and expressed as mean ± 1 s.d. of the percentage cells showing positive cell surface IgG binding by flow cytometry analysis. The keratinocytes were irradiated with 100 mJ/cm2 of UVB.*P < 0.001 (versus cells from adult controls, versus cells from neonatal foreskin); P < 0.01 (versus cells from discoid lupus erythematosus (DLE)); **P < 0.01 (versus cells from adult controls, versus cells from neonatal foreskin); ***P < 0.05 (versus cells from neonatal foreskin).
Cells from SLE patients showed the highest IgG binding (P < 0.001 versus adult controls; P < 0.001 versus neonatal foreskin; P < 0.01 versus DLE), and cells from SCLE showed similarly significant binding (P < 0.01 versus adult controls; P < 0.01 versus neonatal foreskin). Cells from DLE showed the lowest IgG binding among three types of LE, comparable to adult controls.
Confocal microscopy revealed that the IgG-fractionated anti-SS-A/Ro antibodies bound to the surface of the keratinocytes, which was compatible with our recent report [26].
UVB also enhances the susceptibility of keratinocytes from lupus patients to ADCC
Further experiments were performed to investigate whether UV-irradiated keratinocytes from lupus patients also showed enhanced ADCC when exposed to autoantibodies and mononuclear effector cells. As illustrated in Fig. 1, autologous mixtures of irradiated cultured keratinocytes and sera from patients with SLE or SCLE incubated with mononuclear effectors induced significantly greater (P < 0.001) ADCC than did autologous mixtures of irradiated keratinocytes and sera from normal controls (SLE 41.7 ± 8.5%, SCLE 38.4 ± 7.2%, control 16.2 ± 7.2).
Fig. 1.
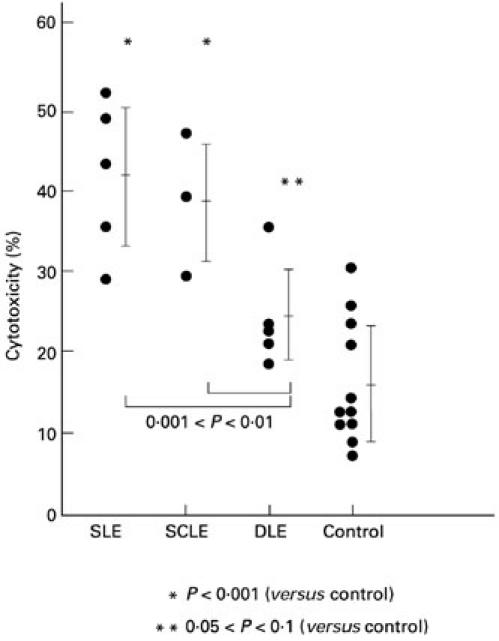
Autologous combinations of targets and serum from lupus patients but not normal controls enhance antibody-dependent cell-mediated cytotoxicity (ADCC) of irradiated keratinocytes. In this assay ADCC (cytotoxicity percentage) was measured using an acridine orange/ethidium bromide assay in 24 h cytotoxicity experiments. Each circle represents an individual data point, and the lines and brackets indicate the mean ± s.d. Each data point is an ADCC experiment containing autologous cultured keratinocytes (T) and sera (A) from one subject plus a standard homologous mononuclear effector (E). The keratinocytes and sera were from patients with systemic lupus erythematosus (SLE), subacute cutaneous lupus erythematosus (SCLE), or discoid lupus erythematosus (DLE) or from normal controls. The keratinocytes were irradiated with 100 mJ/cm2 of UVB light 24 h before addition of sera and effectors. The percentage cytotoxicity was determined by the following formula comparing different combinations of T, A and E. ADCC of irradiated targets = (TUVRAE − TUVRE) − (TAE − TE), where TUVR are targets irradiated with UVB 24 h before the experiment was performed, and T are keratinocytes which were sham-irradiated. Irradiated targets incubated with anti-SS-A/Ro antibody did show some cytotoxicity without added mononuclear effectors, but it represented only a fraction of the cytotoxicity observed with antibody plus effectors (data not shown). Statistical significance of different comparisons is indicated by P values.
Among the LE patients, the SLE and SCLE cells had higher cytotoxicity than DLE cells (0.001 < P < 0.01). When the dilution of autologous fractionated serum probes was adjusted to the mean level of IgG of NHS, the percentage cytotoxicities of UVB-irradiated self-derived keratinocytes from SLE, SCLE and DLE were 35.6 ± 8.3%, 35.1 ± 5.5% and 19.5 ± 3.0%, respectively. There were significant differences between SLE and adult controls (P < 0.001) and between SCLE and adult controls (0.001 < P < 0.01).
There was no clear association between percent cytotoxicity and the titre of anti-SS-A/Ro antibody in sera of patients with SLE and SCLE (data not shown). SLE autologous combinations in Fig. 1 included four anti-SS-A/Ro+ sera and one anti-SS-A/Ro− serum. The serum without anti-SS-A/Ro antibody showed 43% cytotoxicity.
Fractionated anti-SS-A/Ro+ preparations produce significant ADCC of irradiated keratinocytes
As shown in Fig. 2, fractionated specific anti-SS-A/Ro or specific anti-ssDNA reagents or fractionated NHS were diluted 1:20, and were added to irradiated cultured neonatal foreskin keratinocytes plus mononuclear effectors and then the percentage cytotoxicity in classic ADCC assays was measured: anti-SS-A/Ro 32.9 ± 8.4%, anti-ssDNA 19.9 ± 3.0% and NHS 15.4 ± 7.5%. The fractionated anti-SS-A/Ro serum probes induced significantly higher lysis of UVB-irradiated keratinocytes than fractionated probes of anti-ssDNA antibodies or NHS. When the dilution of serum probes was adjusted to the mean level of IgG of NHS, the percentage cytotoxicity of UVB-irradiated keratinocytes by fractionated anti-SS-A/Ro serum probes and fractionated anti-ssDNA serum probes was 27.0 ± 6.3% and 15.9 ± 3.8%, respectively. The elevated cytotoxicity was reduced (by 40.5%, 56.1% and 68.0% in three separate experiments) by competition with purified native SS-A/Ro protein. As shown in Fig. 2, there were no differences in cytotoxicity between UVB-irradiated keratinocytes with fractionated NHS probes and non-irradiated keratinocytes with fractionated probes of anti-SS-A/Ro serum or NHS.
Fig. 2.
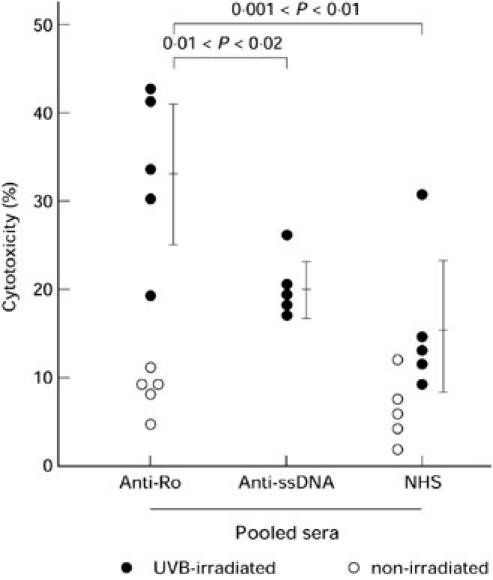
Purified IgG from anti-SS-A/Ro pooled sera significantly increase the level of antibody-dependent cell-mediated cytotoxicity (ADCC) against normal neonatal keratinocyte targets. In this assay, ADCC of normal neonatal foreskin keratinocytes was measured by ethidium bromide/acridine orange assay. Each circle represents one experimental point, each line and bracket shows the mean ± 1 s.d. of the points derived from three experiments. Twenty-four-hour ADCC experiments were performed using combinations of target (T), antibody (A) and effectors (E). The ADCC experiments were performed using normal neonatal keratinocyte cultures (T) and normal control mononuclear effectors (E). The antibodies used to induce ADCC were present in pooled sera from autoimmune patients specific for anti-SS-A/Ro or anti-ssDNA, or from normal human sera. The IgG fractions from the sera were obtained by fractionation with protein-A Sepharose. The specificity of the anti-SS-A/Ro fraction was verified by immunoblot and by blocking with purified SS-A/Ro. The targets were either irradiated keratinocytes (TUVR) exposed to 100 mJ/cm2 of UVB light 24 h prior to combination with antibody plus effectors, or non-irradiated keratinocytes (T). ○, Percentage cytotoxicity of TAE − TE; •, percentage cytotoxicity of TUVRAE − TUVRE. Irradiated targets incubated with anti-SS-A/Ro antibody did show some cytotoxicity without added mononuclear effectors, but it represented only a fraction of the cytotoxicity observed with antibody plus effectors (data not shown).
Based on these results, we directly measured whether UV-irradiated keratinocytes from SLE, SCLE and DLE patients were also more susceptible to ADCC mediated by anti-SS-A/Ro. As shown in Table 3, the keratinocytes from SLE, SCLE, and DLE patients all showed significant ADCC when incubated with anti-SS-A/Ro plus mononuclear effectors, but the keratinocytes from SLE and SCLE patients were more susceptible to anti-SS-A/Ro antibody-dependent damage than cells from DLE patients and adult controls. Fractionated anti-ssDNA serum probes induced significant ADCC only in keratinocytes from SLE patients. Compared with the ADCC induced by anti-SS-A/Ro against cultured human neonatal foreskin keratinocytes as shown in Fig. 2, cultured normal keratinocytes from adult controls showed more variable cytotoxicity and no significant cytotoxicity with any antibody source. However, cultured keratinocytes from SLE and SCLE patients showed enhanced cytotoxicity, as seen in Fig. 1, using autologous serum as the source of antibody.
Table 3.
Keratinocytes from lupus patients show enhanced antibody-dependent cell-mediated cytotoxicity (ADCC) with anti-SS-A/Ro antibody
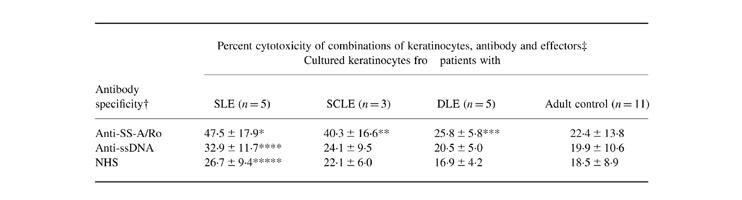
†Antibody specificity: pooled sera specific for anti-SS-A/Ro, anti-ssDNA and pooled normal human sera (NHS) were fractionated; the IgG-enriched fractions were used as antibody-enriched reagents. The anti-SS-A/Ro reagent showed largely anti-60-kD activity blocked by competition with purified SS-A/Ro.‡ADCC of irradiated targets = (TUVRAE − TUVRE) − (TAE − TE), where TUVR are targets irradiated with UVB 24 h before the experiment was performed, and T are keratinocytes which were sham-irradiated. The enriched sera are the antibody (A) source and the effectors (E) are homologous mononuclear effectors. The irradiated keratinocytes were exposed to 100 mJ/cm2 UVB.Values represent the mean percentage cytotoxicity and 1 s.d.Irradiated targets incubated with anti-SS-A/Ro antibody did show some cytotoxicity without added mononuclear effectors, but it represented only a fraction of the cytotoxicity observed with antibody plus effectors (data not shown).Statistical significance: *P < 0.005 (versus anti-SS-A/Ro plus adult control keratinocytes), P < 0.025 (versus anti-SS-A/Ro plus discoid lupus erythematosus (DLE) keratinocytes), P < 0.05 (versus NHS plus systemic lupus erythematosus (SLE) keratinocytes); **P < 0.025 (versus anti-SS-A/Ro plus adult control keratinocytes; ***P < 0.025 (versus NHS plus DLE keratinocytes); ****P < 0.001 (versus anti-SS-A/Ro plus adult control keratinocytes), P < 0.05 (versus anti-SS-A/Ro plus DLE keratinocytes); *****P < 0.025 (versus anti-SS-A/Ro plus adult control keratinocytes), P < 0.05 (versus anti-SS-A/Ro plus DLE keratinocytes).
DISCUSSION
The experiments presented here demonstrate three aspects of keratinocyte cytotoxicity relevant to photosensitive lupus erythematosus: (i) keratinocytes from SLE and SCLE patients show increased susceptibility to UVR-induced cytotoxicity and UVR-induced binding of anti-SSA/Ro antibodies; (ii) UVR-irradiated keratinocytes from SLE and SCLE patients, incubated in their own serum, show significantly increased ADCC when exposed to MNC from normal individuals; and (iii) anti-SS-A/Ro IgG fractions induce significant ADCC of normal irradiated keratinocytes and even greater cytotoxicity of irradiated keratinocytes from SCLE and SLE patients. Although keratinocytes can be killed both by complement-mediated lysis and ADCC [28, 29], keratinocytes repair complement-mediated damage well, and ADCC seems more relevant to the immunopathology seen in numerous skin diseases, including photosensitive lupus [5].
Several mechanisms have been proposed for the pathogenesis of cutaneous lupus erythematosus, such as direct autoantibody effects, ultraviolet effects, and the involvement of cell-mediated immunity [5]. The characteristic deposition of immunoglobulins and complement at the dermoepidermal junction suggests the participation of these antibodies in the pathogenesis. Furthermore, this disease can manifest clinically de novo after extended exposure to sunlight, and an ongoing disease might also be exacerbated after exposure, and ultraviolet exposure can trigger photosensitive lupus under controlled laboratory conditions [1–3].
Recently, induction of keratinocyte apoptosis by ultraviolet radiation has been proposed as an important trigger for production of autoantibodies in the development of skin lesions in SLE [30]. The changes in the distribution of autoantigen on the UVB-irradiated keratinocyte surface are now believed to be time-dependent. As early as 8 h after UVB irradiation, an enrichment of lupus autoantigens such as SS-A/Ro, SS-B/La, snRNP and Sm is induced within apoptotic surface blebs on the surface of cultured keratinocytes [30]. This finding has led to the notion that the surface blebs of apoptotic cells are important immunogenic structures in lupus. Apoptotic cells release large amounts of oligonucleosomes (the DNA-histone chromatin constituent), and patients with lupus have high concentrations of apoptotic cells and circulating nucleosomes. Anti-nucleosome antibodies are produced at high titres before the appearance of anti-DNA and anti-histone antibodies [31]. At present, the nucleosomes are considered to be the primary antigen in SLE [32], which seems to be in agreement with photosensitive cutaneous lupus.
However, basal keratinocytes are relatively resistant to the induction of apoptosis by UVR and can be induced to undergo necrosis by high doses of UVR. Suprabasal keratinocytes do undergo apoptosis in vivo following UV radiation [33], and it has been shown that apoptotic cells in SCLE lesions correspond to suprabasal keratinocytes and not the basal keratinocytes which are believed to be the targets of ADCC in SCLE skin lesions [34]. It appears that UVR has two major effects on keratinocytes in photosensitive lupus: induction of apoptosis and nucleoprotein translocation which might induce autoantibody production [30]; and effects on viable keratinocytes, rendering them more susceptible to ADCC. The results reported in this study may be relevant to both of these mechanisms, since keratinocytes from LE patients show increased cell death when exposed to UVR and also increased ADCC following UVR.
Twenty or 24 h after UVB irradiation, autoantibodies against SS-A/Ro, SS-B/La and U1RNP have been reported to bind to UVB-damaged but living human keratinocytes, both in vitro and in vivo [9–11,13]. The binding of these antibodies to the keratinocytes secondary to UVB irradiation is UVB-dependent and glycosylation-dependent, but cell cycle-independent [10]. Golan et al. [12] demonstrated the enhanced membrane binding of autoantibodies to keratinocytes cultured from SLE patients after UVB/UVA irradiation. These results indicated the possibility that this process of antibody binding is an important inducer of keratinocyte damage in photosensitive cutaneous lupus erythematosus.
The demonstration by Gershwin et al. [21] of the ADCC-mediated destruction of DNA-coated targets by lupus antisera and monocyte/lymphocyte effectors raised the possibility that ADCC might be the mechanism of tissue change in LE, especially the epidermal cell destruction associated with the mononuclear infiltration in lupus skin lesions. The autoantibodies in sera from LE patients specific for non-histone nuclear protein antigens such as SS-A/Ro, RNP and Sm are capable of inducing the ADCC of nuclear antigen-coated erythrocyte targets by monocyte, lymphocyte or low-density lymphocyte (K cell) effectors [35]. Taking into account the results of our present study, this augmented ADCC activity is strongly suspected to be involved in the pathomechanisms of cutaneous LE, especially with respect to photosensitivity. Although it is likely that complement-mediated cytotoxicity is also involved in photosensitive cutaneous lupus [36], ADCC would be likely to be more effective against viable basal keratinocytes [30].
Recently, the disparate localization of the 52- and 60-kD SS-A/Ro antigens was reported by Yell et al. [37] in cultured keratinocytes. It is still controversial whether these antigens are responsible for the development of cutaneous lupus. Our present study used antibody preparations reactive with 60-kD protein, and showed enhanced ADCC using these preparations, consistent with the described translocation of the 60-kD protein in cultured keratinocytes treated with estradiol or UVB light [10, 17]. Furthermore, Lee and associates reported that the autoantibody response to SS-A/Ro in SCLE and NLE may include antibodies to epitopes created by the complexing of the 60-kD SS-A/Ro protein with Y RNA [38]. Furthermore, the 60-kD SS-A/Ro protein and Y RNAs are concentrated in discrete areas of the nucleoplasm, nucleolus and cytoplasm [39]. The role of antibodies against the 52-kD protein is now under investigation.
Enhanced susceptibility to UVR-induced cytotoxicity of cells from lupus patients is not a new observation. Both fibroblasts [40, 41] and leucocytes [41] from SLE patients show increased UVR cytotoxicity, and it was not related to decreased DNA repair mechanisms [40]. In autoimmune mice, fibroblast cultures also show increased cytotoxic damage when exposed to UVR [23]. Golan et al. demonstrated that keratinocyte cultures from LE patients showed enhanced translocation of autoantigens following UVR, as expressed by enhanced binding of cell surface antibodies from anti-SS-A/Ro+ sera [12]. In the present study, we demonstrate that cultured keratinocytes from SLE and SCLE patients have a number of important sensitivities to UVR which are expressed as increased binding of autoantibodies following UVR radiation, enhanced direct cytotoxicity and enhanced ADCC with autologous sera and anti-SS-A/Ro reagents. These findings furnish new insight into the pathomechanisms underlying cutaneous lupus erythematosus, and may yield novel strategies for the treatment and prevention of skin lesions, and prevention of the development of autoimmune phenomena.
Acknowledgments
This work was supported by a grant from the Japanese Ministry of Science, Culture and Education B11470182, C11897013 (F.F.) and by a grant from the National Institutes of Health RO1-AR26427-18 (D.A.N.).
References
- 1.Cripps DJ, Rankin J. Action spectra of lupus erythematosus and experimental immunofluorescence. Arch Dermatol. 1973;107:563–7. [PubMed] [Google Scholar]
- 2.Freeman RG, Knox JM, Owen DW. Cutaneous lesions of lupus erythematosus induced by monochromatic light. Arch Dermatol. 1969;100:677–82. [PubMed] [Google Scholar]
- 3.Walchner M, Messer G, Kind P. Phototesting and photoprotection in LE. Lupus. 1997;6:176–84. doi: 10.1177/096120339700600212. [DOI] [PubMed] [Google Scholar]
- 4.Tebbe B, Orfanos CE. Epidemiology and socioeconomic impact of skin disease in lupus erythematosus. Lupus. 1997;6:96–104. doi: 10.1177/096120339700600204. [DOI] [PubMed] [Google Scholar]
- 5.Norris DA, Lela LA. Pathogenesis of cutaneous lupus erythematosus. Clin Dermatol. 1985;3:20–35. doi: 10.1016/0738-081x(85)90075-6. [DOI] [PubMed] [Google Scholar]
- 6.Sontheimer RD, Thomas JR, Gilliam JN. Subacute cutaneous lupus erythematosus. A cutaneous marker for a distinct lupus erythematosus subset. Arch Dermatol. 1979;115:1409–15. [PubMed] [Google Scholar]
- 7.Sontheimer RD, Maddison PJ, Reichlin M, et al. Serological and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset for neonatal lupus erythematosus. Ann Intern Med. 1982;97:664–71. doi: 10.7326/0003-4819-97-5-664. [DOI] [PubMed] [Google Scholar]
- 8.Weston WL. Significance and character of SS-A (Ro) and SS-B (La) antigens. J Invest Dermatol. 1985;84 doi: 10.1111/1523-1747.ep12274943. [DOI] [PubMed] [Google Scholar]
- 9.LeFeber WP, Norris DA, Ryan SR, et al. Ultraviolet light induces binding of antibodies to selected nuclear antigens on cultured human keratinocytes. J Clin Invest. 1984;74:1545–51. doi: 10.1172/JCI111569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furukawa F, Kashihara-Sawami M, Lyons MB, Norris DA. Binding of antibodies to the extractable nuclear antigens SS-A/Ro and SS-B/La is induced on the surface of human keratinocytes by ultraviolet light (UVL): implications for the pathogenesis of photosensitive cutaneous lupus. J Invest Dermatol. 1990;94:77–85. doi: 10.1111/1523-1747.ep12873930. [DOI] [PubMed] [Google Scholar]
- 11.Furukawa F, Ikai K, Matsuyoshi N, et al. Relationship between heat shock protein induction and the binding of antibodies to the extractable nuclear antigens on cultured human keratinocytes. J Invest Dermatol. 1993;101:191–5. doi: 10.1111/1523-1747.ep12363785. [DOI] [PubMed] [Google Scholar]
- 12.Golan TD, Elkon KB, Gharavi AE, Krueger JG. Enhanced membrane binding of autoantibodies to cultured keratinocytes of systemic lupus erythematosus patients after ultraviolet B/ultraviolet A irradiation. J Clin Invest. 1992;90:1067–76. doi: 10.1172/JCI115922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones SK. Ultraviolet radiation (UVR) induces cell-surface Ro/SSA antigen expression by human keratinocytes in vitro: a possible mechanism for the UVR induction of cutaneous lupus lesions. Br J Dermatol. 1992;126:546–53. doi: 10.1111/j.1365-2133.1992.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 14.Furukawa F, Kanauchi H, Imamura S. Susceptibility to UVB light in cultured keratinocytes of cutaneous lupus erythematosus. Dermatology. 1994;189(Suppl. 1):18–23. doi: 10.1159/000246922. [DOI] [PubMed] [Google Scholar]
- 15.Furukawa F, Huff JC, Weston WL, Norris DA. Serum-free serial culture of adult human keratinocytes from suction-blister roof epidermis. J Invest Dermatol. 1987;89:460–3. doi: 10.1111/1523-1747.ep12460904. [DOI] [PubMed] [Google Scholar]
- 16.Furukawa F, Huff JC, Lyons MB, et al. Characterization and practical benefits of keratinocytes cultured in strontium-containing serum-free medium. J Invest Dermatol. 1988;90:690–6. doi: 10.1111/1523-1747.ep12560908. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa F, Lyons MB, Lee LA, et al. Estradiol enhances binding to cultured human keratinocytes of antibodies specific for SS-A/Ro and SS-B/La. J Immunol. 1988;141:1480–8. [PubMed] [Google Scholar]
- 18.Miyagawa S, Fukumoto T, Hashimoto K, et al. Maternal autoimmune response to recombinant Ro/SSA and La/SSB proteins in Japanese neonatal lupus erythematosus. Autoimmunity. 1995;21:277–82. doi: 10.3109/08916939509001947. [DOI] [PubMed] [Google Scholar]
- 19.Oi VT, Herzenberg LA. Antibody purification: protein A-sepharose column chromatography. In: Mishell BB, Shiigi SM, editors. San Francisco: W. H. Freeman; pp. 368–70. Selected methods in cellular immunology. [Google Scholar]
- 20.Furukawa F, Kanauchi H, Wakita H, et al. Spontaneous autoimmune skin lesions of MRL/n mice: autoimmune disease-prone genetic background in relation to Fas-defect MRL/lpr mice. J Invest Dermatol. 1996;107:95–100. doi: 10.1111/1523-1747.ep12298305. [DOI] [PubMed] [Google Scholar]
- 21.Gershwin ME, Glinski W, Chused T, Steinberg AD. Lymphocyte-dependent antibody-mediated cytolysis of DNA-anti-DNA-coated target cells using human and murine SLE effector populations. Clin Immunol Immunopathol. 1977;8:280–91. doi: 10.1016/0090-1229(77)90118-0. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Reichlin M. Some autoantibodies to Ro/SS-A and La/SS-B are antiidiotypes to anti-double-stranded DNA. Arthritis Rheum. 1996;39:522–31. doi: 10.1002/art.1780390321. [DOI] [PubMed] [Google Scholar]
- 23.Furukawa F, Lyons MB, Norris DA. Susceptible cytotoxicity to ultraviolet B light in fibroblasts and keratinocytes cultured from autoimmune-prone MRL/Mp−lpr/lpr mice. Clin Immunol Immunopathol. 1989;52:460–72. doi: 10.1016/0090-1229(89)90160-8. [DOI] [PubMed] [Google Scholar]
- 24.Tokura Y, Nishijima T, Yagi H, et al. Photohaptenic properties of fluoroquinolones. Photochem Photobiol. 1996;64:838–44. doi: 10.1111/j.1751-1097.1996.tb01844.x. [DOI] [PubMed] [Google Scholar]
- 25.Wakita H, Tokura Y, Furukawa F, Takigawa M. Staphylococcal enterotoxin B upregulates expression of ICAM-1 molecules on IFN-γ-treated keratinocytes and keratinocyte cell lines. J Invest Dermatol. 1995;105:536–42. doi: 10.1111/1523-1747.ep12323426. [DOI] [PubMed] [Google Scholar]
- 26.Furukawa F. Mechanisms of cutaneous injury in photosensitive cutaneous lupus erythematosus. In: Dyall-Smith D, Marks R, editors. Lancs: Parthenon Publishing; pp. 278–82. Dermatology at the millennium. [Google Scholar]
- 27.Norris DA, Kissinger RM, Naughton GM, Bystryn JC. Evidence for immunologic mechanisms in human vitiligo: patients' sera induce damage to human melanocytes in vitro by complement-mediated damage and antibody-dependent cellular cytotoxicity. J Invest Dermatol. 1988;90:783–9. doi: 10.1111/1523-1747.ep12461505. [DOI] [PubMed] [Google Scholar]
- 28.Norris DA, Lee LA. Antibody-dependent cellular cytotoxicity and skin disease. J Invest Dermatol. 1985;85:165s–175s. doi: 10.1111/1523-1747.ep12276370. [DOI] [PubMed] [Google Scholar]
- 29.Norris DA, Ryan SB, Kissinger RM, et al. Systematic comparison of antibody-mediated mechanisms of keratinocyte lysis in vitro. J Immunol. 1985;135:1073–79. [PubMed] [Google Scholar]
- 30.Casiola-Rosen LA, Anhalt G, Rosen A. Autoantigen targets in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burlingame RW, Rubin RL, Balderas RS, Theofilopoulos AN. Genesis and evolution of antichromatin autoantibodies in murine lupus implicates T-dependent immunization with self-antigen. J Clin Invest. 1993;91:1687–96. doi: 10.1172/JCI116378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bach JF, Koutouzov S. New clues to systemic lupus. Lancet. 1997;350(Suppl. III):11. doi: 10.1016/s0140-6736(97)90044-1. [DOI] [PubMed] [Google Scholar]
- 33.Norris DA, Middleton MH, Whang K, et al. Human keratinocytes maintain reversible anti-apoptotic defenses in vivo and in vitro. Apoptosis. 1997;2:136–48. doi: 10.1023/a:1026456229688. [DOI] [PubMed] [Google Scholar]
- 34.Norris DA, Whang K, David-Bajar K, Bennion SD. The influence of ultraviolet light on immunological cytotoxicity in the skin. Photochem Photobiol. 1997;65:636–46. doi: 10.1111/j.1751-1097.1997.tb01905.x. [DOI] [PubMed] [Google Scholar]
- 35.Norris DA, Ryan SR, Fritz KA, et al. The role of RNP, Sm, and SS-A/Ro-specific antisera from patients with lupus erythematosus in inducing antibody-dependent cellular cytotoxicity (ADCC) of targets coated with nonhistone nuclear antigens. Clin Immunol Immunopathol. 1984;31:311–20. doi: 10.1016/0090-1229(84)90084-9. [DOI] [PubMed] [Google Scholar]
- 36.Yu HS, Chiang LC, Chang CH, et al. The cytotoxic effect of neonatal lupus erythematosus and maternal sera on keratinocyte cultures is complement-dependent and can be augmented by ultraviolet irradiation. Br J Dermatol. 1996;135:297–301. [PubMed] [Google Scholar]
- 37.Yell AJ, Wang L, Yin H, McCauliffe DP. Disparate locations of the 52- and 60-kDa Ro/SS-A antigens in cultured keratinocytes. J Invest Dermatol. 1996;107:622–6. doi: 10.1111/1523-1747.ep12584223. [DOI] [PubMed] [Google Scholar]
- 38.Lee LA, Alvarez K, Gross T, Harley JB. The recognition of human 60-kDa Ro ribonucleoprotein particles by antibodies associated with cutaneous lupus and neonatal lupus. J Invest Dermatol. 1996;107:225–8. doi: 10.1111/1523-1747.ep12329677. [DOI] [PubMed] [Google Scholar]
- 39.Farris AD, Puvion-Dutilleul F, Puvion E, et al. The ultrastructural localization of 60-kDa Ro protein and human cytoplasmic RNAs: association with novel electron-dense bodies. Proc Natl Acad Sci USA. 1997;94:3040–5. doi: 10.1073/pnas.94.7.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zamansky GB. Recovery from UV-induced potentially lethal damage in systemic lupus erythematosus skin fibroblasts. Int J Radiat Biol Relat Stud Phys Chem Med. 1986;50:305–12. doi: 10.1080/09553008614550681. [DOI] [PubMed] [Google Scholar]
- 41.Golan TD, Foltyn V, Roueff A. Increased susceptibility to in vitro ultraviolet B radiation in fibroblasts and lymphocytes cultured from systemic lupus erythematosus patients. Clin Immunol Immunopathol. 1991;58:289–304. doi: 10.1016/0090-1229(91)90143-x. [DOI] [PubMed] [Google Scholar]


