Abstract
It is widely accepted that type 2 helper T (Th2) lymphocytes play a crucial role in the pathogenesis of atopic dermatitis (AD) as well as bronchial asthma (BA). We measured the amounts of IL-5 and interferon-gamma (IFN-γ) produced by PBMC upon stimulation with house dust mite (HDM) or Candida albicans (CA) in 17 children (3–15 years) with AD, and compared these values with those of 16 children with BA. Although IL-5 production by PBMC upon stimulation with HDM in patients with AD was significantly higher than that in 13 non-atopic controls (geometric mean = 23.4 pg/ml versus 5.9 pg/ml, P < 0.05), it was significantly lower than that in patients with BA (177.8 pg/ml, P < 0.001). The amount of IL-5 produced by PBMC upon stimulation with CA was also significantly lower in patients with AD than in those with BA (7.2 pg/ml versus 100.0 pg/ml, P < 0.001). The production of IFN-γ by PBMC stimulated with HDM or CA was also significantly lower in patients with AD than in those with BA (HDM 4.3 pg/ml versus 12.6 pg/ml, P < 0.05; CA 6.5 pg/ml versus 60.3 pg/ml, P < 0.001). Consequently the ratio of IL-5 to IFN-γ production was high not only in patients with BA but also in those with AD. These findings suggest that there are some differences in the regulation of in vivo cytokine production between patients with AD and those with BA, although a Th2-dominant profile is common to both.
Keywords: atopic dermatitis, bronchial asthma, IL-5, interferon-gamma, peripheral blood mononuclear cells
INTRODUCTION
To date, it is widely accepted that helper T (Th) cells consist of two functionally different subsets, Th1 and Th2 [1,2]. These subsets are characterized by the profile of involved cytokines: Th1 cells mainly produce interferon-gamma (IFN-γ), while Th2 cells selectively secrete IL-4 and IL-5. The profile of cytokine production by PBMC from patients with bronchial asthma (BA) upon stimulation with house dust mite (HDM) was demonstrated to be compatible with that of Th2 cells [3]. The number of Th2 cells was also shown to be increased in the bronchial tissue and bronchoalveolar lavage fluid (BALF) of patients with BA [4–6]. Recently, the recruitment of allergen-specific Th2 cells from the peripheral blood into the bronchial tissue after an inhalant allergen challenge was also demonstrated [7,8]. These findings suggest that Th2 cells play a principal role in the pathogenesis of BA.
Similarly, the dominance of Th2 cells in PBMC was demonstrated in patients with atopic dermatitis (AD) by establishing T cell clones [9,10]. The pattern of cytokine production by PBMC from patients with AD upon stimulation with polyclonal activators was also reported to correspond to that of Th2 cells [11]. The dominance of Th2-like cells in the affected skin tissue of patients with AD was also demonstrated [12,13]. Thus, the dominance of Th2 cells appears to be essential for the development of AD [14]. To date, however, allergen-specific cytokine production by PBMC in primary culture, which may reflect the state of in vivo cytokine production, has not yet been examined in patients with AD. In this study, we measured the amounts of IL-5 and IFN-γ produced by PBMC from patients with AD in primary culture upon stimulation with HDM or Candida albicans (CA) and compared the amounts with those produced by PBMC from patients with BA. We found that the production of these cytokines was distinctly lower in patients with AD than in those with BA.
PATIENTS AND METHODS
Subjects
Heparinized peripheral blood was obtained at the time of routine blood examination from 17 patients with AD and 16 patients with BA who visited our institute between October 1996 and September 1998, after informed consent was obtained (Table 1). Subjects were limited to those who had experienced either AD or BA, excluding those affected by both. The severity of AD and BA was estimated according to the criteria of Rajka & Langeland [15] and the Japanese Society of Paediatric Allergy and Clinical Immunology [16], respectively. Since it was reported that allergen-specific helper T cells were recruited from the peripheral blood to the lung after exposure to allergens [7,8], the test was performed during the remission phase not only in patients with BA, but also in those with AD. Because cytokine production by PBMC is less in younger children [17–19], the subjects were confined to 3–15-year-olds. No patients had been administered systemic corticosteroids. As a control, heparinized blood was collected similarly from 13 age-matched non-atopic children without any haematologic, immunological or infectious diseases. This study was approved by the Local Ethical Committee.
Table 1.
Profile of subjects
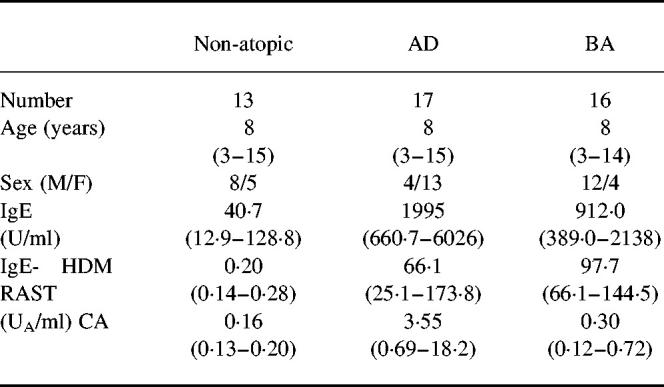
Age (years) is shown as the median and range. The levels of IgE and IgE-RAST are presented as the geometric mean and range of 1 s.d.
AD, Atopic dermatitis; BA, bronchial asthma; HDM, house dust mite; CA, Candida albicans.
Cell culture
PBMC were collected by gradient centrifugation over Ficoll–Paque (Pharmacia, Uppsala, Sweden) and resuspended at a concentration of 1 × 106 cells/ml in RPMI 1640 medium (Iwaki Glass Co., Chiba, Japan) supplemented with 10% heat-inactivated fetal calf serum (FCS; Xavier Investments, Wacoal, Australia), 2 mm glutamine and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin). Then, 0.5 ml cell suspension was added to each well in duplicate on a 48-well culture plate (Falcon 3078; Becton Dickinson, Lincoln Park, NJ) and cultured for 6 days in a humidified atmosphere of 5% CO2 at 37°C with or without 50 μg/ml HDM (Der f) [20–23] or 100 μg/ml CA crude extract (Torii Pharmaceutical Co., Tokyo, Japan). Under these conditions, the level of endotoxin measured by an endotoxin-specific test kit (Endospecy, Seikagaku Kogyo Co., Ltd, Tokyo, Japan) was < 1 ng/ml in HDM- as well as CA-containing medium. After incubation, the supernatant was collected in an Eppendorf tube (Iwaki Glass) and stored at −80°C until the measurement of cytokines.
The production of IL-5 by PBMC stimulated with 50 μg/ml HDM and 100 μg/ml CA was comparable to the peak levels obtained by stimulation with 10 μg/ml HDM and 20 μg/ml CA, respectively (data not shown). The maximum IFN-γ production was induced by stimulation of PBMC with 50 μg/ml HDM or 100 μg/ml CA. The production of IL-5 as well as IFN-γ increased with culture time up to 6 days. Thereafter, the amounts of these cytokines produced by PBMC began to decrease in some subjects.
Measurement of IL-5, IFN-γ and IgE antibody
IL-5 and IFN-γ in the culture supernatants were measured with ELISA kits (Cytoscreen human IL-5 and IFN-γ; BioSource Int., Camarillo, CA), whose sensitivities were both 1 pg/ml. Serum IgE was measured by a radioimmunoassay kit (Ab beads IgE kit Eiken; Eiken Chemical Co., Tokyo, Japan). Radioallergosorbent test for IgE antibody (IgE-RAST) against HDM (Der f) or CA was done using a CAP-RAST radioimmunoassay kit (Pharmacia).
Statistical analysis
The results of IgE-RAST, IL-5 and IFN-γ are presented as the geometric mean and range of 1 s.d. (from the mean −1 s.d. to the mean + 1 s.d.). For the purposes of statistical analysis, if the level of cytokine production was < 1 pg/ml, 0.5 was arbitrarily allocated. The significance of the difference in the data was examined by Student's t-test and judged as significant if P < 0.05.
RESULTS
Cytokine production by PBMC upon stimulation with HDM
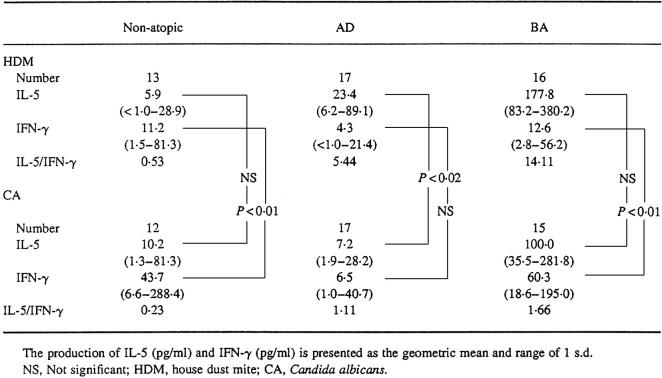
In 16 patients with BA, the amount of IL-5 produced by PBMC upon stimulation with HDM was significantly larger than that in 13 non-atopic controls (177.8 (83.2–380.2) pg/ml versus 5.9 (< 1.0–28.9) pg/ml; P < 0.001) (Fig. 1a). Although IL-5 production by HDM-stimulated PBMC in 17 patients with AD (23.4 (6.2–89.1) pg/ml) was also significantly greater than that in non-atopic controls (P < 0.05), it was significantly lower than that in patients with BA (P < 0.001) (Fig. 1a).
Fig. 1.
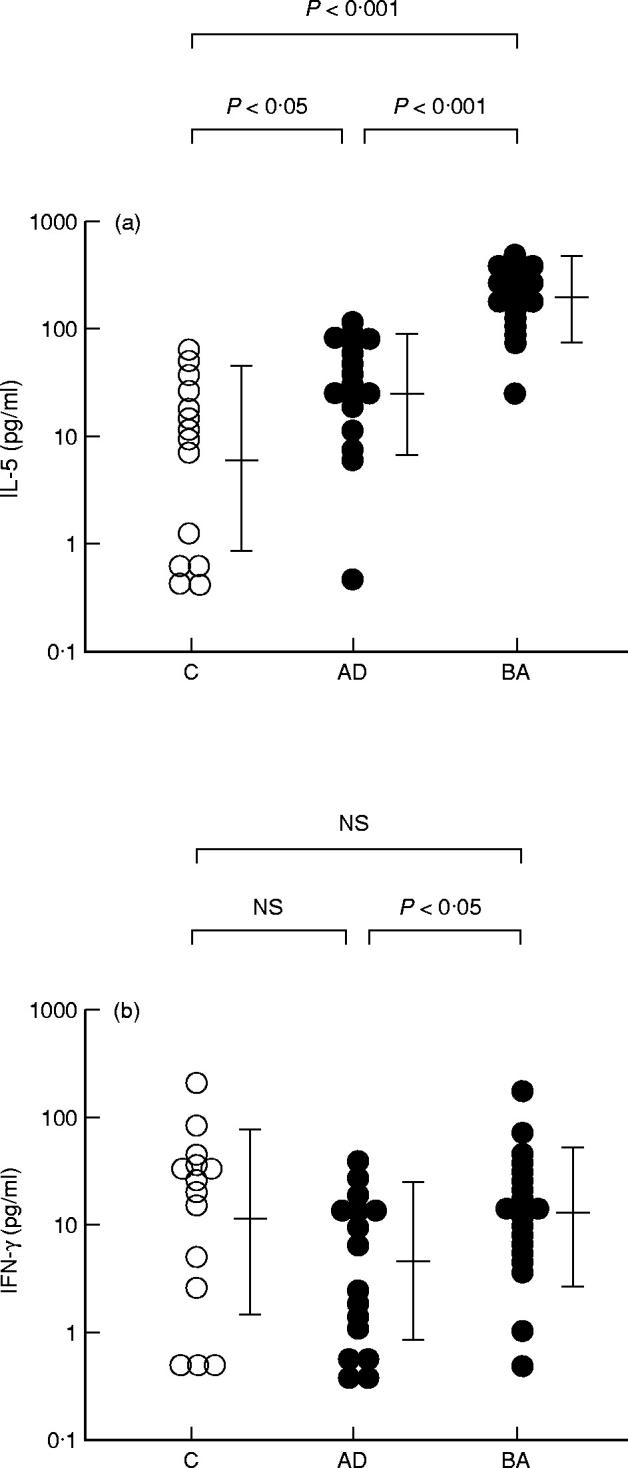
IL-5 and IFN-γ production by PBMC upon stimulation with house dust mite (HDM). IL-5 (a) and IFN-γ (b) production in patients with atopic dermatitis (AD) and those with bronchial asthma (BA) by PBMC upon stimulation with HDM is presented. C, Non-atopic controls; NS, not significant.
IFN-γ production by HDM-stimulated PBMC in patients with BA was 12.6 (2.8–56.2) pg/ml, comparable to that in non-atopic controls (11.2 (1.5–81.3) pg/ml) (Fig. 1b). On the other hand, the amount of IFN-γ produced by HDM-stimulated PBMC in patients with AD (4.3 (< 1.0–21.4) pg/ml) was less than that in non-atopic controls, although the difference was not significant. IFN-γ production by HDM-stimulated PBMC was significantly lower in patients with AD than in those with BA (P < 0.05) (Fig. 1b). Therefore, the ratio of IL-5/IFN-γ production was higher not only in patients with BA (14.11) but also in those with AD (5.44) than that in non-atopic controls (0.53) (Table 2).
Table 2.
IL-5 and IFN-γ production by PBMC in patients with atopic dermatitis (AD) and bronchial asthma (BA)
The production of IL-5 (pg/ml) and IFN-γ (pg/ml) is presented as the geometric mean and range of 1 s.d.
NS, Not significant; HDM, house dust mite; CA, Candida albicans.
IFN-γ production by PBMC upon stimulation with CA
CA has been reported to induce Th1 clones which selectively produce a large amount of IFN-γ [10]. Actually, IFN-γ production by CA-stimulated PBMC from the present non-atopic controls was significantly higher than that produced by their HDM-stimulated PBMC (43.7 (6.6–288.4) pg/ml versus 11.2 (1.5–81.3) pg/ml; P < 0.01) (Table 2). PBMC from patients with BA also produced a significantly higher amount of IFN-γ by stimulation with CA than that produced by stimulation with HDM (60.3 (18.6–195.0) pg/ml versus 12.6 (2.8–56.2) pg/ml; P < 0.01) (Table 2). In contrast, the amount of IFN-γ produced by CA-stimulated PBMC was not notably larger than that produced by HDM-stimulated PBMC in patients with AD (6.5 (1.0–40.7) pg/ml versus 4.3 (< 1.0–21.4) pg/ml) (Table 2).
The amount of IFN-γ produced by PBMC upon stimulation with CA in patients with BA was comparable to that in non-atopic controls (Fig. 2b). In contrast, IFN-γ production by PBMC stimulated with CA was significantly lower in patients with AD than that in patients with BA (P < 0.001) as well as in non-atopic controls (P < 0.02) (Fig. 2b).
Fig. 2.
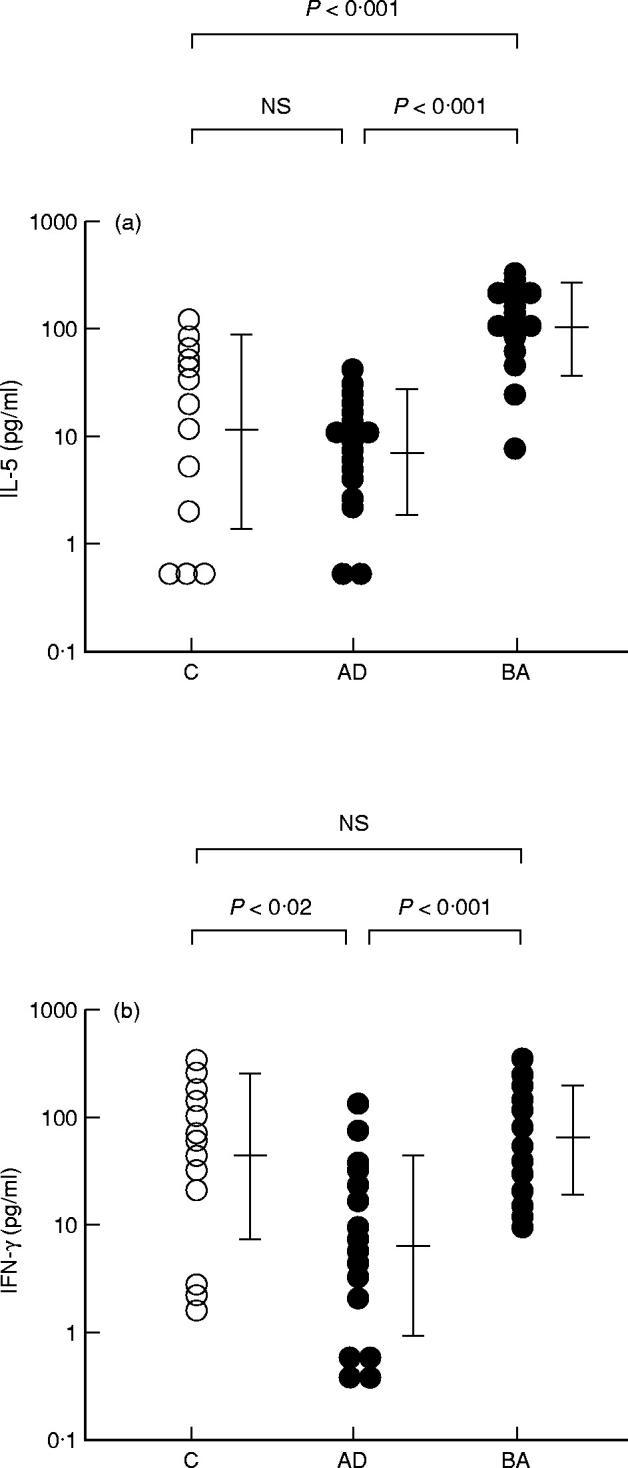
IL-5 and IFN-γ production by PBMC upon stimulation with Candida albicans(CA). IL-5 (a) and IFN-γ (b) production in patients with atopic dermatitis (AD) and those with bronchial asthma (BA) by PBMC upon stimulation with CA is shown. C, Non-atopic controls; NS, not significant.
IL-5 production by PBMC upon stimulation with CA
CA induced a high level of IL-5 production by PBMC from patients with BA (100.0 (35.5–281.8) pg/ml), comparable to that produced by HDM-stimulated PBMC from the same patients (177.8 (83.2–380.2) pg/ml) (Table 2) and significantly higher than that produced by CA-stimulated PBMC from non-atopic controls (10.2 (1.3–81.3) pg/ml; P < 0.001) (Fig. 2a). On the other hand, the amount of IL-5 produced by CA-stimulated PBMC from patients with AD was significantly smaller than that produced by HDM-stimulated PBMC from the same patients (7.2 (1.9–28.2) pg/ml versus 23.4 (6.2–89.1) pg/ml; P < 0.02) (Table 2). It was also significantly smaller than that produced by CA-stimulated PBMC from patients with BA (P < 0.001) (Fig. 2a). The production of IL-5 by CA-stimulated PBMC did not differ significantly between patients with AD and non-atopic controls (Fig. 2a).
The IL-5/IFN-γ ratio of CA-stimulated PBMC in patients with AD (1.11) was comparable to that in patients with BA (1.66) and higher than that in non-atopic controls (0.23) (Table 2).
Relation of cytokine production to severity of disease
The production of IL-5 and IFN-γ by PBMC upon stimulation with HDM or CA did not differ markedly between the patient groups with moderate and severe BA (Table 3). In contrast, cytokine production in patients with severe AD was remarkably lower than that in patients with moderate AD. There were significant differences in the amounts of IL-5 and IFN-γ produced by PBMC upon stimulation with CA between the patient groups with moderate and severe AD (IL-5, 19.1 (7.9–45.7) pg/ml versus 3.2 (< 1.0–11.2) pg/ml, P < 0.01; IFN-γ, 20.4 (3.1–134.9) pg/ml versus 2.2 (< 1.0–7.1) pg/ml, P < 0.02).
Table 3.
Correlation between cytokine production and severity of disease
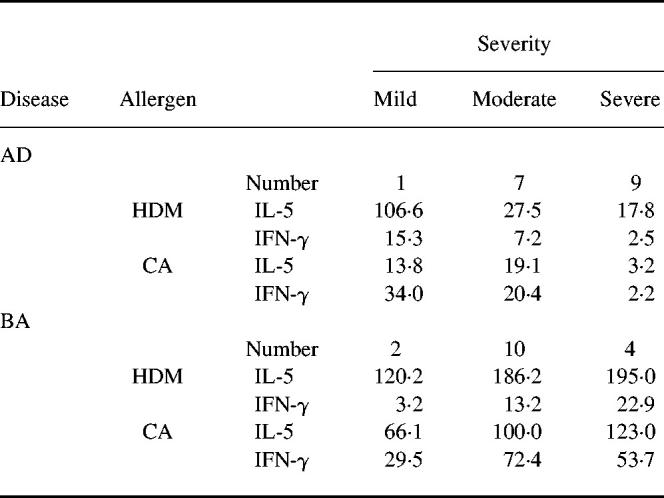
The production of IL-5 (pg/ml) and IFN-γ (pg/ml) is presented as the geometric mean.
AD, Atopic dermatitis; BA, bronchial asthma; HDM, house dust mite; CA, Candida albicans.
DISCUSSION
Many investigators have demonstrated that Th2 cells, probably playing a crucial role in the pathogenesis of atopic disorders, were dominant in PBMC as well as the affected tissues of patients with BA and AD [2–14]. The profile of cytokine production has usually been analysed by establishing cell lines. This method is useful for magnifying the production of cytokines. However, the original characteristics of lymphocytes may be considerably altered during the process of establishing cell lines. In this study, cytokine production by PBMC was measured in primary culture to minimize alterations in the nature of lymphocytes compared with established cell lines. This method also enabled the comparison between patients with AD and BA of the production of cytokines by a constant number of PBMC, probably reflecting the state of in vivo cytokine production.
HDM was shown to stimulate mainly Th2 cells [9,10]. Till et al. reported that a large amount of IL-5 was produced by PBMC from patients with BA upon stimulation with HDM in primary culture [3]. In this study, our findings confirmed this (Fig. 1a). The profiles of cytokine production estimated by the ratios of IL-5 to IFN-γ production in our patients with BA were Th2-dominant (Table 2), comparable to the results of other investigators. We also demonstrated that the ratio of HDM-specific IL-5/IFN-γ production by PBMC in primary culture was elevated in patients with AD. These findings may support the hypothesis that AD and BA develop from common immunological abnormalities.
In this study, however, we demonstrated that the amount of IL-5 as well as IFN-γ produced by PBMC upon stimulation with HDM was notably smaller in patients with AD than in those with BA (Fig. 1). Although females were dominant in the patients with AD (Table 1), there were no significant sexual differences in the production of IL-5 or IFN-γ (data not shown). Although we presented data on cytokine production during the remission phase, essentially the same results were obtained in the acutely aggravated period (unpublished observation). Moreover, the decrease in IL-5 and IFN-γ production was more evident in patients with severe AD than in those with mild to moderate AD (Table 3). Therefore, decreased IL-5 and IFN-γ production seems not to be an accessory phenomenon, but rather related to an essential immunological abnormality which results in AD.
In contrast to HDM, CA was demonstrated to activate mainly Th1 cells even in atopic patients [10]. The difference in cytokine production by PBMC between patients with AD and patients with BA was also demonstrated by the use of CA. Thus, a relatively decreased cytokine production by PBMC from patients with AD does not seem to be a peculiar response to HDM, but a common one to various allergens. Confirmation of this phenomenon and clarification of the cause may largely contribute to a comprehensive understanding of the pathogenesis of AD. It remains to be elucidated whether the decrease in cytokine production by PBMC from patients with AD is due to decreased cytokine production per cell, or to a decrease in the number of cytokine-producing cells in PBMC. It is also unknown whether any active suppression is at work on cytokine production by PBMC from patients with AD.
Although the level of IgE-RAST for HDM in patients with AD was similar to that in patients with BA, the level of IgE-RAST against CA was apparently higher in the former than in the latter (Table 1). The level of serum IgE was also higher in patients with AD than in those with BA (Table 1). These findings however are not peculiar to the subjects of this study, because essentially the same result was obtained by a multicentre study in Japan [24]. The cause of these differences in IgE antibody synthesis is as yet unknown.
REFERENCES
- 1.Mosmann TR, Cherwinski H, Bond MW, et al. Two types of murine helper T cell clones. I. Definition according to profiles of lymphokine activities and secretory proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 2.Umetsu DT, DeKruyff RH. TH1 and TH2 CD4+ cells in human allergic diseases. J Allergy Clin Immunol. 1997;100:1–6. doi: 10.1016/s0091-6749(97)70186-6. [DOI] [PubMed] [Google Scholar]
- 3.Till S, Dickason R, Huston D, et al. IL-5 secretion by allergen-stimulated CD4+ T cells in primary culture: relationship to expression of allergic disease. J Allergy Clin Immunol. 1997;99:563–9. doi: 10.1016/s0091-6749(97)70085-x. [DOI] [PubMed] [Google Scholar]
- 4.Robinson D, Hamid Q, Bentley A, et al. Activation of CD4+ T cells, increased TH2-type cytokine mRNA expression, and eosinophil recruitment in bronchoalveolar lavage after allergen inhalation challenge in patients with atopic asthma. J Allergy Clin Immunol. 1993;92:313–24. doi: 10.1016/0091-6749(93)90175-f. [DOI] [PubMed] [Google Scholar]
- 5.Hamid Q, Azzawi M, Ying S, et al. Expression of mRNA for interleukin-5 in mucosal bronchial biopsies from asthma. J Clin Invest. 1991;87:1541–6. doi: 10.1172/JCI115166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamid Q, Song Y, Kotsimbos TC, et al. Inflammation of small airways in asthma. J Allergy Clin Immunol. 1997;100:44–51. doi: 10.1016/s0091-6749(97)70193-3. [DOI] [PubMed] [Google Scholar]
- 7.Crimi E, Gaffi D, Frittoli E, et al. Depletion of circulating allergen-specific TH2 T lymphocytes after allergen exposure in asthma. J Allergy Clin Immunol. 1997;99:788–97. doi: 10.1016/s0091-6749(97)80013-9. [DOI] [PubMed] [Google Scholar]
- 8.Borgonovo B, Casorati G, Frittoli F, et al. Recruitment of circulating allergen-specific T lymphocytes to the lung on allergen challenge in asthma. J Allergy Clin Immunol. 1997;100:669–78. doi: 10.1016/s0091-6749(97)70172-6. [DOI] [PubMed] [Google Scholar]
- 9.Parronchi P, Macchia D, Poccinni M-P, et al. Allergen- and bacterial antigen-specific T-cell clones established from atopic donors show a different profile of cytokine production. Proc Natl Acad Sci USA. 1991;88:4538–42. doi: 10.1073/pnas.88.10.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wierenga EA, Snoek M, De Groot C, et al. Evidence for compartmentalization of functional subsets of CD4+ T lymphocytes in atopic patients. J Immunol. 1990;144:4651–6. [PubMed] [Google Scholar]
- 11.Jujo K, Renz H, Abe J, et al. Decreased interferon gamma and increased interleukin-4 production in atopic dermatitis promotes IgE synthesis. J Allergy Clin Immunol. 1992;90:323–31. doi: 10.1016/s0091-6749(05)80010-7. [DOI] [PubMed] [Google Scholar]
- 12.Kay AB, Ying S, Varney V, et al. Messenger RNA expression of the cytokine gene cluster, interleukin 3 (IL-3), IL4, IL5, and granulocyte/macrophage colony-stimulating factor, in allergen-induced late-phase cutaneous reactions in atopic subjects. J Exp Med. 1991;173:775–8. doi: 10.1084/jem.173.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kägi MK, Wüthrich B, Montano E, et al. Differential cytokine profiles in peripheral blood lymphocyte supernatants and skin biopsies from patients with different forms of atopic dermatitis, psoriasis and normal individuals. Int Arch Allergy Immunol. 1994;103:332–40. doi: 10.1159/000236651. [DOI] [PubMed] [Google Scholar]
- 14.Leung DYM. Atopic dermatitis: the skin as a window into the pathogenesis of chronic allergic diseases. J Allergy Clin Immunol. 1995;96:302–18. doi: 10.1016/s0091-6749(95)70049-8. [DOI] [PubMed] [Google Scholar]
- 15.Rajka G, Langeland T. Grading of the severity of atopic dermatitis. Acta Derm Venereol. 1989;144(Suppl.):13–14. doi: 10.2340/000155551441314. [DOI] [PubMed] [Google Scholar]
- 16.Furusho K, Nishima S, Akasaka T, et al. Report of the committee on criteria defining the severity and prognosis of childhood asthma. Jpn J Pediatr Allergy Clin Immunol. 1996;10:114–9. (in jpn) [Google Scholar]
- 17.Lewis DB, Yu CC, Meyer J, et al. Cellular and molecular mechanisms for reduced interleukin 4 and interferon-γ production by neonatal T cells. J Clin Invest. 1991;87:194–202. doi: 10.1172/JCI114970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang MLK, Kemp AS. Ontogeny of IL4 production. Pediatr Allergy Immunol. 1995;6:11–19. doi: 10.1111/j.1399-3038.1995.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 19.Kimura M, Tsuruta S, Yoshida T. Unique profile of IL-4 and IFN-γ production by peripheral blood mononuclear cells in infants with atopic dermatitis. J Allergy Clin Immunol. 1998;102:238–44. doi: 10.1016/s0091-6749(98)70092-2. [DOI] [PubMed] [Google Scholar]
- 20.Hiratani M, Muto K, Oshida Y, et al. Lymphocyte responsiveness to Dermatophagoides farinae extract in mite sensitive patients: effect of immunotherapy on cellular proliferative response and specific immunoglobulin E antibody (RAST score) J Allergy Clin Immunol. 1981;68:205–11. doi: 10.1016/0091-6749(81)90185-8. [DOI] [PubMed] [Google Scholar]
- 21.Haida M, Okudaira H, Ogita T, et al. Allergens to the house dust mite Dermatophagoides farinae—immunochemical studies of four allergenic fractions. J Allergy Clin Immunol. 1985;75:686–92. doi: 10.1016/0091-6749(85)90094-6. [DOI] [PubMed] [Google Scholar]
- 22.Yasueda H, Mita H, Yui Y, et al. Isolation and characterization of two allergens from Dermatophagoides farinae. Int Arch Allergy Immunol. 1986;81:214–23. doi: 10.1159/000234137. [DOI] [PubMed] [Google Scholar]
- 23.Kimura M, Tsuruta S, Yoshida T. Correlation of house dust mite-specific lymphocyte proliferation with interleukin 5 production, eosinophilia, and the severity of symptoms in infants with atopic dermatitis. J Allergy Clin Immunol. 1998;101:84–89. doi: 10.1016/S0091-6749(98)70197-6. [DOI] [PubMed] [Google Scholar]
- 24.Okudaira H, Ito K, Miyamoto T, et al. Evaluation of new system for the detection of IgE antibodies (CAP) in atopic diseases. Jpn J Allergol. 1991;40:544–54. (in jpn) [PubMed] [Google Scholar]