Abstract
In the present study, the sensitivity of LMVEC and human umbilical vein endothelial cells (HUVEC) to lipopolysaccharide (LPS) and the proinflammatory cytokines IL-1, tumour necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ) was compared. To this end, the production of the CC- (MCP-1), CXC- (IL-8, ENA-78, Groα, NAP-2, GCP-2) and CX3C (fractalkine) chemokines was studied. A low basal production of these chemokines was observed in both cell types. TNF-α, IL-1 and LPS up-regulated all chemokines tested. IFN-γ however was only able to up-regulate MCP-1 production. LMVEC were more sensitive to IL-1 and LPS compared with HUVEC, since LMVEC produced significantly more MCP-1, ENA-78 and Groα (P < 0.01) under these conditions. Maximal production of MCP-1 in LMVEC was achieved with TNF-α (28.4 ng/ml, P < 0.01), whereas IL-1 was the most potent stimulator of ENA-78 (2.78 ng/ml, P < 0.001) and Groα (29.2 ng/ml, P < 0.001). IL-8 production in LMVEC cells was maximal after LPS stimulation (28.4 ng/ml), but lower than on HUVEC (P < 0.01). LPS, TNF-α and IL-1 stimulation strongly up-regulated all chemokine mRNA. No quantitative differences in mRNA expression between LMVEC and HUVEC were detected for MCP-1 and Groα after LPS stimulation. mRNA expression of ENA-78, GCP-2, CX3C and NAP-2 was however higher in LMVEC under LPS stimulation. In contrast, IL-8 mRNA was slightly more expressed in HUVEC under these conditions. These results further support the hypothesis that the microvascular lung endothelium plays an active role in the induction and perpetuation of acute lung injury.
Keywords: microvascular endothelial cells, chemokines, inflammation
INTRODUCTION
Acute respiratory distress syndrome (ARDS) is one of the major organ manifestations of the multisystem organ failure syndrome (MOFS) with a mortality ranging from 10 to 90% [1]. The initial phase of ARDS is characterized by a profound accumulation of neutrophils and macrophages in airspace, bronchoalveolar lavage fluid (BALF) and macro- and microcirculation. Further diapedesis of neutrophils through the endothelial cell layer is facilitated by an increased endothelial permeability. and in this ‘general leakage’ the interaction of polymorphonuclear leucocytes (PMN) and LMVEC is thought to play a pivotal role. Most of the data concerning endothelial cell function with respect to acute lung injury have been obtained by analysing macrovascular endothelium. However, the migration of neutrophils into inflamed lung tissue takes place in microvascular endothelial cells because of lower bloodstream velocity. LMVEC may differ in many aspects from macrovascular cells and these differences may be relevant to the pathogenesis of ARDS. Microvascular endothelium is described as a target for lipopolysaccharide (LPS) during Gram-negative sepsis, for cytokines like IL-1 and tumour necrosis factor-alpha (TNF-α), released by macrophages and monocytes during inflammatory processes. In response to these factors, endothelial cells up-regulate the expression of adhesion molecules and release chemokines which are recognized by leucocytes. Chemokines that are predominantly attractant for neutrophils belong to the CXC-chemokine family (IL-8, growth-related cytokine family (Groα), epithelial neutrophil activation peptide (ENA-78), neutrophil activating peptide (NAP) and granulocyte chemotactic protein-2 (GCP-2)) and to the CC-chemokine family (monocyte chemotactic peptide-1 (MCP-1)).
Recently a new class of chemokines with a CX3C-motif (fractalkine) was identified. CX3C can be induced on human umbilical vein endothelial cells (HUVEC), which leads to a strong adhesion and migration of leucocytes in vitro [2]. It has a special position within the groups of chemokines, since it is not only secreted like all chemokines, but it is also expressed as membrane-bound antigen [3].
The extreme susceptibility of lung tissue, compared with other tissues, to develop inflammation during endotoxaemia may be related to the high sensitivity of LMVEC to produce chemokines upon exposure to LPS or proinflammatory cytokines. To investigate this hypothesis, chemokine production by LMVEC and HUVEC was compared. Inasmuch as neutrophil accumulation is a prominent feature in the initial phase of ARDS, neutrophil attractant chemokines were mainly studied. Understanding the mechanisms that primarily lead to lung inflammation is of major importance for clinical practice, since therapeutic options are limited and patient improvement rarely occurs. LMVEC may be a promising in vitro model to study ARDS, in order to develop new therapeutic modalities.
MATERIALS AND METHODS
Isolation and culture of cells
HUVEC were isolated as has been described previously [4]. The isolated cells were cultured in Medium 199 (Gibco BRL, Eggenstein, Germany) supplemented with 10% fetal bovine serum (FBS), 20 μg/ml heparin, 100 U/ml penicillin, 100 μg/ml streptomycin and endothelial cell growth factor (ECGF) (all from Sigma, Deisenhofen, Germany) in gelatin-coated culture flasks (Falcon; Becton Dickinson GmbH, Heidelberg, Germany) at 37°C, 95% relative humidity and 5% CO2. Confluent monolayers were passaged by trypsin 0.025%/EDTA 0.01% (CellSystems, Remagen, Germany).
LMVEC were purchased from Clonetics (San Diego, CA) at passage 4. The cells were seeded at a density of 5000 cells/cm2 in T25 flasks (Falcon) and maintained according to the manufacturer's specification in Microvascular Endothelial Growth Medium (CellSystems) supplemented with FBS 5%, human epidermal growth factor 10 ng/ml, human fibroblast growth factor 4 ng/ml, vascular endothelial growth factor 2 ng/ml, ascorbic acid 75 μg/ml, hydrocortisone 0.2 μg/ml, heparin 1 μg/ml, and insulin 5 ng/ml. After confluence they were subcultured as described for HUVEC.
Both types of endothelial cell were characterized on the basis of their cobblestone morphology, uptake of acetylated low-density lipoprotein (LDL), positive staining for Factor VIII-related antigen and platelet-endothelial cell adhesion molecule-1 (PECAM) (CD31) and negative staining for α smooth muscle actin. A stabile expression of F VIII and CD31 was observed even after the passage 9.
Detection of chemokine production by ELISA
LMVEC or HUVEC (1 × 105 cells/ml) were seeded in 24-well plates (for HUVEC coated with gelatin) and grown to confluence. The cells were either unstimulated or stimulated with LPS from Escherichia coli (0.005–1 μg/ml) or human recombinant IL-1α (250 pg/ml) for 24 h and with human recombinant TNF-α (25 ng/ml) or interferon-gamma (IFN-γ; 250–100 ng/ml) (all from Sigma) for 48 h. Supernatants were collected and production of ENA-78, Groα, IL-8 and MCP-1 was measured by sandwich ELISA assay (R&D Systems GmbH, Wiesbaden, Germany). All ELISAs were performed according to the manufacturer's instructions.
Cyclohexamide treatment
Subcultured LMVEC were grown till confluence and either unstimulated or stimulated for 24 h with IL-1 1 ng/ml, LPS 0.5 μg/ml or TNF-α 25 ng/ml in the presence or absence of cyclohexamide (CX). Supernatants were harvested and chemokine production was measured by ELISA. To exclude a possible toxic effect of CX, cells that had been cultured in the presence of CX were washed and restimulated with the same cytokines and LPS in the absence of CX. After 24 h the supernatants were harvested again and used for chemokine determination.
RNA isolation and reverse transcriptase-polymerase chain reaction
LMVEC were grown to confluence in T25 flasks and incubated in the presence or absence of LPS 0.5 μg/ml, IL-1 1 ng/ml, TNF-α 25 ng/ml or IFN-γ 100 ng/ml. Total RNA was extracted using Trizol-Reagent (Gibco BRL) according to the manufacturer's instructions. To eliminate contamination of genomic DNA, all RNA preparations were treated with DNase I (Gibco BRL). Total RNA (2 μg) was reverse transcribed using SuperScript TM II Preamplification System (Life Technologies, Karlsruhe Germany) according to the manufacturer's instructions.
The oligonucleotides (primers) used for polymerase chain reaction (PCR) are listed in Table 1. Primers were purchased from Perkin Elmer (Perkin Elmer Applied Biosystems, Weiterstadt, Germany). cDNA (1 μl), 50 pmol of each primer and 0.5 pmol Taq-DNA-polymerase were added to a final volume of 50 μl (10 mm Tris–HCl (pH 8.8 for CX3C, GCP-2, ENA-78, Groα, and pH 9.2 for MCP-1, IL-8 and NAP-2), 75 mm KCl, 1.5 mm MgCl2 and 200 μm dNTPs. A control, containing no cDNA, was always included. The mixture was heated at 94°C for 5 min followed by 28–35 cycles, each consisting of incubation for 2.5 min at 94°C, 1.5 min at 55°C or 56°C and 1 min at 72°C. After termination of the last cycle the samples were chilled at 4°C. For each primer pair the optimal cycle number was determined by plotting the intensity of the bands against the cycle number. Quantification of PCR products was performed by serial dilutions of cDNA and standardized for equal expression of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene PCR product. PCR products were subjected to electrophoresis in 1% agarose (Serva, Boehringer Ingelheim, Heidelberg, Germany).
Table 1.
Primers used in this study
Prirner sequences and length in base pairs (bp) of the arnplicon are depicted.
Statistical analysis
Statistical analysis was performed using Stata Statistical software (Mann–Whitney test).P < 0.05 was considered significant.
RESULTS
Chemokine production by LMVEC and HUVEC
The production of neutrophil attractant chemokines was studied in LMVEC and HUVEC. A basal production of MCP-1, IL-8, ENA-78 and Groα was found in both cell lines. Statistically significant differences in basal production were found for Groα (P < 0.01), IL-8 (P < 0.01) and MCP-1 (P < 0.001). Chemokine production was strongly up-regulated by TNF-α, IL-1 or LPS. The production of ENA-78 was relatively low in comparison with MCP-1, IL-8 or Groα in both cell types. Whereas TNF-α was more potent in stimulating MCP-1 and IL-8 production in HUVEC compared with LMVEC, IL-1 and LPS were significantly more potent in the up-regulation of MCP-1, ENA-78 and Groα in LMVEC. IL-8 production however was significantly higher in HUVEC (P < 0.01) in comparison with LMVEC. IFN-γ did not influence ENA-78 or Groα, IL-8 only weakly MCP-1 production in LMVEC (Fig. 1).
Fig. 1.
MCP-1, IL-8, ENA-78 and Groα production by LMVEC and human umbilical vein endothelial cells (HUVEC). LMVEC (□) and HUVEC (▪) were stimulated with lipopolysaccharide (LPS; 0.5 μg/ml) or IL-1 (1 ng/ml) for 24 h and tumour necrosis factor-alpha (TNF-α; 25ng/ml) or IFN-γ (250 ng/ml) for 48 h. Thereafter the supernatants were collected and assessed for chemokine production. Results are expressed as mean production (ng/ml) of triplicate cultures. Basal production for MCP-1, ENA-78, IL-8 and Groα in LMVEC versus HUVEC were: MCP-1, 1.5 ng/ml versus 0.64 ng/ml, P < 0.01; ENA-78, 0.04 ng/ml versus 0.027 ng/ml, NS; IL-8, 1.3 ng/ml versus 0.49 ng/ml, P < 0.01; Groα, 1.5 ng/ml versus 0.23 ng/ml, P < 0.01. *P < 0.05, statistically significant difference compared with unstimulated control group.
Up-regulation of chemokine production by LPS and IL-1 was time- and dose-dependent in both cell lines. Maximal production was achieved after 72 h of stimulation with 0.5 μg/ml LPS and 1 ng IL-1 (data not shown).
To study if the up-regulation of chemokine production was due to de novo synthesis, LMVEC were stimulated or not with LPS, TNF-α or IL-1 in the presence or absence of CX. CX completely blocked chemokine production. After removal of CX, LMVEC were still able to respond to LPS, TNF-α and IL-1, although to a lesser extend than LMVEC that were not treated with CX (Table 2).
Table 2.
Reversible inhibition of MCP-1 production by cyclohexamide (CX)

Column A: LMVEC were stimulated or not with IL-1 (1 ng/ml) (2a), tumour necrosis factor-alpha (TNF-α; 25 ng/ml) (2b) or lipopolysaccharide (LPS; 0.5 μg/ml) (2c) in the presence or absence of CX. Thereafter supernatants were collected and chemokine production was measured by ELISA. Column B: LMVEC were washed and stimulated as in A with IL-1, TNF-α or LPS in the absence of CX. Supernatants were collected and immediately assessed for chemokine production. The results are expressed as mean production (ng/ml) of triplicate cultures. Similar findings were observed for ENA-78 and Groα (data not shown).
Chemokine mRNA expression in LMVEC and HUVEC
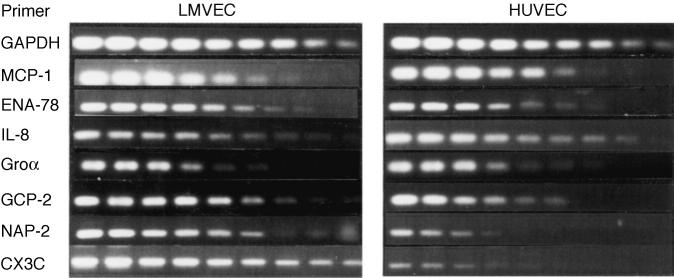
A basal mRNA expression for all chemokines, except NAP-2 and CX3C, was found in both cell lines (data not shown). LPS, TNF-α and IL-1 stimulation strongly up-regulated all chemokine mRNA. No quantitative differences in mRNA expression between LMVEC and HUVEC were detected for MCP-1 and Groα after LPS stimulation. The mRNA expression of ENA-78, GCP-2, CX3C and NAP-2 was however higher in LMVEC under LPS stimulation. In contrast, IL-8 mRNA was slightly more expressed in HUVEC under these conditions (Fig. 2). Similar findings were observed in IL-1- or TNF-α-stimulated LMVEC and HUVEC (data not shown).
Fig. 2.
Chemokine mRNA expression in lipopolysaccharide (0.5 μg/ml)-stimulated LMVEC and human umbilical vein endothelial cells (HUVEC). Total RNA was isolated and reverse transcribed in cDNA as described in Materials and Methods. Serial dilutions of cDNA were amplified and standardized for equal expression of glyceraldehyde-3-phosphate dehydrogenase.
Involvement of endogenously produced TNF-α in LPS stimulation
Since TNF-α itself is able to up-regulate chemokine production, we examined whether LPS-induced chemokine production was mediated via endogenously produced TNF-α. To this end, LMVEC were stimulated with TNF-α or LPS in the presence or absence of anti-TNF-α antibodies. Up-regulation of MCP-1, ENA-78 or Groα production by TNF-α was completely blocked by these antibodies. However, anti-TNF-α antibodies were only able to block the up-regulation of ENA-78 production after LPS stimulation, suggesting that this was mediated by endogenously produced TNF-α (Fig. 3).
Fig. 3.

. MCP-1, ENA-78 and Groα production by LMVEC after stimulation with either lipopolysaccharide (LPS) or tumour necrosis factor-alpha (TNF-α) in the presence or absence of anti-TNF-α antibodies. Cells were grown to confluence in 24-well plates and stimulated with either TNF-α 25 ng/ml or LPS 0.5 μg/ml in the presence or absence of anti-TNF-α antibodies. Supernatants were collected and assessed for chemokine production by ELISA. Results are expressed as mean production (ng/ml) of triplicate cultures. *P < 0.05, statistically significant difference compared with unstimulated control group.
DISCUSSION
A common pathophysiologic denominator in the development of ARDS seems to be the microvascular system. The participation of LMVEC and their elevated expression of chemoattractant cytokines is likely to be critical for the orchestration of the directed migration of leucocytes into the lung [5]. Apart from endotoxin, ‘early response cytokines' as well as IFN have been implicated in the inflammatory process [6–8]. Enhanced levels of TNF-α, IL-1 and IL-8 in the plasma of patients with ARDS have been described and are associated with patient outcome [9]. Moreover, it was found that high concentrations of IL-8 and ENA-78 significantly correlated with PMN concentrations in BALF [10]. These facts underline the clinical relevance of studies such as this on the regulation of chemokine production by LMVEC. Susceptibility of lung tissue to inflammation may be associated with differences in the regulation of cytokine production between endothelial cells of different organs. In the present study the chemokine spectrum of unstimulated and stimulated LMVEC and HUVEC was therefore compared.
The chemokine spectra of unstimulated LMVEC and HUVEC are both qualitatively and quantitatively different. Whereas CX3C and NAP-2 mRNA was detected in unstimulated LMVEC, it was absent in HUVEC. In addition it was found in this study that the basal production of Groα, IL-8 and MCP-1 was significant higher in LMVEC. These findings underline the differences in cytokine response between lung endothelial cells and endothelial cells of other tissues, and thus may explain the susceptibility of lung tissue in inflammation. MCP-1 is described to be chemoattractant for monocytes, T lymphocytes, basophils and neutrophils. It is expressed by a variety of cell types in response to different stimuli. Similar to previous findings obtained in HUVEC [11], an up-regulation of MCP-1 production was observed after stimulation of LMVEC with IFN-γ. In contrast, IFN-γ did not influence the production of ENA-78, IL-8 or Groα.
MCP-1 was de novo synthesized upon stimulation with LPS, IL-1 or TNF-α, since no MCP-1 could be detected in supernatants of stimulated or unstimulated LMVEC that were cultured in the presence of CX.
IL-8 is the prototypic and most extensively studied CXC-chemokine. It is present in many tissues and is up-regulated during inflammation, thereby supporting the migration of neutrophils through endothelial and epithelial cell layers [12]. Furthermore, IL-8 may directly stimulate endothelial cell chemotaxis and angiogenesis [13]. High concentrations of IL-8 were found in both BALF and serum of patients with ARDS [10,14,15]. In our study it was found that IL-8 production was higher in HUVEC than in LMVEC under all stimulatory conditions tested. These findings are in contrast to the results obtained with ENA-78, MCP-1 and Groα, that are preferentially more highly expressed in LMVEC after IL-1, LPS, but not after TNF-α stimulation. ENA-78, MCP-1 and Groα may therefore play an important role in the local recruitment of neutrophils into the lung during sepsis.
Groα and ENA-78 are potent chemoattractants for neutrophils in vivo and in vitro [16,17]. Groα was originally described as endogenous growth factor for human melanoma cells [18]. It binds, like IL-8, ENA-78 and other neutrophil activating chemokines, to the CXCR2 receptor. Up-regulation of Groα production after stimulation with LPS or proinflammatory cytokines has previously been demonstrated in macrovascular endothelial cells [19]. In LMVEC, a low basal production of Groα was measured, which was strongly up-regulated after LPS, TNF-α and IL-1 stimulation. Similar to MCP-1 production, LMVEC produced significantly higher amounts of Groα than HUVEC. ENA-78 was also detected in supernatants of IL-1-, LPS- and TNF-α-stimulated LMVEC or HUVEC.
The stimulatory effect of LPS on ENA-78 production was due to the endogenous production of TNF-α, since ENA-78 was not up-regulated by LPS in the presence of anti-TNF antibodies.
Similar to other studies, it was found that endothelial cells produce more Groα than ENA-78 [20]. ENA-78 is expressed by many cells, such as epithelial cells and stimulated macrovascular cells [21]. To our knowledge this is the first report on ENA-78 production by LMVEC.
Expression of NAP-2 and GCP-2 mRNA was studied by reverse transcriptase (RT)-PCR. GCP-2 induces chemotaxis, enzyme release and calcium mobilization in human leucocytes [22]. NAP-2 was found to be secreted by monocytes and platelets after LPS treatment [23]. It is chemotactic for neutrophils, although less efficiently than the homologous chemokine IL-8 [24]. In patients with ARDS, an increased concentration of NAP-2 in BALF, but not in plasma, has been reported [25,26]. It thus seems that the local production of NAP-2 may be of relevance in the onset or perpetuation of the inflammatory process. NAP-2 mRNA but not GCP-2 mRNA was detected in unstimulated LMVEC. Both were up-regulated after stimulation with IL-1, TNF-α and LPS. Interestingly, it was found that NAP-2 mRNA expression was only weakly influenced by LPS in HUVEC, again underlining the heterogeneity in vascular endothelium.
CX3C has a special position among chemokines, because it is not only secreted but also expressed as membrane antigen. Since relatively little is known about the regulation of fractalkine expression, this was investigated in this study. Significantly more CX3C mRNA was detected in LMVEC after IL-1, TNF-α and LPS stimulation in comparison with HUVEC. Under basal conditions, CX3C mRNA was only expressed in LMVEC, but not in HUVEC.
In conclusion, our data demonstrate that human LMVEC are more sensitive to LPS and IL-1 than HUVEC. Our results provide evidence for an active role of microvascular endothelial cells in the induction or perpetuation of acute lung injury. These specific properties of LMVEC may explain, at least partially, why the lung is so vulnerable to damage in situations with generalized cytokine release or endotoxaemia. The high sensitivity to LPS might be particularly important in this respect.
REFERENCES
- 1.Bernard G, Artigas A, Brigham K. Report of the American-European Consensus Conference on Acute Respiratory Distress Syndrome. J Crit Care. 1994;9:72–81. doi: 10.1016/0883-9441(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 2.Bazan JF, Bacon KB, Hardiman G, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–4. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 3.Imai T, Hieshima K, Haskell C, et al. Identification and molecular characterisation of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–30. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 4.Jaffe EA, Nachmann RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins: identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–56. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCuscey R, Urbaschek R, Urbaschek B. The microcirculation during endotoxemia. Cardiovasc Res. 1996;32:752–63. [PubMed] [Google Scholar]
- 6.Demling R. The modern version of adult respiratory distress syndrome. Annu Rev Med. 1995;46:193–202. doi: 10.1146/annurev.med.46.1.193. [DOI] [PubMed] [Google Scholar]
- 7.Ward PA. Role of complement, chemokines and regulatory cytokines in acute lung injury. Ann NY Acad Sci. 1996;31:104–12. doi: 10.1111/j.1749-6632.1996.tb32572.x. [DOI] [PubMed] [Google Scholar]
- 8.Crockett-Torabi E, Ward PA. The role of leukocytes in tissue injury. Eur J Anaest. 1996;13:235–46. doi: 10.1046/j.1365-2346.1996.00982.x. [DOI] [PubMed] [Google Scholar]
- 9.Meduri UG, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, Leeper K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Chest. 1995;107:1062–73. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 10.Goodman RB, Strieter RM, Martin DP, Steinberg KP, Martin TR. Inflammatory cytokines in patients with persistence of acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154:602–11. doi: 10.1164/ajrccm.154.3.8810593. [DOI] [PubMed] [Google Scholar]
- 11.Douglas MS, Ali S, Rix A, Zhang JG, Kirby JA. Endothelial production of MCP-1: modulation by heparin and consequences for mononuclear cell activation. Immunol. 1997;92:512–8. doi: 10.1046/j.1365-2567.1997.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smart SJ, Casale TB. Interleukin-8-induced transcellular neutrophil migration is facilitated by endothelial and pulmonary epithelial cells. Am J Respir Cell Mol Biol. 1993;9:489–95. doi: 10.1165/ajrcmb/9.5.489. [DOI] [PubMed] [Google Scholar]
- 13.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 (IL-8) as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 14.Schutte H, Lohmeyer J, Rosseau S, et al. Bronchoalveolar and systemic cytokine profiles in patients with ARDS, severe pneumonia and cardiogenic pulmonary oedema. Eur Respir J. 1996;9:1858–67. doi: 10.1183/09031936.96.09091858. [DOI] [PubMed] [Google Scholar]
- 15.Grau GE, Mili N, Lou JN, Morel DR, Ricou B, Lucas R, Suter PM. Phenotypic and functional analysis of pulmonary microvascular endothelial cells from patients with acute respiratory distress syndrome. Lab Invest. 1996;74:761–70. [PubMed] [Google Scholar]
- 16.Murakami K, Ueno A, Yamanouchi K, Kondo T. Thrombin induces GROα/mGSA production in human umbilical vein endothelial cells. Thrombosis Res. 1995;79:387–94. doi: 10.1016/0049-3848(95)00127-d. [DOI] [PubMed] [Google Scholar]
- 17.Imaizumi T, Albertine K, Jicha DL, McIntyre TM, Prescott SM, Zimmermann GA. Human endothelial cells synthesize ENA-78: relationship to IL-8 and to signaling of PMN adhesion. Am J Respir Cell Mol Biol. 1997;17:181–92. doi: 10.1165/ajrcmb.17.2.2818. [DOI] [PubMed] [Google Scholar]
- 18.Thomas HG, Richmond A. High yield purification of melanoma growth stimulatory activity. Mol Cell Endocrinol. 1988;57:69–76. doi: 10.1016/0303-7207(88)90033-0. [DOI] [PubMed] [Google Scholar]
- 19.Wen DZ, Rowland A, Derynck R. Expression and secretion of Gro/mGSA by stimulated endothelial cells. EMBO J. 1989;8:1761. doi: 10.1002/j.1460-2075.1989.tb03569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Proost P, De Wolf-Peeters C, Conings R, Opdenakker G, Billiau A, Van Damme J. Identification of a novel granulocyte chemotactic protein (GCP-2) from human tumor cells. In vitro and in vivo comparison with natural forms of GRO, IP-10 and IL-8. J Immunol. 1993;150 [PubMed] [Google Scholar]
- 21.Walz A, Burgener R, Car B, Baggiolini M, Kunkel SL, Strieter RM. Structure and neutrophil-activating properties of a novel inflammatory peptide (ENA-78) with homology to interleukin-8. J Exp Med. 1991;174:1355–62. doi: 10.1084/jem.174.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Proost P, Wuyts A, Conings R, Lenaerts JP, Billau A, Openakker G, Van Damme J. Human and bovine granulocyte chemotactic protein-2: complete amino acid sequence and functional characterisation as chemokines. Biochemistry. 1993;32:10170–7. doi: 10.1021/bi00089a037. [DOI] [PubMed] [Google Scholar]
- 23.Walz A, Baggiolini M. A novel cleavage product of betathromboglobulin formed in cultures of stimulated mononuclear cells activates human neutrophils. Biochem Biophys Res Commun. 1989;180:969–75. doi: 10.1016/0006-291x(89)92203-1. [DOI] [PubMed] [Google Scholar]
- 24.Walz A, Dewald B, Vontscharner V, Baggiolini M. Effects of the neutrophil-activating peptide NAP-2, platelet basic protein, connective tissue-activating peptide III and platelet factor 4 on human neutrophils. J Exp Med. 1998;170:1745–50. doi: 10.1084/jem.170.5.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen AB, Stevens MD, Miller EJ, et al. Neutrophil-activating peptide-2 in patients with pulmonary edema from congestive heart failure or ARDS. Am J Physiol. 1993;264:L490–L495. doi: 10.1152/ajplung.1993.264.5.L490. [DOI] [PubMed] [Google Scholar]
- 26.Miller EJ, Cohen AB, Nagao S, et al. Elevated levels of NAP/interleukin-8 are present in the airspace of patients with adult respiratory distress syndrome and are associated with increased mortality. Am Rev Respir Dis. 1992;146:427–32. doi: 10.1164/ajrccm/146.2.427. [DOI] [PubMed] [Google Scholar]