Abstract
Recent studies have identified a key role for macrophage migration inhibitory factor (MIF) in a number of immune cell-mediated diseases. The current study investigated the potential role of MIF in acute allograft rejection. Lewis rats underwent bilateral nephrectomy and then received an orthotopic DA renal allograft or an orthotopic Lewis renal isograft. Groups of six animals were killed at day 1 or 5 after transplantation. No immunosuppression was used. Animals receiving a renal allograft exhibited severe rejection on day 5, as shown by high levels of serum creatinine, very low rates of creatinine clearance, and severe tubulitis with a dense macrophage and T cell infiltrate. In contrast, isografts had normal renal function on day 5 with no histological evidence of rejection. Northern blotting showed that renal MIF mRNA expression was unchanged at day 1, but was increased 3.5-fold on day 5. In situ hybridization showed a marked increase in MIF mRNA expression by tubular cells and MIF mRNA expression by many infiltrating mononuclear cells in day 5 allografts. Immunostaining confirmed an increase in tubular MIF protein expression, particularly in areas of severe tubular damage with prominent leucocytic infiltration. Double staining showed that many infiltrating macrophages and T cells expressed the MIF protein in day 5 allografts. There was only a minor increase in MIF expression in day 5 isografts, demonstrating that neither surgical injury nor stress cause significant up-regulation of MIF expression in allograft rejection. In conclusion, this study has demonstrated that local MIF production is specifically increased in acute renal allograft rejection. These results suggest that MIF may play an important role in the cellular immune response mediating acute allograft rejection.
Keywords: macrophage migration inhibitory factor, transplantation, macrophage, T cell, immunohistochemistry
INTRODUCTION
Macrophage migration inhibitory factor (MIF) was originally described as a product of activated lymphocytes which inhibits the migration of guinea pig macrophages in vitro, and promotes the skin DTH reaction [1,2]. It is now apparent that MIF is produced by a wide range of cell types, including intrinsic renal glomerular and tubular epithelial cells, and is synthesized in most tissues [3–6]. As exemplified by experimental glomerulonephritis, local MIF production is up-regulated in association with macrophage and T cell infiltration and tissue damage in immune-mediated disease [6]. Using blocking antibodies, it has been demonstrated that MIF plays a pivotal role in experimental models of endotoxaemia, the skin DTH reaction, crescentic glomerulonephritis and arthritis [7–13].
An unexpected and unique function of MIF is its ability to counter-regulate glucocorticoid action. MIF can overcome glucocorticoid-mediated inhibition of endotoxin-induced macrophage cytokine production [14]. This is also evident in vivo with administration of recombinant MIF overriding dexamethasone inhibition of lethal endotoxaemia in mice [14]. Furthermore, MIF appears to be involved in the response to stress on the basis that physiological doses of dexamethasone up-regulate macrophage MIF production, endotoxic shock causes release of MIF into the circulation, and that MIF is released from the AtT-20 pituitary corticotrophic cell line following stimulation with corticotrophin-releasing factor [4,7,14].
Acute allograft rejection is a T cell-dependent process in which the graft becomes infiltrated with large numbers of T cells and macrophages [15,16]. T cells can cause graft injury through cytotoxic mechanisms and indirectly via the recruitment and activation of macrophages in a DTH mechanism. Furthermore, macrophage infiltration within human renal allografts is the single best histological predictor of graft survival [17]. Based upon its known functions, it is postulated that MIF may play a pathological role in acute allograft rejection.
The aim of this study was to examine MIF mRNA and protein expression in a rat model of acute renal allograft rejection. This model was performed in the absence of immunosuppressive treatment so as to give a clear picture of MIF expression in the rejection process. In addition, an isograft control was included to assess the potential contribution of surgical stress to MIF expression within the graft.
MATERIALS AND METHODS
Model of acute renal allograft rejection
A group of 24 inbred male Lewis rats (RT11) (200–385 g) underwent bilateral nephrectomy of which 12 received an orthotopic DA (RT1a) renal allograft and 12 received an orthotopic Lewis renal isograft. The donor rat was anaesthetized, the left kidney removed and flushed in situ with cold preservative solution and stored in that solution on ice until transplantation (approx. 30 min). The recipient rat was anaesthetized, the left kidney removed and then replaced orthotopically with the graft. The recipient left renal artery was telescoped into the donor renal artery, and the recipient renal vein joined to the donor renal vein using an external cuff. The donor and recipient ureters were joined over a short polyethylene stent after the kidney was revascularized. The right kidney was then removed. Groups of six animals were killed on day 1 and on day 5 post-transplantation. No immunosuppression was administered. Kidney specimens were collected from Lewis rats at the time of the bilateral nephrectomy and used as the normal kidney control.
Renal function
Blood samples and 24 h urine collections were taken from six Lewis rats prior to the transplantation to serve as the normal controls. Blood and 24 h urine collections were taken on day 1 or day 5 after transplantation. Serum and urine creatinine levels were measured using a modified Jaffe method and urinary protein excretion was determined by the Ponceau red method. All analyses were performed by the Department of Biochemistry, Monash Medical Centre.
Histopathology
Renal tissues were fixed in 4% buffered formalin and 4-μm paraffin sections were stained with haematoxylin and eosin. The degree of tubular rejection was graded on a scale of 0–4 on the basis of the percentage of the cortex containing necrotizing tubulitis and mononuclear cell infiltration: 0, no lesions; 1, lesions in 0–10%; 2, 10–25%; 3, 25–50%; and 4, > 50% of the cortex.
Probes
Plasmids containing a 420-bp fragment of mouse MIF cDNA [18], and a 358-bp fragment of rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA [19], were used to prepare digoxigenin (DIG)-labelled anti-sense and sense cRNA probes according to the manufacturer's protocol (Boehringer Mannheim GmbH, Mannheim, Germany). Probes were precipitated and DIG incorporation assessed by dot blotting. For Northern blotting, a 440-bp DIG-labelled, single-stranded cDNA probe for rat MIF was prepared by polymerase chain reaction (PCR).
Northern blotting
Northern blotting was performed as previously described [20]. Total cellular RNA from a half kidney was extracted using the RNAzol reagent (Gibco BRL, Gaithersburg, MD), and 10-μg samples were denatured with glyoxal and dimethylsulphoxide, size fractionated on 1.2% agarose gels and capillary blotted onto positively charged nylon membranes (Boehringer Mannheim). Membranes were hybridized overnight with DIG-labelled probes in DIG Easy Hyb solution (Boehringer Mannheim), washed, and bound probes were detected using sheep anti-DIG antibody (Fab) conjugated with alkaline phosphatase and development with CPD-star enhanced chemiluminescence (Boehringer Mannheim). Chemiluminescence emissions were captured on Kodak XAR film and densitometry analysis performed using the Gel-Pro Analyser program (Media Cybernetics, Silver Spring, MD).
In situ hybridization
In situ hybridization was performed on 4-μm paraffin sections of formalin-fixed tissue using a microwave-based protocol as described previously [5,10]. Sections were heated for 10 min in a microwave oven in 10 mm sodium citrate pH 6.0, incubated in 0.2 m HCl for 15 min and then 1% Triton X-100 for 15 min before digestion for 20 min with 10 μg/ml proteinase-K (Boehringer Mannheim) at 37°C. Sections were washed in 2× SSC, prehybridized and then hybridized with MIF cRNA sense or anti-sense probes overnight at 42°C in a hybridization buffer containing 50% deionized formamide, 4 × SCC, 1 mg/ml salmon sperm DNA and 1 mg/ml yeast tRNA. Sections were then washed in 0.1 × SCC at 42°C and the probe detected using alkaline phosphatase-conjugated sheep anti-DIG F(ab) fragments and colour development with NBT/X-phosphate. Sense control probes gave no signal.
Antibodies
The following MoAbs were used in this study: IIID9, mouse MoAb raised against recombinant human MIF which cross-reacts with rat MIF [10]; ED1, anti-rat CD68 labels most monocytes and macrophages [21]; R73, recognizes a non-polymorphic epitope of the αβ T cell receptor [22]. Peroxidase and alkaline phosphatase-conjugated goat anti-mouse IgG, mouse peroxidase–anti-peroxidase complexes (PAP), and mouse alkaline phosphatase–anti-alkaline phosphatase complexes (APAAP) were all purchased from Dakopatts (Glostrup, Denmark).
Immunohistochemistry
One- and two-colour immunohistochemical staining was performed as described previously [5,10,23]. Paraffin sections (4 μm) were treated with 10 min of microwave oven heating in 10 mm sodium citrate pH 6.0, preincubated with 10% fetal calf serum (FCS) and 10% normal sheep serum in PBS for 20 min, drained and incubated with either III.D.9 or ED1 MoAb overnight at 4°C. Sections were then washed (× 3) in PBS, endogenous peroxidase-inactivated in 0.3% H2O2 in methanol, incubated with peroxidase-conjugated goat anti-mouse IgG followed by mouse PAP and developed with 3,3-diaminobenzidine to produce a brown colour. Detection of T cells using the R73 MoAb was performed on cryostat sections (6 μm) of tissue fixed in 2% paraformaldehyde-lysine-periodate using the same method, except for omission of the microwave step.
For two-colour immunostaining, sections were stained using ED1 or R73 MoAbs as above and then treated by microwave heating to denature bound antibodies and prevent antibody cross-reactivity [23]. Tissues were then preincubated as above, followed by a second preincubation in 10% bovine serum albumin for 20 min, drained and incubated sequentially with III.D.9, alkaline phosphatase-conjugated goat anti-mouse IgG and mouse APAAP and developed with fast blue BB salt (Ajax Chemicals, Melbourne, Australia) to produce a blue colour. Sections were mounted in an aqueous medium.
Quantification of immunohistochemistry staining
The number of immunostained cells was scored under high power (× 400) in 20 glomerular cross-sections per animal. Immunostaining of tubules and interstitial cells was scored in 20 high power fields (× 400) moving sequentially from the outer to inner to outer cortex in randomly selected areas, avoiding only glomeruli and large vessels. No adjustment was made for the tubular area. On average, 1000 tubules were scored for each animal. All scoring was performed on coded slides.
RESULTS
Renal function and histological damage
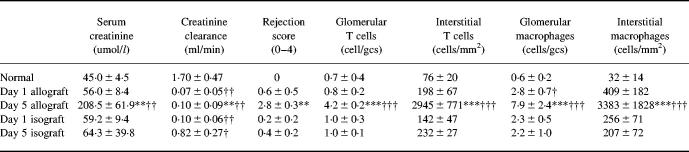
All animals receiving renal allografts exhibited severe rejection on day 5 post-transplantation, with highly elevated serum creatinine and very low rates of creatinine clearance (Table 1). Animals receiving a renal isograft had reduced levels of creatinine clearance during the first 24 h post-transplantation, but this recovered to 50% of the normal (baseline) creatinine clearance by day 5.
Table 1.
Renal function, histological analysis and leucocyte infiltration in renal allograft rejection
Data are mean plusmn; s.d.
†P < 0.05; ††P < 0.01; †††P < 0.001 versus normal; *P < 0.05; **P < 0.01; ***P < 0.001 versus day 5 isograft by anova.
gcs, Glomerular cross-section.
All allograft recipient animals exhibited histological evidence of severe rejection on day 5, with severe necrotic tubulitis and dense interstitial mononuclear cell infiltration (Fig. 1). Quantification of immunostaining showed a significant glomerular macrophage and T cell infiltrate and an intense interstitial macrophage and T cell infiltrate in day 5 allografts (Table 1). No significant vascular rejection was seen at day 5, despite the severe interstitial rejection. In contrast, little damage was seen in the renal isografts on day 5, with no significant macrophage or T cell infiltration (Fig. 1 and Table 1).
Fig. 1.
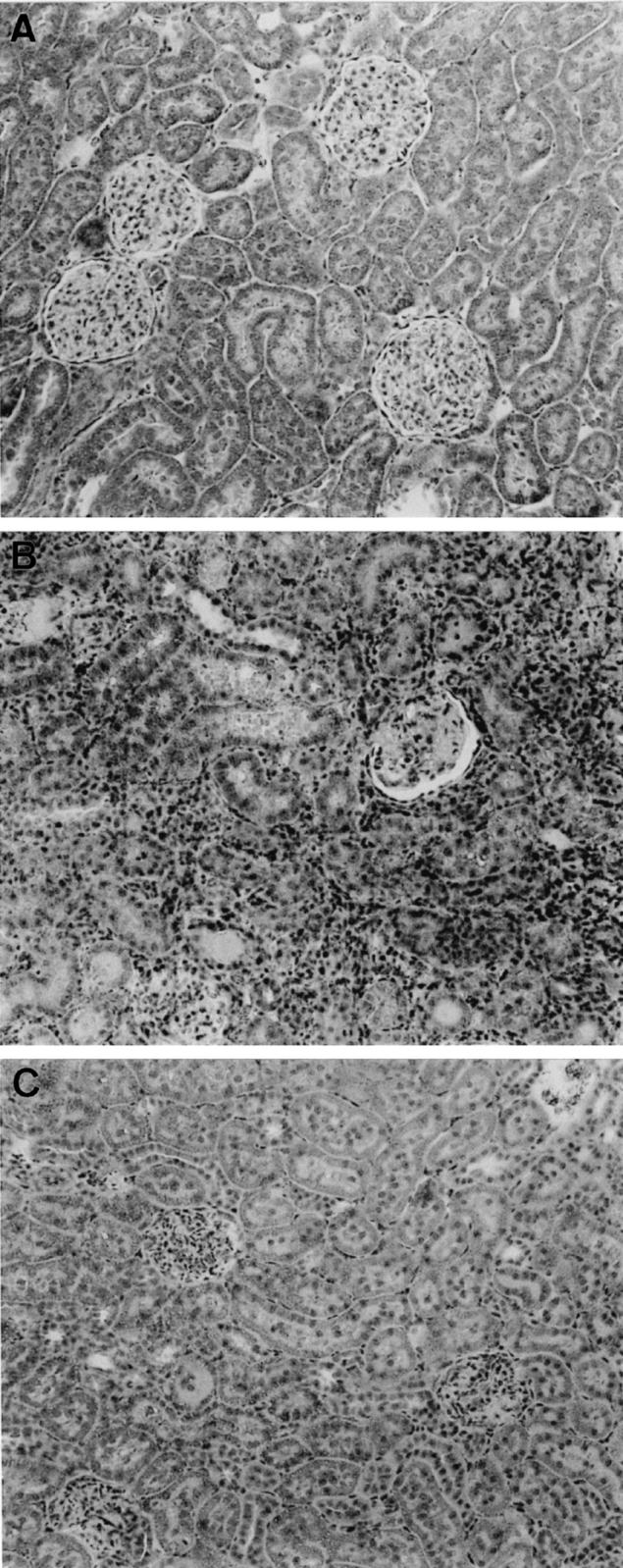
Haematoxylin and eosin staining of acute renal allograft rejection in the rat. (A) Normal rat kidney. (B) Day 5 renal allograft rejection showing severe tubulitis and a dense interstitial mononuclear cell infiltrate. (C) Day 5 renal isograft showing minimal tubulitis and no significant interstitial mononuclear cell infiltrate. (Mag. × 160.)
MIF gene expression
Northern blotting showed constitutive MIF mRNA expression in normal rat kidney (Fig. 2). There was no change in MIF gene expression in day 1 isografts or allografts. However, there was a 3.5-fold increase in MIF mRNA expression in day 5 allografts compared with normal rat kidney (Fig. 2). There was a two-fold increase in MIF mRNA levels in day 5 isografts, but this was not statistically significant (Fig. 2).
Fig. 2.
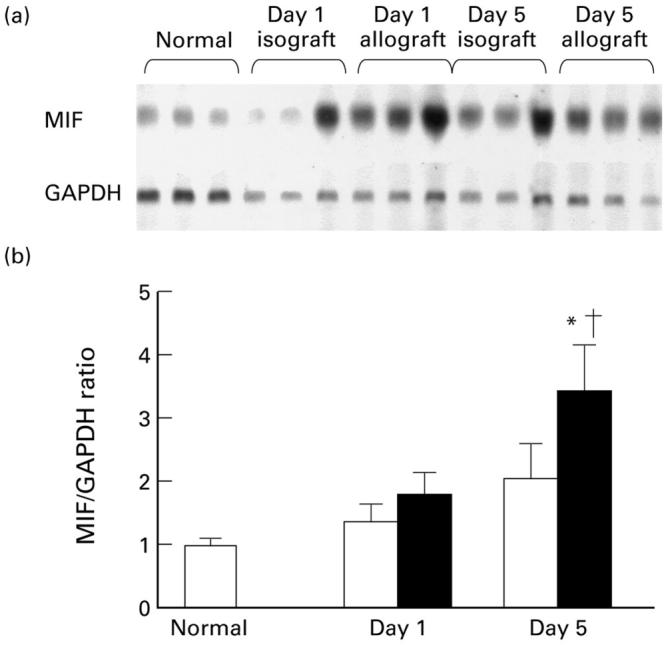
Renal macrophage migration inhibitory factor (MIF) mRNA expression in acute renal allograft rejection in the rat. (a) Northern blot of whole kidney RNA probed for MIF and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (b) Densitometric analysis of Northern blots showing the ratio of MIF to GAPDH mRNA expression in normal rats, isografts (□) and allografts (▪). Data are mean ± s.e.m. for groups of six animals. †P < 0.05 versus normal; *P < 0.05 versus day 5 isograft.
The distribution of MIF gene expression within grafts was assessed by in situ hybridization. There was constitutive expression of MIF mRNA by tubular epithelial cells and a few glomerular cells (mostly podocytes) in normal rat kidney (Fig. 3A). There was little change in the pattern of MIF mRNA expression in day 1 allografts and isografts compared with normal kidney. However, there was a marked increase in renal MIF mRNA expression on day 5 of allograft rejection (Fig. 3B). Many tubules, particularly in areas of severe damage, showed a strong hybridization signal MIF mRNA. Many of the infiltrating interstitial cells in areas of damage showed MIF mRNA expression (Fig. 3B). In contrast, MIF gene expression in the day 5 isograft controls was similar to that seen in normal kidney (Fig. 3C).
Fig. 3.
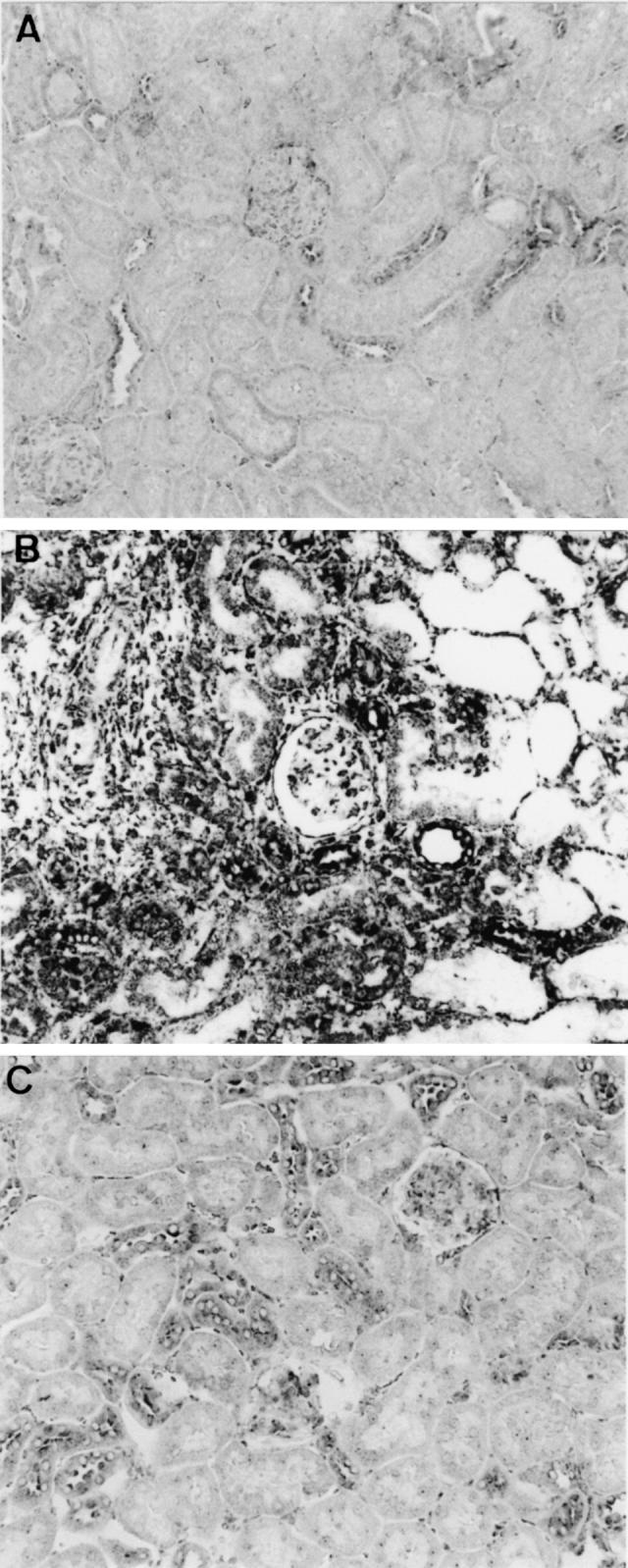
In situ hybridization using a digoxigenin (DIG)-labelled macrophage migration inhibitory factor (MIF) riboprobe in acute renal allograft rejection in the rat. (A) Normal rats kidney showing MIF mRNA expression in cortical tubular epithelial cells and some glomerular cells. (B) Day 5 allograft showing a dramatic increase in both the intensity and area of MIF mRNA expression. Most tubules show strong staining for MIF mRNA, especially in areas of severe damage. Many infiltrating interstitial cells are positive for MIF and there is an increase in the number of glomerular cells expressing MIF mRNA. (C) Day 5 isograft showing a small increase in the number of tubules expressing MIF mRNA compared with normal kidney, but the intensity of staining is much less than that seen in day 5 allografts. (Mag. × 160.)
MIF protein expression
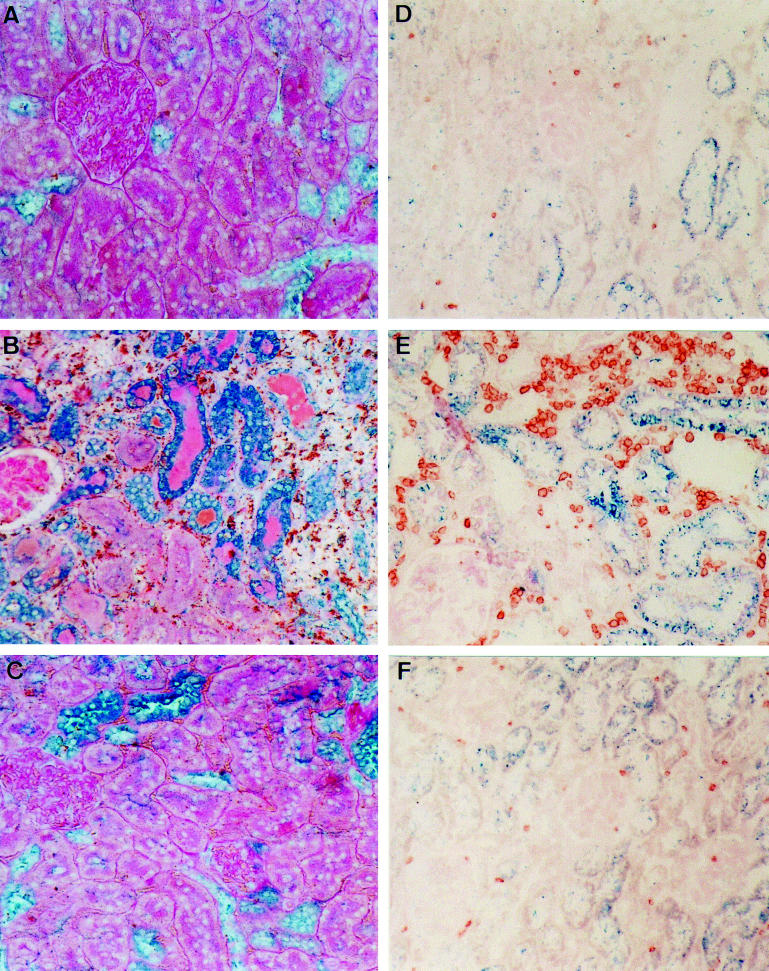
Consistent with the pattern of in situ hybridization, immunostaining with an anti-MIF MoAb showed MIF protein expression cortical tubules (35–51%), and a small number of glomerular cells (mostly podocytes) in normal Lewis rat kidney (Figs 4A and 5). Day 1 renal allografts and isografts showed a significant increase in the percentage of MIF+ tubules (Fig. 5). On day 5 of allograft rejection, there was a further increase in the percentage of tubules expressing MIF protein (79–96%), and a marked increase in the intensity of tubular MIF immunostaining (Figs 4B and 5). Strong tubular MIF immunostaining was seen in areas of severe damage in association with focal macrophage and T cell infiltration (Fig. 4B). Double immunostaining showed that many interstitial macrophages and T cells expressed MIF (Fig. 4B). In contrast, in areas where tubular morphology was reasonably well preserved there was weak MIF expression and mild leucocyte infiltration. In day 5 isografts, the pattern of MIF immunostaining was little changed compared with that seen in normal rat kidney (Figs 4C and 5).
Fig. 4.
(See previous page) Double immunohistochemistry staining of macrophage migration inhibitory factor (MIF) and leucocytes in acute renal allograft rejection in the rat. (A–C) Paraffin sections double stained for MIF (blue) and ED1+ macrophages (brown) and counterstained with periodic acid-Schiff (PAS) minus haematoxylin. (A) Normal rat kidney showing constitutive MIF expression by cortical tubular epithelial cells and the presence of occasional macrophages. (B) Day 5 renal allograft rejection showing a marked up-regulation in MIF immunostaining. Damaged tubules show strong MIF staining in association with prominent ED1+ macrophage infiltration. Many of the infiltrating macrophages are also stained blue for MIF protein. (C) Day 5 renal isograft showing little change in tubular MIF expression and few ED1+ macrophages. (D–F) Frozen sections double-stained for MIF (blue) and R73+ T cells (brown) with no counterstain. (D) Normal rat kidney showing MIF immunostaining in cortical tubules and the presence of occasional T cells. (E) Day 5 allograft rejection showing marked up-regulation of MIF staining in damaged tubules in association with prominent T cell infiltration. Some infiltrating T cells are double-stained for MIF. (F) Day 5 isograft showing a minor increase in the number of MIF-stained tubules, little damage and no significant T cell infiltrate. (Mag. × 160.)
Fig. 5.
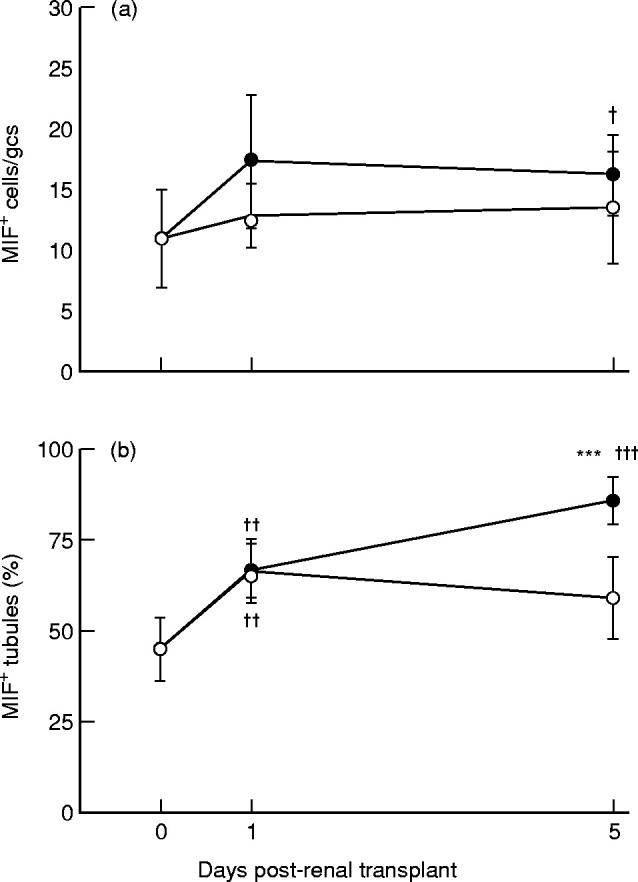
Quantification of macrophage migration inhibitory factor (MIF) immunostaining in acute renal allograft rejection in the rat. The number of: (a) glomerular cells, and (b) the percentage of tubules stained with the anti-MIF antibody were scored in normal rats (day 0), isografts (○) and allografts (•). gcs, Glomerular cross-section. Data are mean ± s.e.m. ***P < 0.001 versus day 5 isograft; †P < 0.05; ††P < 0.01; †††P < 0.001 versus normal by anova.
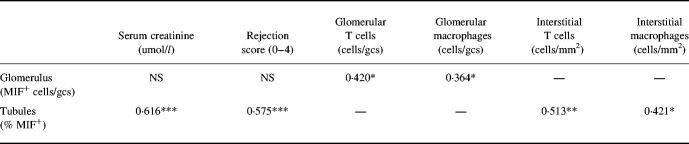
Correlation between MIF expression and transplant rejection
Tubular MIF expression was found to correlate with the severity of rejection, loss of renal function and interstitial T cell and macrophage infiltration (Table 2). There was a minor increase in glomerular MIF expression which correlated with the mild glomerular T cell and macrophage infiltrate, although little glomerular damage was evident. Glomerular MIF expression did not correlate with the severity of rejection or loss of renal function (Table 2).
Table 2.
Correlation macrophage of migration inhibitory factor (MIF) expression with serum creatinine, severity of graft rejection and macrophage and T cell accumulation
Data from all animals were analysed. The Pearson single correlation coefficient was used for all data except the rejection score (Spearman correlation coefficient).
*P < 0.05; **P < 0.01; ***P < 0.001.
gcs, Glomerular cross-section.
DISCUSSION
This study has demonstrated that MIF gene and protein expression is markedly up-regulated during acute renal allograft rejection in the rat. This pattern of increased MIF expression is similar to that previously described in rat crescentic glomerulonephritis [5]. The ability of neutralizing anti-MIF antibodies to substantially inhibit crescentic glomerulonephritis [10,11] suggests that the increased MIF expression in acute renal allograft rejection may also be of pathological significance. This postulate is supported by three observations. First, up-regulation of MIF expression was most prominent in areas with severe tubular damage and focal macrophage and T cell infiltration, whereas areas with little damage had weak MIF expression and only mild leucocytic infiltration. Second, there was a highly significant correlation between tubular MIF expression and loss of renal function, histological damage and interstitial leucocytic infiltration. Third, the up-regulation of MIF expression was specific to the rejection process. This latter point is supported by two findings. The increase in renal MIF mRNA and protein expression paralleled the development of allograft rejection, with little change on day 1 and marked up-regulation on day 5 when severe rejection was apparent. In addition, there was only a small increase in renal MIF expression in day 5 isografts, demonstrating that the non-specific effects of surgical trauma and stress make only a minor contribution to the increased renal MIF expression seen in allograft rejection.
There are several possible mechanisms by which MIF may participate in the rejection process. First, local MIF production may promote macrophage and T cell accumulation in the rejecting graft. This is based upon the ability of MIF to induce macrophage and T cell recruitment in the skin DTH response and in experimental glomerulonephritis [9,10]. Indeed, MIF can exert direct chemotactic effects upon macrophages [1], and it can induce expression of IL-1 and leucocyte adhesion molecules which play a crucial role in the recruitment process [10]. Second, MIF may promote T cell activation within the graft given the ability of anti-MIF antibodies to block primary T cell activation in vitro and in vivo [24]. Third, MIF is well known as a potent macrophage activator. Therefore, MIF production by T cells, or by intrinsic renal cells, may activate macrophages leading to the release of mediators that cause allograft damage.
It was interesting that there was increased MIF expression by intrinsic renal cells (glomerular and tubular epithelial cells) in addition to MIF expression by infiltrating macrophages and T cells. Assuming that MIF plays a pathogenic role in transplant rejection, it will be important to delineate the relative contribution of MIF production by intrinsic renal cells versus infiltrating leucocytes.
The results of the current study are consistent with a recent examination of MIF expression in human renal allograft rejection. Increased MIF expression was seen in biopsies of rejecting renal allografts, whereas biopsies from non-rejecting grafts showed normal levels of MIF expression [25]. In addition, the percentage of MIF+ tubules correlated with the loss of renal function, the severity of graft rejection, and leucocytic infiltration.
In conclusion, MIF expression is up-regulated during acute rat renal allograft rejection. This was specific to the rejection process, since isografts showed little change in MIF expression. These data suggest a pathological role for MIF in allograft rejection, a postulate which needs to be tested by cytokine blocking studies.
Acknowledgments
We acknowledge the expert assistance of Brian Howden, Department of Surgery, Monash Medical Centre, who performed the transplant surgery. This study was supported by the National Health and Medical Research Council (grant no. 960106).
REFERENCES
- 1.David JR. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell–antigen interaction. Proc Natl Acad Sci USA. 1996;56:72–7. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloom BR, Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966;153:80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- 3.Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:1895–902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishino T, Bernhagen J, Shiiki H, Calandra T, Dohi K, Bucala R. Localization of macrophage migration inhibitory factor (MIF) to secretory granules within the corticotrophic and thyrotrophic cells of the pituitary gland. Mol Med. 1995;1:781–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Bacher M, Meinhardt A, Lan HY, et al. Migration inhibitory factor expression in experimentally induced endotoxemia. Am J Pathol. 1997;150:235–46. [PMC free article] [PubMed] [Google Scholar]
- 6.Lan HY, Mu W, Yang N, Meinhardt A, Nikolic-Paterson DJ, Bacher M, Atkins RC, Bucala R. De novo renal expression of macrophage migration inhibitory factor (MIF) during the development of rat crescentic glomerulonephritis. Am J Pathol. 1996;149:1119–27. [PMC free article] [PubMed] [Google Scholar]
- 7.Bernhagen J, Calandra T, Mitchell RA, et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–9. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 8.Calandra T, Spiegel LA, Metz CN, Bucala R. Macrophage migration inhibitory factor is a critical mediator of the activation of immune cells by exotoxins of Gram-positive bacteria. Proc Natl Acad Sci USA. 1988;95:11383–8. doi: 10.1073/pnas.95.19.11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernhagen J, Bacher M, Calandra T, Metz CN, Doty S, Donnelly T, Bucala R. An essential role for macrophage migration inhibitory factor (MIF) in the tuberculin delayed-type hypersensitivity reaction. J Exp Med. 1996;183:277–82. doi: 10.1084/jem.183.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lan HY, Bacher M, Yang N, et al. The pathogenic role of macrophage migration inhibitory factor in immunologically induced kidney disease in the rat. J Exp Med. 1997;185:1455–65. doi: 10.1084/jem.185.8.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang N, Nikolic-Paterson DJ, Ng Y-Y, et al. Reversal of established rat crescentic glomerulonephritis by blockade of macrophage migration inhibitory factor (MIF): potential role of MIF in regulating glucocorticoid production. Mol Med. 1998;4:413–24. [PMC free article] [PubMed] [Google Scholar]
- 12.Mikulowska A, Metz NC, Bucala R, Holmdahl R. Macrophage migration inhibitory factor is involved in the pathogenesis of collagen type II-induced arthritis in mice. J Immunol. 1997;158:5514–7. [PubMed] [Google Scholar]
- 13.Leech M, Metz C, Santos L, Peng T, Holdsworth SR, Bucala R, Morand EF. Involvement of macrophage migration inhibitory factor in the evolution of rat adjuvant arthritis. Arthritis Rheum. 1988;41:910–7. doi: 10.1002/1529-0131(199805)41:5<910::AID-ART19>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 14.Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, Cerami A, Bucala R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;376:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- 15.Hancock WW, De Gee DMP, Rickles FR, Ewan VA, Atkins RC. Immunohistological analysis of serial biopsies taken during human renal allograft rejection. Changing profile of infiltrating cells and activation of the coagulation system. Transplantation. 1985;39:430–8. doi: 10.1097/00007890-198504000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Dallman MJ. Molecular biology of the allograft response. Transplant Proc. 1996;28(Suppl. 1):2–6. [PubMed] [Google Scholar]
- 17.Bogman MJ, Dooper IM, van de Winkel J, et al. Diagnosis of renal allograft rejection by macrophage immunostaining with a CD14 monoclonal antibody, WT14. Lancet. 1989;2:235. doi: 10.1016/s0140-6736(89)90427-3. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell R, Bacher M, Bernhagen J, Pushkarskaya T, Seldin MF, Bucala R. Cloning and characterization of the gene for mouse macrophage migration inhibitory factor (MIF) J Immunol. 1995;154:3863–70. [PubMed] [Google Scholar]
- 19.Fort P, Marty L, Piechaczyk M, el Sabrouty S, Dani C, Jeanteur P, Blanchard JM. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucl Acids Res. 1985;13:1431–42. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hattori M, Nikolic-Paterson DJ, Lan HY, Kawaguchi H, Ito K, Atkins RC. Up-regulation of ICAM-1 and VCAM-1 expression during macrophage recruitment in lipid induced glomerular injury in ExHC rats. Nephrology. 1995;1:221–32. [Google Scholar]
- 21.Dijkstra CD, Dopp EA, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985;54:589–99. [PMC free article] [PubMed] [Google Scholar]
- 22.Hunig T, Wallny HJ, Hartley JK, Lawetzky A, Tiefenthaler G. A monoclonal antibody to a constant determinant of the rat T cell antigen receptor that induces T cell activation. Differential reactivity with subsets of immature and mature T lymphocytes. J Exp Med. 1989;169:73–86. doi: 10.1084/jem.169.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lan HY, Mu W, Nikolic-Paterson DJ, Atkins RC. A novel, simple, reliable and sensitive method of multiple immunoenzymic staining: use of microwave oven heating to block antibody cross-reactivity and retrieve antigens. J Histochem Cytochem. 1995;43:97–102. doi: 10.1177/43.1.7822770. [DOI] [PubMed] [Google Scholar]
- 24.Bacher M, Metz CN, Calandra T, et al. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci USA. 1996;93:7849–54. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lan HY, Yang N, Brown FG, et al. MIF expression in human renal allograft rejection. Transplantation. 1998;66:1465–71. doi: 10.1097/00007890-199812150-00009. [DOI] [PubMed] [Google Scholar]