Abstract
The normal microbiota plays an important role in the health of the host, but little is known of how the human immune system recognizes and responds to Gram-positive indigenous bacteria. We have investigated cytokine responses of peripheral blood mononuclear cells (PBMC) to Gram-positive cell walls (CW) derived from four common intestinal indigenous bacteria, Eubacterium aerofaciens (Eu.a.), Eubacterium limosum(Eu.l.), Lactobacillus casei(L.c.), and Lactobacillus fermentum (L.f.). Our results indicate that Gram-positive CW of the normal intestinal microbiota can induce cytokine responses of the human PBMC. The profile, level and kinetics of these responses are similar to those induced by lipopolysaccharide (LPS) or CW derived from a pathogen, Streptococcus pyogenes (S.p.). Bacterial CW are capable of inducing production of a proinflammatory cytokine, tumour necrosis factor-alpha (TNF-α), and an anti-inflammatory cytokine, IL-10, but not that of IL-4 or interferon-gamma (IFN-γ). Monocytes are the main cell population in PBMC to produce TNF-α and IL-10. Induction of cytokine secretion is serum-dependent; both CD14-dependent and -independent pathways are involved. These findings suggest that the human cytokine responses induced by Gram-positive CW of the normal intestinal microbiota are similar to those induced by LPS or Gram-positive CW of the pathogens.
Keywords: cell wall, cytokine, human, monocytes/macrophage, normal microbiota
INTRODUCTION
The complex relationship between a host and the normal microbiota within the host presents an intriguing question how the human immune system recognizes and reacts, for example, with the indigenous bacteria in the gut [1]. The organisms, mainly bacteria, which reside in those parts of the human body that are exposed to the external environment, and usually coexist peacefully with the host, are termed as ‘normal’, ‘indigenous’ or ‘autochthonous’ microbiota [2,3]. There is ample evidence that the normal microbiota has beneficial effects, such as production of enzymes for food digestion, synthesis of vitamins, and importantly, maintenance of a microenvironment preventing pathogenic colonization. Nevertheless, members of the normal microbiota may also behave as pathogens, especially in newborn, traumatized or immunocompromised individuals [2,4]. Further, they may be involved in the pathogenesis of inflammatory and autoimmune diseases, such as inflammatory bowel disease (IBD) [5].
The outer surface structures of bacteria are the major components to interact with the immune system. Lipopolysaccharide (LPS), a common constitute of the outer membrane of Gram-negative bacteria, is able to induce expression of cytokines, adhesion molecules and enzymes that are active in the inflammatory cascade [6]. Recently, it has been shown that components of some Gram-positive bacterial cell walls (CW), including peptidoglycans and teichoic acids, could stimulate human monocytes/macrophages to release proinflammatory cytokines, for instance, tumour necrosis factor-alpha (TNF-α), IL-1β and IL-6 [7–9]. However, almost all the human studies have concentrated on pathogenic bacteria, with very few studies of Gram-positive CW derived from members of the normal microbiota [4,10].
Cytokines are the essential endogenous signals induced by infections. Proinflammatory cytokines, including TNF-α and interferon-gamma (IFN-γ), mediate the inflammatory response [11,12], whereas anti-inflammatory cytokines, including IL-4 and IL-10, are negative regulators [13,14]. All these cytokines are important mediators in human diseases such as septic shock, rheumatoid arthritis and IBD [5,15,16].
Eubacterium spp
and Lactobacillus spp. are Gram-positive rods and among the most common bacterial genera occurring in the normal intestinal microbiota of humans, and they rarely cause diseases [17,18]. Members of Lactobacillus spp. are the most commonly used probiotics for animals and humans [4]. Streptococcus pyogenes(S.p.) is a Gram-positive group A streptococcus and one of the most important human pathogens. It has been reported that a single i.p. injection of the CW from Eubacterium aerofaciens (Eu.a.), Lactobacillus casei (L.c.) or S.p. induces chronic polyarthritis in susceptible rat strains, whereas CW derived from Eubacterium limosum(Eu.l.) or Lactobacillus fermentum (L.f.) do not have this capacity [19–21]. In the present work, we have used CW derived from these bacteria to study cytokine responses by the human immune system to the normal microbiota.
MATERIALS AND METHODS
Bacterial strains and their cultivation
Eu.a. (ATCC 25986), Eu.l. (ATCC 8486), L.c. B (ATCC 11578), L.f. (ATCC 14931) and S.p. (ATCC 10389) were obtained from the American Type Culture Collection (Manassas, VA). Eu.a. and Eu.l. were grown on menadion vitamin K3 agar plates, then inoculated into BBL Schaedler broth (Becton Dickinson Microbiology System, Cockeysville, MD) at 37°C under anaerobic conditions. L.c. and L.f. were grown on Rogosa SL agar plates (Difco, Detroit, MI) in anaerobic jars and transferred to Lactobacillus MRS broth (Difco) for culture. S.p. was grown on blood agar base (Pronadisa, Madrid, Spain) in a 5% CO2 incubator at 37°C and inoculated into Todd–Hewitt broth (BBL; Becton Dickinson) for culture. All bacteria were collected in the late log phase of the growth.
Preparation of bacterial CW
The bacterial CW were prepared as described before [22]. Briefly, the heat-killed bacteria were disrupted in a Braun MSK cell homogenizer (B. Braun Biotech International, Melsungen, Germany) shaking with sterile glass beads (0.45 mm diameter) to disrupt over 95% of the cells as determined by Gram-staining.
The CW were further purified by treatment with RNase (25 μg/mg of CW) and DNase (2.5 μg/mg) and trypsin (25 μg/mg) for 4 h at 37°C. All enzymes used were purchased from Sigma Chemical Co. (St Louis, MO). After enzyme treatments, the washed CW were dialysed against water and lyophilized.
The lyophilized CW were subjected to sonic vibration for approx. 80 min in a Branson Sonifier Model B15 (Smith Kline Co., Danbury, CT). After sonication, the supernatants were centrifuged at 10 000 g for 30 min (Sorvall RC-5C centrifuge, SS-34 rotor). The supernatants from 10 000 g were centrifuged at 100 000 g, 4°C for 60 min in a Sorvall OTD-65B ultracentrifuge (Du Pont Co., Wilmington, DE; Ti60 rotor). The pellets from 100 000 g were resuspended in PBS for a 30-s sonication and labelled as 100p60. This 100p60 preparation of different bacterial CW was used in all experiments. It is known that this CW preparation with proper bacterial strains can induce chronic arthritis in a rat model [22]. Finally, CW solutions were filtered through 0.45 μm pore size filters (Millipore S. A., Molsheim, France) and stored at 4°C before use.
The carbohydrate contents of CW preparations of Eu.a., Eu.l., L.c., L.f. and S.p. were 56%, 50%, 38%, 40% and 40%, respectively; a phenol assay as described before was applied [23]. The protein contents, as determined by Bradford protein assay [24] using a BioRad kit (BioRad Labs, Hercules, CA), were 12.5%, 14.5%, 6.8%, 10% and 9.5%, respectively.
Endotoxin assay, LPS and polymyxin B
E-TOXATE, LPS from Escherichia coli serotype 0127:B8, and polymyxin B were purchased from Sigma. To exclude the possible LPS contamination in our reagents and bacterial CW preparations, the endotoxin content of all reagents and CW preparations was determined by Limulus Amebocyte Lysate assay. The endotoxin concentration was < 0.3 EU/ml in all reagents and CW preparations used.
Cell isolation
Human peripheral blood mononuclear cells (PBMC) were isolated from fresh buffy coats of healthy donors (Finnish Red Cross Blood Transfusion Service, Turku, Finland) by density gradient centrifugation with Ficoll–Paque (Pharmacia LKB, Uppsala, Sweden). RPMI 1640 (Gibco BRL, Paisley, UK) supplemented with 10 mm HEPES, 2 mml-glutamine, and 10% heat-inactivated pooled human AB serum was used as a culture medium in all experiments unless otherwise specified.
Adherent cells were isolated by the adherence of PBMC on Petri dishes (Costar, Cambridge, MA) at 37°C for 1 h. After five washes by 10 ml warm Hanks' balanced salt solution, monolayers of adherent cells were collected and centrifuged at 200 g for 3 min. This procedure yields a population consisting of > 75% monocytes, as determined by CD14 expression. Non-adherent cells were transferred to new Petri dishes. After another 1 h incubation, non-adherent cells in the supernatants were collected for the experiments; 60–70% of cells were CD3+, 10–20% CD19+, and < 15% CD14+.
Cytokine induction and detection
Cells were stimulated with CW preparations of Eu.a., Eu.l., L.c., L.f. or S.p. at 37°C for 24 h in flat-bottomed 48-well tissue culture plates (Costar). Culture supernatants were collected and stored at −70°C prior to the cytokine determination. Cytokine concentrations of the supernatants were measured by using ELISA kits from CLB (Amsterdam, The Netherlands) for human IL-10, TNF-α and IFN-γ, or ELISA kit from R&D Systems Europe Ltd. (Abingdon, UK) for human IL-4. The sensitivity of assays was 20 pg/ml for IL-10 and TNF-α, 4 pg/ml for IFN-γ and 0.5 pg/ml for IL-4. All cytokine assays were carried out in duplicate.
CD14 blocking assay
Cultured adherent and non-adherent PBMC were preincubated for 2 h at 37°C with 10 μg/ml of anti-human CD14 MoAb (clone MY4, IgG2b mouse) or isotype-matched control (clone MPC-11, IgG2b mouse) obtained from Coulter Immunology (Hialeah, FL). Thereafter, cells were incubated with different CW or LPS, and supernatants were collected for cytokine determinations.
Statistical analysis
To test for differences between the groups, anova was used. Samples with equal distributions were compared using Student's t-test. Differences were considered statistically significant when P < 0.05.
RESULTS
Cytokine profiles induced by Gram-positive CW
To study the cytokine response of PBMC to Gram-positive CW of members of the normal microbiota, PBMC were incubated with 10 μg/ml CW of Eu.a., Eu.l., L.c., or L.f. CW of S.p. was selected to represent CW from pathogenic species, and LPS (1 μg/ml) served as a control. The results show that all CW used and LPS are able to stimulate human PBMC to produce IL-10 and TNF-α, but not IL-4 and IFN-γ. The differences in cytokine production induced by different CW (10 μg/ml) or LPS (1 μg/ml) were not statistically significant (Fig. 1). To confirm the negative findings with IL-4 and IFN-γ, different concentrations of CW or LPS were incubated with PBMC for 6 h, 3 days and 5 days; no detectable cytokine production was observed (data not shown).
Fig. 1.
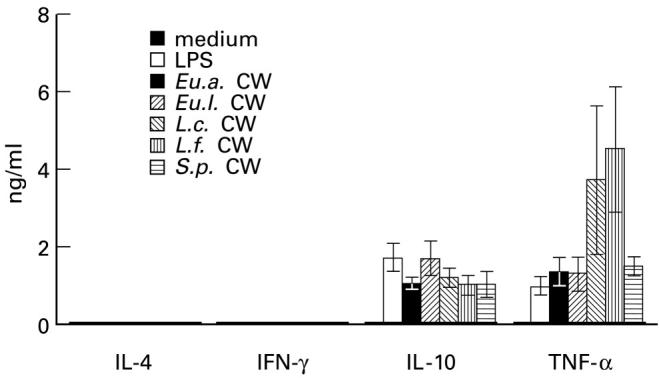
Production of IL-10 and tumour necrosis factor-alpha (TNF-α) but not of IL-4 and IFN-γ by human peripheral blood mononuclear cells (PBMC) in response to Gram-positive cell walls (CW) derived from members of intestinal normal microbiota or from Streptococcus pyogenes(S.p.). Lipopolysaccharide (LPS) was used as a control. Cells (2 × 106/ml) were incubated with CW (10 μg/ml) or LPS (1 μg/ml) at 37°C for 24 h, thereafter the supernatants were assessed for cytokines by ELISA. Each column represents a mean ± s.d. of eight separate experiments.
Kinetics of IL-10 and TNF-α production
To study kinetics of IL-10 and TNF-α production, PBMC were stimulated with CW of Eu.a. or S.p. or with LPS for 6 h, 24 h and 48 h (Fig. 2, upper panels). The kinetics of the production appeared to be similar for both cytokines, independent of whether the cells were stimulated by CW of Eu.a. or S.p. or by LPS. The peak level of IL-10 production was at 24 h incubation, while TNF-α reached the highest level at 6 h.
Fig. 2.
Kinetics and dose effects of IL-10 and tumour necrosis factor-alpha (TNF-α) release triggered by cell walls (CW) from Eubacterium aerofaciens(Eu.a.) or Streptococcus pyogenes(S.p.). Lipopolysaccharide (LPS) was used for comparison. Upper panels: peripheral blood mononuclear cells (PBMC; 2 × 106/ml) were stimulated with CW (10 μg/ml) or LPS (1 μg/ml) for 6 h, 24 h and 48 h at 37°C. Lower panels: cells were stimulated with various concentrations of bacterial CW or LPS (1 μg/ml) at 37°C for 24 h. Each dot represents a mean ± s.d. from two (upper panels) or three (lower panels) separate experiments. •, LPS; ▪, Eu.a. CW; ▴, S.p. CW.
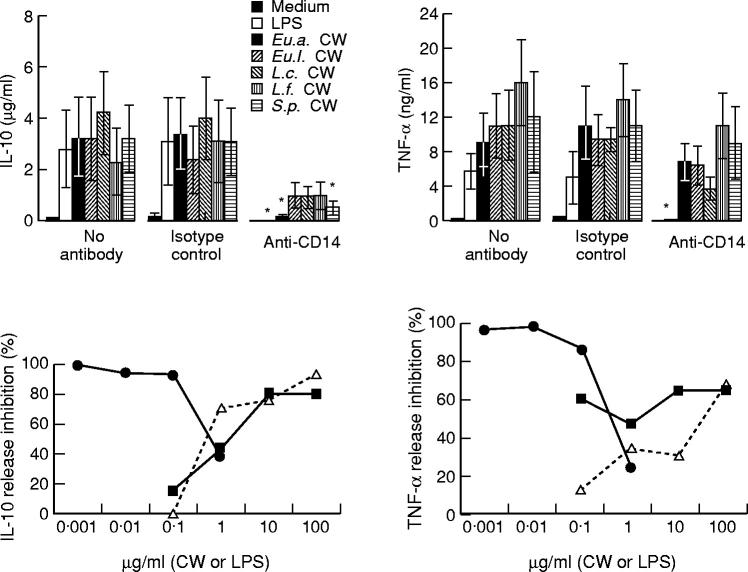
Dose-dependence of IL-10 and TNF-α production induced by CW
To study the dose effect on IL-10 and TNF-α production, PBMC were stimulated with various concentrations of CW from Eu.a. or S.p. or LPS (Fig. 2, lower panels). Regarding IL-10, the optimal CW concentration was 10 μg/ml, while LPS was able to induce the same level of IL-10 with a dose of only 1 μg/ml. Regarding TNF-α, the highest concentration was achieved with a CW dose of 100 μg/ml (larger CW doses were practically not possible). Altogether, these results indicate that IL-10 and TNF-α production stimulated by bacterial CW occurs in a dose-dependent manner.
Influence of polymyxin B on cytokine induction by bacterial CW
It is known that polymyxin B inhibits cytokine induction by LPS. To exclude the possible LPS contamination in our bacterial CW preparations, CW and different concentrations of LPS were mixed with polymyxin B (10 μg/ml) 30 min before incubation with PBMC. The results (Fig. 3) show that polymyxin B inhibited IL-10 production (by > 98%) and TNF-α production (by 77%) stimulated by LPS at a concentration as high as 1 μg/ml. Regarding stimulation by CW, polymyxin B inhibited IL-10 induction (by 46%), but increased TNF-α production (by 35%). These results suggest that effects of bacterial CW on cytokine induction are not caused by LPS contamination.
Fig. 3.
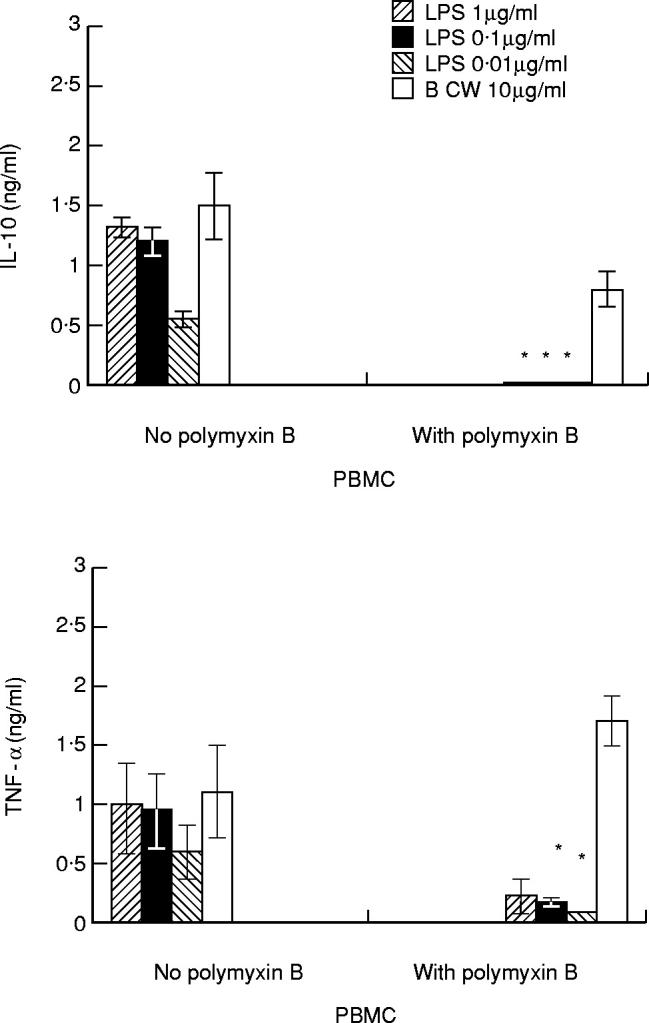
Influence of polymyxin B on cytokine induction by cell walls (CW) or lipopolysaccharide (LPS). Peripheral blood mononuclear cells (PBMC; 2 × 106/ml) were incubated with CW (10 μg/ml) or different concentrations of LPS. CW or LPS were first mixed with polymyxin B (10 μg/ml) 30 min before incubation with PBMC. Each column represents a mean ± s.d. of three separate experiments. The results for the CW are pooled data obtained with CW of Eubacterium aerofaciens(Eu.a.), Eubacterium limosum(Eu.l.), Lactobacillus casei(L.c.), Lactobacillus fermentum(L.f.) and Streptococcus pyogenes(S.p.). *P < 0.05 compared with the control with no polymyxin B.
Induction of IL-10 and TNF-α release by CW is serum-dependent
It has been documented that the PBMC response to LPS, especially to low concentrations of LPS, is dramatically enhanced by a soluble serum factor, LPS-binding protein (LBP) [25]. To determine whether the cytokine induction by Gram-positive bacterial CW from indigenous bacteria is also serum dependent, PBMC were stimulated with CW from Eu.a. or S.p., or LPS in the presence or absence of 10% human AB serum (Fig. 4). The results obtained indicate that induction of IL-10 by CW or LPS is serum-dependent; > 88% inhibition was observed in the absence of serum. In contrast to IL-10, TNF-α induction by CW was inhibited by 35–49% in the absence of serum, indicating only partial serum dependence.
Fig. 4.

The serum dependence of IL-10 and tumour necrosis factor-alpha (TNF-α) induction by Eubacterium aerofaciens(Eu.a.) or Streptococcus pyogenes(S.p.) cell walls (CW) or by lipopolysaccharide (LPS). Peripheral blood mononuclear cells (PBMC; 2 × 106/ml) were stimulated with CW or LPS at 37°C for 24 h with or without 10% human AB serum. Each column represents a mean ± s.d. of three separate experiments. *P < 0.05 compared with the experiments with serum.
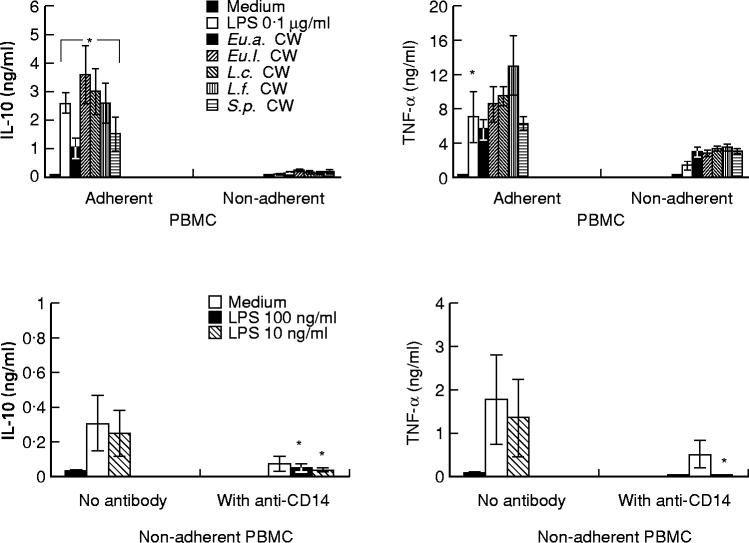
IL-10 and TNF-α are mostly produced by adherent monocytes
To find the main source among the PBMC to produce IL-10 and TNF-α, adherent cells were incubated with different bacterial CW (Fig. 5, upper panels). The results show that adherent PBMC produced 7–17-fold more IL-10 than non-adherent cells. For TNF-α, the difference was two-to-five-fold. Adherent PBMC used consisted of > 75% monocytes as determined by CD14 expression in flow cytometry analysis. From non-adherent PBMC approx. 65% were CD3+, 15% were CD19+ and < 15% were CD14+ (data not shown). In separate experiments, non-adherent cells were stimulated by LPS in the presence or absence of anti-CD14 MoAb (10 μg/ml) (Fig. 5, lower panels). IL-10 and TNF-α production from non-adherent PBMC stimulated by LPS was inhibited by 88% and 98%, respectively, by the presence of anti-CD14 MoAb (10 μg/ml). These results indicate that IL-10 and TNF-α were mostly produced by adherent monocytes, and cytokine production by the non-adherent cell population was largely due to contaminated monocytes.
Fig. 5.
Comparison of cytokine production from adherent and non-adherent peripheral blood mononuclear cells (PBMC) stimulated with cell walls (CW) or lipopolysaccharide (LPS). Upper panels: the cells (106/ml) were incubated with CW (10 μg/ml) or LPS (0.1 μg/ml) at 37°C for 24 h. *P < 0.05 compared with that of adherent PBMC. Lower panels: non-adherent cells (106/ml) were incubated with LPS in the presence or absence of anti-CD14 MoAb (10 μg/ml). *P < 0.05 compared with the experiments with no antibody. Each column represents a mean ± s.d. of three separate experiments.
Anti-CD14 MoAb partially inhibits IL-10 and TNF-α production by adherent PBMC
To investigate the possible role of CD14 in cytokine induction by bacterial CW, adherent PBMC were incubated with 10 μg/ml anti-CD14 MoAb for 2 h, then cultured with different bacterial CW (Fig. 6, upper panels). The capacity of CW to stimulate production of IL-10 and TNF-α was strongly inhibited by anti-CD14 MoAb. Regarding IL-10, the inhibition was lowest with L.c. CW (57%) and highest with Eu.a. CW (96%). For TNF-α production, the inhibition ranged from 24% (Eu.a. CW) to 66% (L.c. CW). As expected, the capacity of LPS (10 ng/ml) to induce IL-10 and TNF-α was blocked by anti-CD14 MoAb (> 98% inhibition). To determine the efficacy of the anti-CD14 MoAb-induced inhibition, different concentrations of CW and LPS were used (Fig. 6, lower panels). Anti-CD14 MoAb (10 μg/ml) could almost totally block IL-10 and TNF-α induction when LPS concentrations of 0.1 μg/ml were applied. However, anti-CD14 MoAb was only partially effective in blocking cytokine induction, especially that of TNF-α. The effect of anti-CD14 MoAb was independent of the concentration of the stimulatory CW.
Fig. 6.
The effect of anti-CD14 MoAb on cytokine production induced by cell walls (CW) or lipopolysaccharide (LPS). Upper panels: adherent peripheral blood mononuclear cells (PBMC; 106/ml) were incubated for 2 h with anti-CD14 MoAb, or with immunoglobulin isotype control, then cultured with CW (10 μg/ml) or LPS (10 ng/ml) at 37°C for 24 h. *P < 0.05 compared with the experiments with no antibody. Lower panels: adherent cells (106/ml) were stimulated with different concentrations of CW of Lactobacillus casei(L.c.) or Streptococcus pyogenes(S.p.) or with LPS in the presence or absence of anti-CD14 MoAb. Results represent means of four separate experiments. •, LPS + anti-CD14; ▪, L.c.+ anti-CD14; Δ, S.p.+ anti-CD14.
DISCUSSION
Our results demonstrate that human PBMC can recognize and respond to Gram-positive CW originating from members of the normal intestinal microbiota. The profile, level, kinetics and main source of the cytokine responses were similar to those induced by LPS or by CW derived from a pathogen (S.p.). These effects were not caused by LPS contamination, as shown by the controls with Limulus amebocyte lysate assays, polymyxin B, culture media and anti-CD14 MoAb. Bacterial CW induce PBMC to produce TNF-α and IL-10, but not IFN-γ and IL-4; monocytes are the major cell population responsible for the production of TNF-α and IL-10. These results are consistent with the finding that live or fixed lactic acid bacteria could induce human PBMC in vitro to produce TNF-α, IL-6 and IL-10 [10]. Among PBMC, IFN-γ and IL-4 are mostly derived from T cells [12,13].
The present data indicate that CW of the normal microbiota may be relatively poor activators of T cells, which is also supported by the observation that in physiological conditions T cells are hyporesponsive against antigens derived from the autologous intestinal microbiota [26]. Likewise, we did not observe any significant proliferative response by PBMC to the CW used in this study, indicating lack of the recall response (data not shown).
An imbalance in the interaction between the immune system and the intestinal normal microbiota may lead to diseases. This has been shown in vivo by using genetically manipulated animals, which can spontaneously develop colitis in conventional but not in germ-free conditions [27]. It has also been reported that inappropriately activated T cells, reacting with enteric microbiota, can mediate chronic inflammation in spontaneously colitic C3H/HeJBir mice [28]. It is obvious that proinflammatory cytokines, such as TNF-α, play an important role in the intestinal pathology of these animal models.
Nevertheless, the normal microbiota usually coexists in a peaceful symbiosis with the host, despite the fact that some indigenous bacteria or their degradation products can translocate into the body [29]. Many presumed mechanisms may be involved. One possible mechanism is that translocated Gram-positive bacteria, which are the major population of the intestinal microbiota, are less efficient inducers of the proinflammatory cytokines when compared with LPS of Gram-negative bacterial species. As suggested by the results obtained in this study and elsewhere [7], the capacity of LPS for cytokine induction is 10–1000-fold stronger than that of Gram-positive bacterial CW. Further, the cytokine balance may serve as a self-limit for the inflammatory process. In our experiments, CW of the normal microbiota first up-regulated TNF-α synthesis, and thereafter TNF-α synthesis decreased along with the increase of IL-10 production, suggesting a self-regulatory mechanism [14,30]. These and other possible mechanisms together maintain a delicate and complex symbiotic relationship between the host and the normal microbiota. The disturbance of one or several of these mechanisms may generate inappropriate immune responses leading to pathological conditions [5,31].
It is believed that the innate immune system recognizes pathogen-associated molecular patterns (PAMP) shared by a large group of pathogens, through pattern recognition receptors (PRR) [32]. CD14 is one of the PRR [8]. Despite the key role of CD14 in response to LPS, it is still controversial whether Gram-positive bacterial CW stimulate monocytes through a CD14-dependent pathway [8,33]. In our experiments, 10 μg/ml anti-CD14 MoAbs could inhibit production of IL-10 by approx. 80% and that of TNF-α by 34%, induced by 10 μg/ml bacterial CW. Moreover, no further inhibition of IL-10 and TNF-α induction was observed when lower CW concentrations were used. These results indicate that most probably both CD14-dependent and -independent pathways are involved in the cytokine induction by Gram-positive CW of the normal microbiota.
It is well documented that the CD14 response to LPS is dramatically enhanced by a soluble serum factor, LBP [25]. Our experiments indicate that IL-10 induction by bacterial CW is serum-dependent, while TNF-α induction is partially serum-dependent. Further investigations are required to identify the serum factor(s) and the pathway involved. Since the human serum used was heat-inactivated, the complement system is probably not involved.
Taken together, our results demonstrate that the human immune system responds to Gram-positive CW of the intestinal normal microbiota by generating cytokine responses, similarly to the responses induced with LPS or with a CW derived from a pathogenic bacterial species (S.p.). Further, cytokine induction is serum-dependent; both CD14-dependent and -independent pathways are involved. These findings extend our understanding of how the human immune system responds to the normal microbiota. They also support the hypothesis that the human innate immune system generally recognizes PAMP through PRR in response to microbes; members of the intestinal normal microbiota are no exceptions.
Acknowledgments
We thank Ms Marju Niskala for excellent technical assistance and Dr Hui Hu for discussion and suggestions. This work was supported by EVO of Turku University Central Hospital.
REFERENCES
- 1.Gaskins HR. Immunological aspects of host/microbiota interactions at the intestinal epithelium. In: Mackie RI, White BA, Isaacson RE, editors. Gastrointestinal microbiology. Vol. 2. London: Chapman & Hall; 1997. pp. 537–87. [Google Scholar]
- 2.Mackowiak PA. The normal microbial flora. N Engl J Med. 1982;307:83–93. doi: 10.1056/NEJM198207083070203. [DOI] [PubMed] [Google Scholar]
- 3.Wilson KH. Biota of human gastointestinal tract. In: Mackie RI, White BA, Isaacson RE, editors. Gastrointestinal microbiology. Vol. 2. London: Chapman & Hall; 1997. pp. 39–58. [Google Scholar]
- 4.Tannock GW. Influences of the normal microbiota on the animal host. In: Mackie RI, White BA, Isaacson RE, editors. Gastrointestinal microbiology. Vol. 2. London: Chapman & Hall; 1997. pp. 466–97. [Google Scholar]
- 5.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 6.Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–57. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 7.Heumann D, Barras C, Severin A, Glauser MP, Tomasz A. Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect Immun. 1994;62:2715–21. doi: 10.1128/iai.62.7.2715-2721.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pugin J, Heumann AB, Tomasz A, et al. CD14 as a pattern recognition receptor. Immunity. 1994;1:509–16. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 9.Wilson M, Seymour R, Henderson B. Bacterial perturbation of cytokine networks. Infect Immun. 1998;66:2401–9. doi: 10.1128/iai.66.6.2401-2409.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miettinen M, Vuopio-Varkila J, Varkila K. Production of human necrosis factor alpha, interleukin-6, and interleukin-10 is induced by lactic acid bacteria. Infect Immun. 1996;64:5403–5. doi: 10.1128/iai.64.12.5403-5405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tracey KJ, Cerami A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]
- 12.Billiau A. Interferon-γ biology and role in pathogenesis. Adv Immunol. 1996;62:61–129. doi: 10.1016/s0065-2776(08)60428-9. [DOI] [PubMed] [Google Scholar]
- 13.Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–8. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 14.Moore KW, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–90. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 15.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 16.Isomäki P, Luukkainen R, Saario R, Toivanen P, Punnonen J. Interleukin-10 functions as an antiinflammatory cytokine in rheumatoid synovium. Arthritis Rheum. 1996;39:386–95. doi: 10.1002/art.1780390306. [DOI] [PubMed] [Google Scholar]
- 17.Moore WEC, Moore LVH. Genus Eubacterium. In: Sneath PHA, Mair NS, Sharpe ME, Holt JG, editors. Bergey's manual of systematic bacteriology. Vol. 2. Baltimore: Williams & Wilkins; 1984. pp. 1353–73. [Google Scholar]
- 18.Kandler ON, Weiss ? Genus Lactobacillus. In: Sneath PHA, Mair NS, Sharpe ME, Holt JG, editors. Bergey's manual of systematic bacteriology. Vol. 2. Baltimore: Williams & Wilkins; 1984. pp. 1209–34. [Google Scholar]
- 19.Cromartie WJ, Craddock JC, Schwab JH, Anderle SK, Yang CH. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977;146:1585–602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehman TJA, Allen JB, Plotz PH, Wilder RL. Polyarthritis in rats following the systemic injection of Lactobacillus casei cell walls in aqueous suspension. Arthritis Rheum. 1983;26:1259–65. doi: 10.1002/art.1780261013. [DOI] [PubMed] [Google Scholar]
- 21.Severijnen AJ, van Kleef R, Hazenberg MP, van de Merwe JP. Chronic arthritis induced in rats by cell wall fragments of Eubacterium species from the human intestinal flora. Infect Immun. 1990;58:523–8. doi: 10.1128/iai.58.2.523-528.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox A, Brown RR, Anderle SK, Chetty C, Cromartie WJ, Gooder H, Schwab JH. Arthropathic properties related to the molecular weight of peptidoglycan-polysaccharide polymers of streptococcal cell walls. Infect Immun. 1982;35:1003–10. doi: 10.1128/iai.35.3.1003-1010.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for the determination of sugars and related substances. Anal Chem. 1956;28:350–6. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- 24.Bradford M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–53. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 25.Jack RS, Fan XL, Bernheiden M, et al. Lipopolysaccharide-binding protein is required to combat a murine Gram-negative bacterial infection. Nature. 1997;389:742–5. doi: 10.1038/39622. [DOI] [PubMed] [Google Scholar]
- 26.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Buschenfelde KH. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease. Clin Exp Immunol. 1995;102:448–55. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morales VM, Snapper SB, Blumberg RS. Probing the gastrointestinal immune function using transgenic and knockout technology. Curr Opin Gastroenterol. 1996;12:577–83. [Google Scholar]
- 28.Cong Y, Brandwein SL, McCabe RP, Lazenby A, Birkenmeier EH, Sundberg JP, Elson CO. CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J Exp Med. 1998;187:855–65. doi: 10.1084/jem.187.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berg RD. Bacterial translocation from the gastrointestinal tract. Trends Microbiol. 1995;3:149–54. doi: 10.1016/s0966-842x(00)88906-4. [DOI] [PubMed] [Google Scholar]
- 30.O'Farrell AM, Liu Y, Moore KW, Mui ALF. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for stat3-dependent and -independent pathways. EMBO J. 1998;17:1006–18. doi: 10.1093/emboj/17.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duchmann R, Schmitt E, Knolle P, Meyer zum Buschenfelde KH, Neurath M. Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur J Immunol. 1996;26:934–8. doi: 10.1002/eji.1830260432. [DOI] [PubMed] [Google Scholar]
- 32.Medzhitov R, Janeway CA. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–8. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 33.Cauwels A, Wan E, Leismann M, Tuomanen E. Coexistence of CD14-dependent and independent pathways for stimulation of human monocytes by gram-positive bacteria. Infect Immun. 1997;65:3255–60. doi: 10.1128/iai.65.8.3255-3260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]