Abstract
Ageing is associated with decreased resistance to bacterial infections and concomitant increased circulating levels of inflammatory cytokines. The purpose of the present study was to research age-related changes in levels of early mediators of the acute-phase response in whole blood supernatants following LPS stimulation, representing an ex vivo model of sepsis. Levels of tumour necrosis factor-alpha (TNF-α), IL-1β and IL-6 in whole blood supernatants were measured after in vitro LPS stimulation for 24 h in 168 elderly humans aged 81 years from the 1914 cohort in Glostrup, Denmark and in 91 young controls aged 19–31 years. Levels of TNF-α and IL-1β were significantly lower in elderly humans compared with young controls, whereas no difference was detected with regard to IL-6. Elderly humans with low body mass index had the lowest levels of IL-1β. Young women had lower levels of proinflammatory cytokines compared with young men, but this difference was blurred by ageing. No relation was found between circulating plasma levels of TNF-α and levels after in vitro LPS stimulation. In conclusion, decreased production of TNF-α and IL-1β after exposure to LPS may reflect impaired host defence against infections in the elderly and be of importance in elderly humans with underlying health disorders. However, the clinical relevance is questionable in healthy elderly people because decreased levels were found compared with young men but not compared with young women.
Keywords: ageing, tumour necrosis factor-alpha, IL-1β, IL-6, lipopolysaccharide
INTRODUCTION
Elderly humans show increased in vivo inflammatory activity in the blood, including increased plasma concentrations of tumour necrosis factor-alpha (TNF-α) [1,2], IL-6 [1,3–8], cytokine antagonists such as IL-1 receptor antagonist and circulating soluble TNF receptors [1,9], acute-phase proteins [10,11], and neopterin [9]. Enhanced levels of these parameters may reflect ongoing pathological processes such as atherosclerosis and dementia [1]. However, it is unclear if the inflammatory response to an acute stimulation is preserved in the elderly. Clinical studies of acute, serious illness have indicated that aged humans are less likely to develop fever and leucocytosis and are more likely to die of the infection than other age groups [12–15]. The paucity of inflammatory signs in the elderly in response to acute stress suggests a defect in early mediators of the acute-phase response such as TNF-α, IL-1β and IL-6.
Studies of age-related differences in the production of proinflammatory cytokines have yielded inconsistent results [16]. In the present study we measured levels of TNF-α, IL-1β, and IL-6 in whole blood supernatants after Escherichia coli LPS stimulation for 24 h in a large number of elderly humans, representing an approximation to a normal population within a narrow age spectrum, and in young controls. The influences of atherosclerosis and body mass index (BMI) were considered. A low BMI has been associated with mortality in elderly humans [17–19], suggesting that BMI may be a general marker of health status in this age group. LPS is a wall constituent of Gram-negative bacteria and represents a natural occurring endotoxin which mainly stimulates monocytes in the blood [20,21].
SUBJECTS AND METHODS
Elderly humans
Eighty-four women and 84 men aged 80–81 years were included in the present study. The elderly people were from the 1914 cohort in Glostrup, which is a longitudinal study of ageing [22]. In 1995, survivors were asked to participate in a survey of 80-year-olds [23]. One hundred and seventy-four subjects accepted an extra visit to the laboratory for the collection of blood samples. No one had dementia. We failed to generate LPS-stimulated supernatants in six subjects who were left out in the present study.
Young humans
Ninety-one healthy voluntary individuals (45/46 women/men), median age 25 years (range 19–31 years) were included as controls.
None of the included individuals suffered from acute illness prior to the collection of blood samples. Statistical analyses were done with and without subjects having severe health disorders and disorders or medical intakes known or suspected to influence LPS-stimulated production of IL-1β, TNF-α or IL-6: cancer at present or previously (n = 25), acute or chronic inflammatory disorders (n = 5); intake of systemic corticosteroids (n = 7), acetyl salicylic acid (> 100 mg, n = 8), or non-steroidal anti-inflammatory drugs (n = 20); low haemoglobin (< 6.5 mmol/l, n = 2), increased concentrations of leucocytes (> 15 × 109/l, n = 1), increased sedimentation rate (> 30, n = 22), increased blood glucose (> 10 mmol/l, n = 2), increased alkaline phosphatase (> 400 U/l, n = 1), increased alanine amino transferase (> 60 U/l, n = 2), or increased carbamide (> 15 mmol/l, n = 1). In total, 64 elderly people were separated due to these criteria. They are referred to as group B. The remaining 81-year-old individuals are named group A.
Missing values are due to technical problems in the laboratory.
Atherosclerosis
Elderly people with one of the following diagnoses were categorized as having atherosclerosis: acute myocardial infarction, angina pectoris, intermittent claudication, aortic aneurism, embolus or thrombosis in the arterial system, stroke, transient cerebral ischaemia or amaurosis fugax. A low ankle-brachial arterial pressure index is indicative of: generalized atherosclerosis [24]—particularly those of < 0.90 [25,26]; increased risk of cardiovascular diseases [27]; and increased mortality [28,29]. The elderly were divided into two groups based on an ankle-brachial arterial pressure < 0.9 versus ≥ 0.9.
Body mass index
BMI was calculated as weight divided by height squared. The elderly were subdivided into two groups depending on BMI: women < 20 and men < 22 compared with women ≥ 20 and men ≥ 22 in accordance with the guidelines for admission criteria for immunogerontological studies in humans [30].
Blood sampling and measurement of levels of TNF-α, IL-1β and IL-6 in whole blood supernatants
Blood samples were drawn between 7 and 10:00 a.m. after an overnight fast in evacuated blood collection tubes supplemented with 25 IE heparin/ml blood. Whole blood was diluted 1:4 in RPMI (Kibbutz Beit Haemen, Biological Industries, Israel) and stimulated for 24 h at 37°C with E. coli LPS (Difco, Detroit, MI) in a final solution of 1 μg/ml. Supernatants were analysed in duplicates by commercially available ELISA kits (Quantikine; R&D Systems, Minneapolis, MN); the mean was used in the statistical analysis. The minimum detection doses were 4.4 pg/ml for TNF-α, 0.7 pg/ml for IL-6, and < 1 pg/ml for IL-1β. The choice of stimulation for 24 h was based on a pilot study including 16 healthy volunteers—eight elderly (age range 77–81 years) and eight young (age range 20–30 years). TNF-α concentrations were measured after LPS stimulation (1 μg/ml) for 4, 6, 12, 24, and 48 h. For both young and elderly humans, TNF-α production peaked within 24 h and was maximal in most subjects after 12 h. The time of maximal concentration was not displaced in the elderly. Declines in the supernatant levels were pronounced after 48 h. Stimulation for 24 h was chosen in the subsequent experimental design in order to choose a time point where TNF-α production was levelling off but before a pronounced breakdown had taken place. Levels in unstimulated blood were below the detection limit of the assays.
Plasma levels of TNF-α in vivo
The concentration of TNF-α in plasma at baseline was measured by a high sensitivity commercial ELISA kit (Quantikine High Sensitivity; R&D Systems). The detection limit was < 180 fg/ml.
Clinical chemistry tests
Leucocytes, lymphocytes, monocytes and neutrophils were determined by a cell counter (Technic H.1; Miles Inc., Tarrytown, NY).
Statistical analysis
Statistical calculations were performed using SYSTAT statistical software 7.0 (SYSTAT, Evanston, IL). Initial analyses revealed that the cytokine data were not distributed normally. Therefore, data are presented as medians and quartiles. The cytokine data were log transformed in the following analyses. Differences between two groups were analysed by a t-test for independent groups. The effect of gender on age-related differences was analysed by a two-way anova including age group, gender, and the interaction between these two parameters. If the P value for the interaction was significant, comparisons in pairs were carried out by a Tukey test. The relationship between LPS-induced levels of TNF-α on one side and levels of IL-1β, IL-6 and in vivo TNF-α on the other was analysed by linear regression analysis for each age group. The relationship between proinflammatory cytokines (dependent variable) and BMI in the elderly cohort (independent variable) was tested by multiple linear regression analysis adjusting for the effect of gender. BMI was tested for an interaction with gender in order to examine if separate slopes for men and women should be calculated. P < 0.05 was considered significant.
RESULTS
Leucocyte subsets
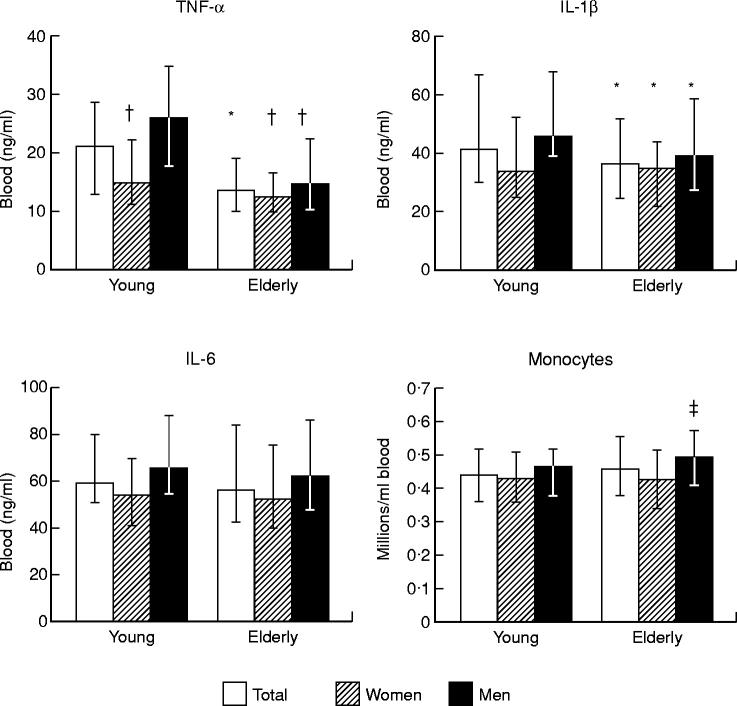
Elderly humans had lower concentrations of lymphocytes and increased concentrations of neutrophils and leucocytes compared with young controls (Table 1). No differences in the concentrations of monocytes (Fig. 1) were found. It made no difference when elderly individuals from group B were left out (data not shown).
Table 1.
Leucocyte subsets in the blood from 81-year-old humans (elderly) and 18–30-year-old controls (young)

Medians (quartiles) are shown.
*Significant diifference (P < 0.05) from young controls.
Fig. 1.
Levels of proinflammatory cytokines in whole blood supernatants following LPS stimulation and blood concentrations of monocytes in young and elderly humans. Whole blood (diluted 1:4) was stimulated with LPS in vitro for 24 h. Total: men and women are pooled; young women, n = 45; young men, n = 46; elderly women, n = 84; elderly men, n = 84. Medians and quartiles are shown. *Significant different (P < 0.05) from young controls (total); †significant different (P < 0.05) from young men; ‡significant different (P < 0.05) from elderly females.
Levels of proinflammatory cytokines in whole blood supernatants following LPS stimulation in young and elderly humans
Levels of TNF-α, IL-1β and IL-6 were linearly interrelated for both age groups (Table 2). Elderly humans had higher levels of TNF-α and IL-1β compared with young controls, whereas no difference was found with regard to IL-6 (Fig. 1). This suggests a functional defect of monocytes (effector cells) in elderly people, considering that the elderly group had a normal monocyte count. In accordance with this hypothesis, statistical analyses adjusting cytokine levels for the concentration of monocytes resulted in the same conclusions as analyses of the unadjusted levels (data not shown).
Table 2.
Correlations between levels of TNF-α, IL-1β and IL-6 in whole blood supernatants following LPS stimulation (24 h) and blood concentrations of monocytes in elderly and young people
r, Pearson's correlation coefficient.
When the elderly from group B were left out in the statistical analysis, elderly from group A were still found to have decreased levels in culture supernatants of TNF-α (P < 0.0005), whereas IL-1β was not significant any more (P = 0.1). Furthermore, the elderly in group B had lower IL-1β levels compared with group A: median (quartiles), 37.8 ng/ml (25.8–53.8 ng/ml), n = 104 for group A versus 32.8 ng/ml (20.3–46.6 ng/ml), n = 64 for group B; P = 0.05. No difference was detected with regard to IL-6 when group B was left out (P = 0.5).
When gender was included in the statistical analysis (two-way anova) a significant interaction between age and sex (P = 0.03) was found for TNF-α. Subsequent comparisons in pairs showed that there was no difference in levels of TNF-α between elderly women and elderly men; young women had lower concentrations of TNF-α than young men; and elderly people (both women and men) had lower levels compared with young men but not compared with young women. The same results were found when group B elderly were excluded (data not shown). No interaction between age group and gender was found with regard to IL-1β (P = 0.6), but the elderly had lower levels than young controls (P = 0.009) and women pooled had lower levels than men pooled (P < 0.0005). Accordingly, parallel decreases for IL-1β were observed in elderly women and men compared with the respective gender in the young controls. The same results were obtained after exclusion of elderly subjects belonging to group B (data not shown). No age-related decrease or interaction between age and gender was found with regard to IL-6 levels, but women had lower levels compared with men (P = 0.001).
It was tested if increased levels of proinflammatory cytokines in women compared with men resulted from different absolute numbers of monocytes in the blood. A significant interaction between sex and age group was detected (two-way anova, P = 0.05) and elderly men were subsequently found to have higher blood concentrations of monocytes than elderly women, whereas there was no difference between men and women in the young group (Fig. 1). Accordingly, lower levels of proinflammatory cytokines in supernatants from young women compared with young men did not result from lower concentrations of monocytes in the blood. This was confirmed by the fact that statistical analyses adjusting cytokine levels for the concentration of monocytes resulted in the same conclusions as analyses of the unadjusted levels (data not shown).
No linear relations were found between TNF-α levels after in vitro LPS stimulation and plasma concentrations of TNF-αin vivo: r (Pearson's correlation coefficient) = – 0.055, n = 165, P = 0.5 for the elderly group and r = −0.065, n = 81, P = 0.6 for the young group.
LPS-induced levels of proinflammatory cytokines in relation to BMI in elderly humans
BMI was only available for 158 81-year-old people: median (range) was 23 (16–37), n = 75 for women and 25 (17–44), n = 83 for men; P = 0.3. LPS-induced levels of IL-1β were linearly related to BMI adjusted for the effect of gender in multiple linear regression analysis (Table 3). The same result was found when elderly from group B were left out (data not shown). No correlations were found between BMI on one hand and TNF-α or IL-6 on the other in similar models (Table 3). Women with BMI < 20 together with men with BMI < 22 had lower production of IL-1β than the remaining elderly subjects: medians (quartiles) 30.4 ng/ml (16.6–37.1), n = 16 versus 37.9 ng/ml (25.2–54.7), n = 142, P = 0.01. No differences were detected with regard to TNF-α and IL-6 (data not shown). Furthermore, the concentration of monocytes did not differ in subjects with low versus normal/high BMI: medians (quartiles) 0.50 (0.40–0.58), n = 15 versus 0.46 (0.38–0.57), n = 147, P = 0.4.
Table 3.
Linear regression of proinflammatory cytokines on body mass index (BMI) and gender in 81-year-old humans

Linear regression modol: dependent variable = constant + BMI + gender. Gender was coded 0,1 for fernales, males. It was checked that BMI did not interact with gender(P < 0.05) from young controls.
LPS-induced levels of proinflammatory cytokines in elderly humans with and without atherosclerosis
No difference was found in supernatant levels of the three proinflammatory cytokines after LPS stimulation between elderly humans with and without clinical manifestations of atherosclerosis or with an ankle-brachial arterial pressure index below versus above 0.9 (Fig. 2). Exclusion of elderly from group B did not influence this result (data not shown).
Fig. 2.

Atherosclerosis and proinflammatory cytokines in 81-year-old humans. Levels of proinflammatory cytokines in whole blood after LPS stimulation for 24 h. □, Clinical diagnosis of atherosclerosis, n = 45; ▪, no clinical diagnosis of atherosclerosis, n = 123. Ankle-brachial blood pressure index: < 0.9 (□) is indicative of peripheral atherosclerosis, n = 70; ≥ 0.9 (▪), n = 97. Medians and quartiles are shown.
DISCUSSION
The major findings in the present study were that: (i) LPS-induced inflammatory activity, reflected by the concentrations of TNF-α and IL-1β in culture supernatants from whole blood after 24 h, was decreased in elderly humans compared with young controls. No difference was found with regard to IL-6, although this cytokine showed a strong linear relation to TNF-α and IL-1β; (ii) young women had lower levels of proinflammatory cytokines after LPS stimulation compared with young men, but this difference was blurred with ageing; (iii) elderly humans with a low BMI had lower LPS-induced levels of IL-1β than elderly with a normal or high BMI; (iv) levels of TNF-α in whole blood supernatants after LPS stimulation were not related to plasma levels of TNF-αin vivo or to atherosclerosis.
It is questionable if the observed age-related decrease in the LPS-induced cytokine response had any clinical relevance, because elderly people had decreased levels compared with young men but not compared with young women. A low BMI has been associated with mortality in elderly humans [17–19]. Accordingly, the association between low IL-1β production and low BMI indicated that decreased production of IL-1β primarily affected those who had an overall bad health status and an age-related effect acted synergistically with a decline resulting from chronic medical disorders. This hypothesis was supported by the finding of lower IL-1β levels in elderly humans with severe health disorders or medical intakes with potential influence on the production of proinflammatory cytokines (group B) compared with the remaining elderly (group A).
In accordance with the data in the present study, others have reported decreased LPS-induced production of TNF-α [31] and IL-1β [31–33] by isolated monocytes from elderly compared with young humans in smaller trials. Decreased monocyte production of these cytokines in response to LPS has also been reported in several animal studies [34–40]. However, one study reported a positive correlation between age and levels of IL-1β, but not TNF-α, in supernatants from isolated human monocyte cultures stimulated for 24 h with LPS [41]. Another study found increased concentrations of TNF-α and IL-1β in whole blood supernatants from elderly humans after ex vivo LPS stimulation for 48 h [42]. The pilot experiment in the present study showed that peak levels of TNF-α were much earlier and pronounced decreases occurred after 48 h in a similar culture system. Accordingly, increased levels of proinflammatory cytokines in whole blood supernatants after 48 h may reflect prolonged low grade inflammatory activity, unstimulated production or decreased cytokine breakdown, and not LPS-stimulated production. No difference was detected in TNF-α and IL-1β production after LPS stimulation of isolated blood mononuclear cells (BMNC) for 22 h in 711 elderly humans and 21 young volunteers [43]. However, as stated by the authors [43], the small control group might have biased the study in favour of finding no difference. These considerations form the basis of stating that most well-controlled and well-designed studies with high n values indicate that monocytes from elderly humans show decreased in vitro production of proinflammatory cytokines in response to an LPS challenge at least when compared with young men. This may be of importance during an inflammatory response. Contradictory results may result from different time kinetics, different assays and differences in the studied populations. In response to stimulation with phorbol myristate acetate (PMA) together with phytohaemagglutinin (PHA) (PHA stimulates mainly CD4+ T cells), BMNC from elderly humans produced more TNF-α, IL-1β and IL-6 after 24–72 h [44]. However, this is a very different model system which involves T cells and it may not reproduce a natural stimulation [20,45].
In the present study, increased plasma concentrations of circulating TNF-αin vivo could not be ascribed to a hyperactivity of monocytes in response to stimulations such as LPS. However, from the present results it cannot be excluded that prolonged activation of monocytes in the blood or in other tissues (e.g. cells in atherosclerotic lesions) are responsible for the increased in vivo levels of circulating proinflammatory cytokines.
The lower production of proinflammatory cytokines in young women compared with men at the same age may be caused by inhibitory effects of female hormones or a stimulatory effect by testosterone. In support of this, studies have shown that human monocytes produced lower levels of proinflammatory cytokines spontaneously [46] and after LPS stimulation [47] in the presence of high levels of female hormones. However, it has also been shown that oestrogen enhanced LPS-stimulated BMNC secretion of IL-1 and IL-6 secretion in men [47], and there were no cyclic changes in LPS-induced TNF production during a menstrual cycle [48]. Testosterone has been shown to reduce the production of IL-6 by human BMNC [49] and the production of IL-1 following LPS stimulation [47]. Accordingly, it is unlikely that the high production of proinflammatory cytokines in young men in the present study is related to testosterone, whereas oestrogens may be responsible for the suppressed cytokine production in young women.
It is possible that lower production of proinflammatory cytokines in young women is beneficial and linked to the preponderance of older women in the elderly population at large. On the other hand, young women may be more susceptible to infections than young men. Increased annual incidence of upper respiratory tract infections have been reported in female versus male swimmers in a retrospective study [50]. Longitudinal studies are necessary to throw light on these hypotheses. However, it has been shown that low circulating levels of TNF-αin vivo are associated with increased mortality in febrile patients [51] and a fatal outcome from meningococcal disease [52]. These findings indicate that a large TNF-α response is important during infections.
Inflammation has been related to the pathogenesis of atherosclerosis [1,53]. However, in the present study subjects with clinical manifestations of atherosclerosis or a low ankle-brachial arterial pressure index did not show different levels of proinflammatory cytokines in response to LPS.
Stimulation for 24 h was chosen to optimize the measurements of LPS-induced levels of TNF-α, but this may not have been the optimal time for IL-6 measurements because IL-6 has been shown to succeed TNF-α [54,55]. This could explain why age-related differences were not detected with regard to IL-6. Furthermore, the choice of 24 h stimulation may have resulted in missing differences in the initiation or duration of cytokine secretion and conclusions have to be tempered by this possibility. Thus, it is possible that the fever and leucocytosis deficit in the aged, resulting in increased susceptibility to infection, is related to a slower induction process of proinflammatory cytokines in the elderly patients rather than the absolute values.
In conclusion, elderly humans have decreased levels of proinflammatory cytokines in whole blood supernatants after exposure to endotoxin from Gram-negative bacteria, representing an ex vivo model of sepsis. This may be of special importance in elderly humans with underlying health disorders, whereas the clinical relevance is questionable in healthy elderly because decreased levels were found compared with young men but not compared with young women. Young women had suppressed levels of proinflammatory cytokines compared with young men, but this difference was blurred with age. We suggest that the low levels of proinflammatory cytokines in response to stimulation may cause a weak local inflammatory response which may play a part in the age-associated increased incidence of severe and invasive infections.
Acknowledgments
The excellent assistance of Hanne Willumsen is acknowledged. The research was supported by the Velux Foundation and The Danish Foundation for the Advancement of Medical Science. H.B. was supported by a grant from The Danish Research Council 9503346.
REFERENCES
- 1.Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhoj P, Pedersen BK. A high plasma concentration of TNF-alfa is associated with dementia in centenarians. J Gerontol Med Scien. 1999;57A:357–64. doi: 10.1093/gerona/54.7.m357. [DOI] [PubMed] [Google Scholar]
- 2.Paolisso G, Rizzo MR, Mazziotti G, et al. Advancing age and insulin resistance: role of plasma tumor necrosis factor-alpha. Am J Physiol, Endocrinol, Metabol. 1998;38:E294–E299. doi: 10.1152/ajpendo.1998.275.2.E294. [DOI] [PubMed] [Google Scholar]
- 3.Wei J, Xu H, Davies JL, Hemmings GP. Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci. 1992;51:1953–6. doi: 10.1016/0024-3205(92)90112-3. [DOI] [PubMed] [Google Scholar]
- 4.Kania DM, Binkley N, Checovich M, Havighurst T, Schilling M, Ershler WB. Elevated plasma levels of interleukin-6 in postmenopausal women do not correlate with bone density. J Am Geriatr Soc. 1995;43:236–9. doi: 10.1111/j.1532-5415.1995.tb07328.x. [DOI] [PubMed] [Google Scholar]
- 5.Ershler WB, Sun WH, Binkley N, et al. Interleukin-6 and aging: blood levels and mononuclear cell production increase with advancing age and in vitro production is modifiable by dietary restriction. Lymph Cytokine Res. 1993;12:225–30. [PubMed] [Google Scholar]
- 6.Hager K, Machein U, Krieger S, Platt D, Seefried G, Bauer J. Interleukin-6 and selected plasma proteins in healthy persons of different ages. Neurobiol Aging. 1994;15:771–2. doi: 10.1016/0197-4580(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 7.James K, Premchand N, Skibinska A, Skibinski G, Nicol M, Mason JI. IL-6, DHEA and the ageing process. Mech Ageing Dev. 1997;93:15–24. doi: 10.1016/s0047-6374(96)01807-6. [DOI] [PubMed] [Google Scholar]
- 8.Baggio G, Donazzan S, Monti D, et al. Lipoprotein(a) and lipoprotein profile in healthy centenarians: a reappraisal of vascular risk factors. FASEB J. 1998;12:433–7. doi: 10.1096/fasebj.12.6.433. [DOI] [PubMed] [Google Scholar]
- 9.Catania A, Airaghi L, Motta P, et al. Cytokine antagonists in aged subjects and their relation with cellular immunity. J Gerontol Med Sci. 1997;52:B93–B97. doi: 10.1093/gerona/52a.2.b93. [DOI] [PubMed] [Google Scholar]
- 10.Ballou SP, Lozanski FB, Hodder S, et al. Quantitative and qualitative alterations of acute-phase proteins in healthy elderly persons. Age Ageing. 1996;25:224–30. doi: 10.1093/ageing/25.3.224. [DOI] [PubMed] [Google Scholar]
- 11.Caswell M, Pike LA, Bull BS, Stuart J. Effect of patient age on tests of the acute-phase response. Arch Pathol Lab Med. 1993;117:906–10. [PubMed] [Google Scholar]
- 12.Gleckman R, Hibert D. Afebrile bacteremia. A phenomenon in geriatric patients. JAMA. 1982;248:1478–81. doi: 10.1001/jama.248.12.1478. [DOI] [PubMed] [Google Scholar]
- 13.Torres JM, Cardenas O, Vasquez A, Schlossberg D. Streptococcus pneumoniae bacteremia in a Community Hospital. Chest. 1998;113:387–90. doi: 10.1378/chest.113.2.387. [DOI] [PubMed] [Google Scholar]
- 14.Finkelstein MS, Petkun WM, Freedman ML, Antopol SC. Pneumococcal bacteremia in adults: age-dependent differences in presentation and in outcome. J Am Geriatr Soc. 1983;31:19–27. doi: 10.1111/j.1532-5415.1983.tb06283.x. [DOI] [PubMed] [Google Scholar]
- 15.Ahkee S, Srinath L, Ramirez J. Community-acquired pneumonia in the elderly: association of mortality with lack of fever and leukocytosis. South Med J. 1997;90:296–8. doi: 10.1097/00007611-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 17.Jarrett RJ, Shipley MJ, Rose G. Weight and mortality in the Whitehall Study. Br Med J Clin Res Ed. 1982;285:535–7. doi: 10.1136/bmj.285.6341.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tayback M, Kumanyika S, Chee E. Body weight as a risk factor in the elderly. Arch Intern Med. 1990;150:1065–72. [PubMed] [Google Scholar]
- 19.Losonczy KG, Harris TB, Cornoni HJ, et al. Does weight loss from middle age to old age explain the inverse weight mortality relation in old age? Am J Epidemiol. 1995;141:312–21. doi: 10.1093/aje/141.4.312. [DOI] [PubMed] [Google Scholar]
- 20.Jaattela M. Biologic activities and mechanisms of action of tumor necrosis factor-alpha/cachectin. Lab Invest. 1991;64:724–42. [PubMed] [Google Scholar]
- 21.Gutierrez-Ramos JC, Bluethmann H. Molecules and mechanisms operating in septic shock: lessons from knockout mice. Immunol Today. 1998;18:329–34. doi: 10.1016/s0167-5699(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 22.Schroll M, Jorgensen T, Ingerslev J. The Glostrup Population Studies, 1964–92. Dan Med Bull. 1992;39:204–7. [PubMed] [Google Scholar]
- 23.Schroll M, Avlund K, Davidsen M. Predictors of five-year functional ability in a longitudinal survey of men and women aged 75–80. The 1914 population in Glostrup, Denmark. Aging Mila. 1997;9:143–52. doi: 10.1007/BF03340140. [DOI] [PubMed] [Google Scholar]
- 24.Ouriel K, McDonnell AE, Metz CE, Zarins CK. Critical evaluation of stress testing in the diagnosis of peripheral vascular disease. Surgery. 1982;91:686–93. [PubMed] [Google Scholar]
- 25.Zheng ZJ, Sharrett AR, Chambless LE, et al. Associations of ankle-brachial index with clinical coronary heart disease, stroke and preclinical carotid and popliteal atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 1997;131:115–25. doi: 10.1016/s0021-9150(97)06089-9. [DOI] [PubMed] [Google Scholar]
- 26.Postiglione A, Cicerano U, Gallotta G, et al. Prevalence of peripheral arterial disease and related risk factors in elderly institutionalized subjects. Gerontol. 1992;38:330–7. doi: 10.1159/000213350. [DOI] [PubMed] [Google Scholar]
- 27.Newman AB, Siscovick DS, Manolio TA, et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993;88:837–45. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 28.McKenna M, Wolfson S, Kuller L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis. 1991;87:119–28. doi: 10.1016/0021-9150(91)90014-t. [DOI] [PubMed] [Google Scholar]
- 29.Criqui MH, Coughlin SS, Fronek A. Noninvasively diagnosed peripheral arterial disease as a predictor of mortality: results from a prospective study. Circulation. 1985;72:768–73. doi: 10.1161/01.cir.72.4.768. [DOI] [PubMed] [Google Scholar]
- 30.Ligthart GJ, Corberand JX, Fournier C, et al. Admission criteria for immunogerontological studies in man: the SENIEUR protocol. Mech Ageing Dev. 1984;28:47–55. doi: 10.1016/0047-6374(84)90152-0. [DOI] [PubMed] [Google Scholar]
- 31.Gon Y, Hashimoto S, Hayashi S, Koura T, Matsumoto K, Horie T. Lower serum concentrations of cytokines in elderly patients with pneumonia and the impaired production of cytokines by peripheral blood monocytes in the elderly. Clin Exp Immunol. 1996;106:120–6. [PubMed] [Google Scholar]
- 32.Rudd AG, Banerjee DK. Interleukin-1 production by human monocytes in ageing and disease. Age Ageing. 1989;18:43–46. doi: 10.1093/ageing/18.1.43. [DOI] [PubMed] [Google Scholar]
- 33.McLachlan JA, Serkin CD, Morrey KM, Bakouche O. Antitumoral properties of aged human monocytes. J Immunol. 1995;154:832–43. [PubMed] [Google Scholar]
- 34.Bruley RM, Vergnon I. Interleukin-1 synthesis and activity in aged mice. Mech Ageing Dev. 1984;24:247–64. doi: 10.1016/0047-6374(84)90111-8. [DOI] [PubMed] [Google Scholar]
- 35.Effros RB, Svoboda K, Walford RL. Influence of age and caloric restriction on macrophage IL-6 and TNF production. Lymphokine Cytokine Res. 1991;10:347–51. [PubMed] [Google Scholar]
- 36.Bradley SF, Vibhagool A, Kunkel SL, Kauffman CA. Monokine secretion in aging and protein malnutrition. J Leuk Biol. 1989;45:510–4. doi: 10.1002/jlb.45.6.510. [DOI] [PubMed] [Google Scholar]
- 37.Wallace PK, Eisenstein TK, Meissler JJJ, Morahan PS. Decreases in macrophage mediated antitumor activity with aging. Mech Ageing Dev. 1995;77:169–84. doi: 10.1016/0047-6374(94)01524-p. [DOI] [PubMed] [Google Scholar]
- 38.Davila DR, Edwards CK, Arkins S, Simon J, Kelley KW. Interferon-gamma-induced priming for secretion of superoxide anion and tumor necrosis factor-alpha declines in macrophages from aged rats. FASEB J. 1990;4:2906–11. doi: 10.1096/fasebj.4.11.2165948. [DOI] [PubMed] [Google Scholar]
- 39.Inamizu T, Chang MP, Makinodan T. Influence of age on the production and regulation of interleukin-1 in mice. Immunology. 1985;55:447–55. [PMC free article] [PubMed] [Google Scholar]
- 40.Norman DC, Castle S, Yamamura RH, Yoshikawa TT. Interrelationship of fever, immune response and aging in mice. Mech Ageing Dev. 1995;80:53–67. doi: 10.1016/0047-6374(94)01543-u. [DOI] [PubMed] [Google Scholar]
- 41.Riancho JA, Zarrabeitia MT, Amado JA, Olmos JM, Gonzalez MJ. Age-related differences in cytokine secretion. Gerontol. 1994;40:8–12. doi: 10.1159/000213568. [DOI] [PubMed] [Google Scholar]
- 42.Born J, Uthgenannt D, Dodt C, et al. Cytokine production and lymphocyte subpopulations in aged humans. An assessment during nocturnal sleep. Mech Ageing Dev. 1995;84:113–26. doi: 10.1016/0047-6374(95)01638-4. [DOI] [PubMed] [Google Scholar]
- 43.Roubenoff R, Harris TB, Abad LW, Wilson PF, Dallal GE, Dinarello CA. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol Med Scien. 1998;53:M20–M26. doi: 10.1093/gerona/53a.1.m20. [DOI] [PubMed] [Google Scholar]
- 44.Fagiolo U, Cossarizza A, Santacaterina S, et al. Increased cytokine production by peripheral blood mononuclear cells from healthy elderly people. Ann N Y Acad Sci. 1992;663:490–3. doi: 10.1111/j.1749-6632.1992.tb38712.x. [DOI] [PubMed] [Google Scholar]
- 45.Desch CE, Kovach NL, Present W, Broyles C, Harlan JM. Production of human tumor necrosis factor from whole blood ex vivo. Lymphokine Res. 1989;8:141–6. [PubMed] [Google Scholar]
- 46.Pacifici R, Brown C, Puscheck E, et al. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci USA. 1991;88:5134–8. doi: 10.1073/pnas.88.12.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li ZG, Danis VA, Brooks PM. Effect of gonadal steroids on the production of IL-1 and IL-6 by blood mononuclear cells in vitro. Clin Exp Rheumatol. 1993;11:157–62. [PubMed] [Google Scholar]
- 48.Angstwurm MW, Gartner R, Ziegler HH. Cyclic plasma IL-6 levels during normal menstrual cycle. Cytokine. 1997;9:370–4. doi: 10.1006/cyto.1996.0178. [DOI] [PubMed] [Google Scholar]
- 49.Kanda N, Tsuchida T, Tamaki K. Testosterone inhibits immunoglobulin production by human peripheral blood mononuclear cells. Clin Exp Immunol. 1996;106:410–5. doi: 10.1046/j.1365-2249.1996.d01-842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fricker PA, Gleeson M, McDonald WA, Pyne DB, Flanagan A, Clancy RL. International Society of Exercise and Immunology. IV. Rome: International Symposium; 1999. Incidence of respiratory and gut infections in elite swimmers; p. 67. (Abstr.) [Google Scholar]
- 51.Van Dissel JT, Van Langevelde P, Westendorp RGJ, Kvappenberg K, Frölich M. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet. 1998;351:950–3. doi: 10.1016/S0140-6736(05)60606-X. [DOI] [PubMed] [Google Scholar]
- 52.Westendorp RGJ, Langermans JAM, de Bel CE, et al. Release of tumor necrosis factor: an innate host characteristic that may contribute to the outcome of meningococcal disease. J Infect Dis. 1995;171:1057–60. doi: 10.1093/infdis/171.4.1057. [DOI] [PubMed] [Google Scholar]
- 53.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 54.De GD, Zangerle PF, Gevaert Y, et al. Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. Cytokine. 1992;4:239–48. doi: 10.1016/1043-4666(92)90062-v. [DOI] [PubMed] [Google Scholar]
- 55.DeForge LE, Remick DG. Kinetics of TNF, IL-6, and IL-8 gene expression in LPS-stimulated human whole blood. Biochem Biophys Res Commun. 1991;174:18–24. doi: 10.1016/0006-291x(91)90478-p. [DOI] [PubMed] [Google Scholar]