Abstract
The interaction between mycobacterial phenolic glycolipids (PGLs) and phagocytes was studied. Human neutrophils were allowed to interact with each of four purified mycobacterial PGLs and the neutrophil production of reactive oxygen metabolites was followed kinetically by luminol-/isoluminol-amplified chemiluminescence. The PGLs from Mycobacterium tuberculosis and Mycobacterium kansasii, respectively, were shown to stimulate the production of oxygen metabolites, while PGLs from Mycobacterium marinum and Mycobacterium bovis BCG, respectively, were unable to induce an oxidative response. Periodate treatment of the M. tuberculosis PGL decreased the production of oxygen radicals, showing the importance of the PGL carbohydrate moiety for the interaction. The activation, however, could not be inhibited by rhamnose or fucose, indicating a complex interaction which probably involves more than one saccharide unit. This is in line with the fact that the activating PGLs from M. tuberculosis and M. kansasii contain tri- and tetrasaccharides, respectively, while the nonactivating PGLs from M. marinum and M. bovis BCG each contain a monosaccharide. The complement receptor 3 (CR3) has earlier been shown to be of importance for the phagocyte binding of mycobacteria, but did not appear to be involved in the activation of neutrophils by PGLs. The subcellular localization of the reactive oxygen metabolites formed was related to the way in which the glycolipids were presented to the cells.
Keywords: Mycobacterium, oxygen radicals, carbohydrate moiety, phagocyte
INTRODUCTION
Pathogenic mycobacteria have the capacity to survive and multiply inside phagocytic cells [1]. Little is known, however, about the molecular mechanisms responsible for the mycobacterial resistance to destruction by the phagocytes. The initial contact between the mycobacteria and the phagocytic cell is in all probability of great importance for the phagocytic process and it is likely that mycobacterial surface molecules, such as the complex cell wall glycolipids, are of importance for the outcome of such an interaction. Several members of the genus Mycobacterium (i.e. Mycobacterium bovis BCG, Mycobacterium haemophilum, Mycobacterium kansasii, Mycobacterium leprae, Mycobacterium marinum, and the so-called Canetti strains of Mycobacterium tuberculosis) contain glycosyl phenolphthiocerol dimycocerosates, or so-called phenolic glycolipids (PGLs) [2–7]. These glycolipids constitute an important part of the bacterial cell envelopes of these bacteria. The long hydrophobic carbon chain of the PGLs carry a para-substituted phenol ring, which is linked to an oligosaccharide moiety containing between one and four sugars that are possibly exposed on the surface of the bacterium [3]. The PGLs are potent specific antigens, with antigenicity located in the oligosaccharide moiety, while the lipid core is thought mainly to provide an anchor in the cell wall [3].
A specific problem when studying glycolipids, such as the PGLs, is their poor solubility in water. To overcome this, the mycobacterial glycolipids may be coated on microtitre plates [8], a method well suited for studying the initial contact between phagocytes and mycobacteria.
Most studies concerning phagocytes and mycobacteria have been performed with macrophages [9], but since neutrophils usually are the first phagocytic cells to encounter bacterial invaders, they are in all probability involved also in the host defence against mycobacteria [10–12]. Upon encounter with a bacterium, the neutrophil employs different lysosomal proteins and produce reactive oxygen radicals to destroy the prey [13]. The oxygen radical producing system in phagocytes is a multicomplex membrane spanning enzyme system, the NADPH-oxidase, that is assembled at two locations during activation, both in the plasma membrane and in intracellular vesicles (e.g. phagosomes). Such activation of the NADPH-oxidase in phagocytes has been shown after interaction with intact mycobacteria or cell wall structures from mycobacteria [14–17].
In the present study, purified PGLs from four mycobacterial strains differing in carbohydrate specificity (M. bovis BCG, M. kansasii, M. marinum, and M. tuberculosis) were investigated with respect to their capacity to induce activation of the NADPH-oxidase in human neutrophils.
MATERIALS AND METHODS
Preparation of mycobacterial cell wall glycolipids
Phenolic glycolipids from M. bovis BCG Danish substrain, M. kansasii ATCC 12478 type strain, M. marinum ATCC 927 type strain and M. tuberculosis MNC 1485 (Canetti strain) were prepared as described [5,18,19]. In short, nonpolar lipids were extracted from a dried bacterial biomass of 2–5 mg using a biphasic mixture of equal amounts of methanolic saline (10 ml 0.3% aqueous NaCl added to 100 ml methanol) and petroleum ether (b.p. 60–80°C). The solvent and bacterial solution was mixed on a tube rotator for 15 min and the petroleum ether layer (top phase) was removed and evaporated to dryness at room temperature under nitrogen. The phenolic glycolipids were purified by two-dimensional preparative thin-layer chromatography (TLC) using petroleum-ether: ethyl acetate (98:2 v/v, thrice) in the first direction and petroleum-ether: acetone (98:2 v/v, once) in the second direction [5,18,19].
The glycosyl units of the PGLs used in this study contain between one and four monosaccharides of arabinohexose, fucose, or rhamnose, substituted with different number of methyl groups. The M. kansasii PGL contains a tetrasaccharide, the M. tuberculosis PGL contains a trisaccharide, while the M. marinum PGL and the M. bovis BCG PGL both contain a monosaccharide [2–7] (Table 1).
Table 1.
Sugar units of the four phenolic glycolipids investigated
Adapted from [3].
Coating of microtitre plates with mycobacterial glycolipids
The PGLs were dissolved in aqueous ethanol (70%), heated to 78°C for 100 min, and diluted in a mixture (1:1 v/v) of phosphate buffered saline (PBS) and ethanol (62.2% ethanol final concentration). The lipids (100 μl of various concentrations) were added to microtitre plate wells (nontransparent rigid styrene; MicroFLUOR, Dynatech Laboratories, USA) and incubated over night at room temperature in a humid chamber. The wells were washed three times with PBS supplemented with bovine serum albumin (BSA; 1% w/v) before use.
The coating concentration of the PGLs was 10 μg/ml, if not stated otherwise.
Coating of latex beads with PGL
Latex beads (2.6 × 109 beads; 3 μm in diameter) were washed twice in PBS and resuspended in 100 μl PBS/ethanol (1:3 v/v) containing M. tuberculosis PGL (50 μg/ml). The beads were incubated with PGL over night at room temperature, washed twice with PBS supplemented with BSA (1% w/v), and resuspended in PBS to a concentration of 5 × 108 per ml. Latex beads coated with BSA (1% w/v) were used as a control.
Isolation of neutrophils
Neutrophils were isolated from buffy coats obtained from healthy blood donors at the Sahlgrenska's Hospital, Göteborg. After dextran sedimentation at 1 g, hypotonic lysis of the remaining erythrocytes, and centrifugation on a Ficoll–Pacque gradient to remove mononuclear cells [20], the neutrophils were washed twice and resuspended (5 × 106 per ml) in Krebs-Ringer phosphate buffer containing 10 mm glucose, 1 mm Ca2+, and 1.5 mm Mg2+ (KRG; pH 7.3). The cell suspension was stored on melting ice and used within 4 h of preparation. The responsiveness of the cell preparations were routinely checked using the neutrophil activating peptide formyl-methionyl-leucyl-phenylalanine (fMLP; 10−7m final concentration) and the extracellular chemiluminescence response (peak value) was always approximately 30 light units (RLU).
Neutrophil respiratory burst measurement
The NADPH-oxidase activity was measured using a luminol/isoluminol-amplified chemiluminescence (CL) system. Two different luminometers were used. A Luminoskan (Labsystem, Helsinki, Finland) apparatus was used to follow the activity in the solid phase microtitre plate system, whereas a six channel Biolumat LB 9505 (Berthold Co., Wildbad, Germany) was used to follow the activity during neutrophil interaction with lipid coated latex beads [21–23]. The results obtained in the Luminoskan are given in arbitrary units and by the Biolumat in counts per minutes (cpm).
To measure the total amount of generated reactive oxygen radicals, neutrophils (2.5 × 105 per well) were mixed with horseradish peroxidase (HRP; 4 U), luminol (56 μm), and KRG in a total volume of 200 μl. In order to differentiate between intracellularly and extracellularly produced oxygen metabolites, two different reaction mixtures were used. The CL system used for measurement of intracellularly generated oxygen metabolites contained neutrophils (2.5 × 105 per well), luminol (56 μm), superoxide dismutase (SOD; a membrane-impermeable scavenger of O2−; 50 U/ml), and catalase (a membrane-impermeable scavenger of H2O2; 2000 U/ml) [23]. The CL system for detection of the extracellularly released reactive oxygen radicals contained HRP (4 U), neutrophils (2.5 × 105/well), and isoluminol (a membrane-impermeable CL substrate; 56 μm), all in KRG [24].
Degradation of the sugar moiety
To determine the involvement of the PGL carbohydrate moieties in the neutrophil interaction, M. tuberculosis PGL coated on microtitre plates was oxidized with sodium meta-periodate (50 mm; 0.1 ml/well) in the dark at room temperature for 16 h [4,7,25,26]. The plates were washed three times with PBS supplemented with BSA (1% w/v) before use. The control plates were treated the same way, but in the abscence of sodium meta-periodate.
Inhibition of the interaction between neutrophils and M. tuberculosis PGL
To evaluate which monosaccharide in the M. tuberculosis PGL that could be responsible for the neutrophil activation, l-rhamnose and methyl-β-l-fucopyranoside, respectively, were added to the microtitre wells (concentrations up to 100 mm were tested) together with neutrophils (2.5 × 105/well). The production of oxygen metabolites was measured by luminol-amplified chemiluminescence (CL).
To evaluate if the PGLs bind and activate the neutrophils through complement receptor 3 (CR3; CD11b/CD18), α-methyl-d-mannoside (10–150 mm final concentration [27–31]), N-acetyl-d-glucosamine (NAG; 10–100 mm final concentration [27,31]), or the tripeptide Arg-Gly-Asp (RGD-peptide; 250 mm final concentration [32,33]); were added to the microtitre wells together with the neutrophils (2.5 × 105 per well). The production of oxygen metabolites was measured by luminol-amplified CL.
Mobilization of neutrophils granules
Neutrophils that exist in a primed state respond to a certain stimulus in an enhanced fashion as compared to unprimed cells [34]. Such primed cells have usually mobilized part of their granules to the cell surface and thus expose an increased amount of receptor structures [35–37]. Here, neutrophils were primed to different extents by use of two different mobilization procedures. One cell population was incubated at 37°C for 30 min without any additive. The other cell population was incubated for 10 min at 15°C with the chemoattractant fMLP (10−7m final concentration) and then transferred to a heated water bath (37°C) for another 5 min incubation. This protocol mobilizes the granules without inducing any activation of the NADPH-oxidase [37,38]. The cells were washed once and resuspended to 5 × 106 cells/ml in KRG and put on ice until use.
Reagents
Catalase, SOD, and HRP were acquired from Boehringer-Mannheim (Mannheim, Germany). Dextran and Ficoll–Pacque were obtained from Pharmacia Fine Chemicals (Uppsala, Sweden). Luminol, isoluminol, cytochalasin B, latex beads, fMLP, Arg-Gly-Asp, α-methyl-d-mannoside, NAG, l-rhamnose, methyl-β-l-fucopyranoside, and BSA were obtained from Sigma (Sigma Chemical Co., St Louis, MO, USA). Sodium meta-periodate was acquired from Merck (Darmstadt, Germany).
RESULTS
Neutrophil NADPH-oxidase activity induced by mycobacterial PGL
The mycobacterial PGLs were analysed regarding their capacity to activate the neutrophil NADPH-oxidase. The PGLs from M. tuberculosis and M. kansasii induced a substantial increase in NADPH-oxidase activity (Fig. 1), while no superoxide anion production was obtained in response to M. bovis BCG or M. marinum PGLs (Fig. 1). The amount of superoxide anion formed upon interaction with M. tuberculosis or M. kansasii PGLs was dependent on the coating concentration, but no further increase was obtained with coating concentrations higher than 10 μg/ml of PGL (Fig. 2).
Fig. 1.
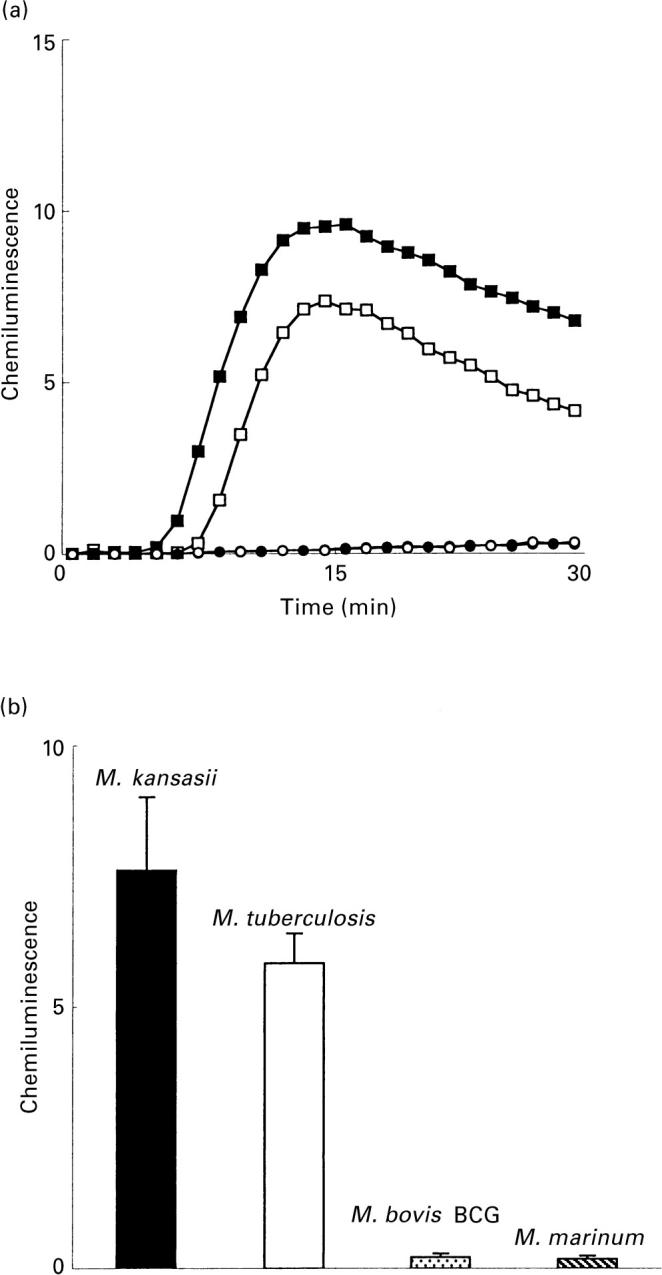
Neutrophil NADPH-oxidase activity triggered by mycobacterial PGL. (a) The time-courses of the neutrophil responses induced by M. tuberculosis PGL (□), M. kansasii PGL (▪), M. bovis BCG PGL (○), and M. marinum PGL (•), are given. The responses were measured with luminol and HRP in microtitre wells coated with PGL (coating concentration of 10 μg/ml). Abscissa, time (min); ordinata, chemiluminescence (arbitrary units). (b) The mean peak value (+ SD) of five (M. bovis BCG;  and M. marinum;
and M. marinum; 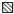 PGL) and 10 (M. tuberculosis; □ and M. kansasii; ▪ PGL) experiments. Ordinata, CL (arbitrary units).
PGL) and 10 (M. tuberculosis; □ and M. kansasii; ▪ PGL) experiments. Ordinata, CL (arbitrary units).
Fig. 2.
Neutrophil NADPH-oxidase activity triggered by PGL from M. tuberculosis and M. kansasii, respectively. The time-courses of the neutrophil responses induced by (a) M. tuberculosis PGL or (b) M. kansasii PGL are given. The responses were measured with luminol and HRP in microtitre wells coated with PGL at coating concentrations of 0.1 μg/ml (•), 10 μg/ml (□), or 30 μg/ml (▪). Abscissa, time (min); ordinata, chemiluminescence (arbitrary units).
Role of the PGL carbohydrate moiety
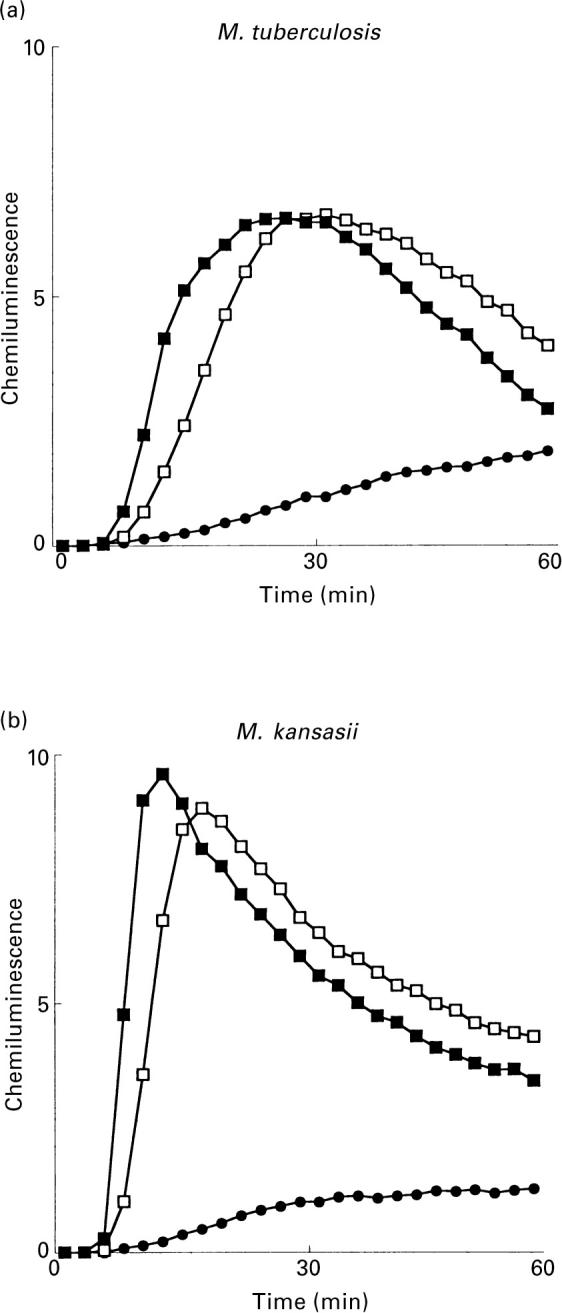
Since the differences between the PGLs tested lies in their suger moieties (Table 1), we investigated whether the carbohydrate moeity of the PGLs is responsible for neutrophil activation. One of the activating PGLs (M. tuberculosis) was pretreated with sodium meta-periodate. The cellular production of reactive oxygen radicals was reduced following this treatment (Fig. 3), indicating that an intact oligosaccharide was crucial for the PGL activation of neutrophils.
Fig. 3.
Effect of sodium meta-periodate treatment on the neutrophil NADPH-oxidase activity triggered by M. tuberculosis PGL. The figure shows the time-course of the CL response induced by periodate-treated (▪) or untreated (□) M. tuberculosis PGL (coating concentration 10 μg/ml). The responses were measured in the presence of luminol and HRP. Abscissa, time (min); ordinata, chemiluminescence (arbitrary units). The inset shows the mean peak value (+ SD) of three experiments.
Effect of monosaccharides
The M. tuberculosis PGL contains fucose and rhamnose. To further evaluate how the carbohydrate moiety of the PGL takes part in the activation of the neutrophils, l-rhamnose or methyl-β-l-fucopyranoside, respectively, were added together with neutrophils to wells coated with M. tuberculosis PGL. Neither of these sugars could inhibit the PGL-induced production of oxygen metabolites (Fig. 4). Concentrations higher than 3 mm of the monosaccharides inhibited the responses unspecifically, as shown by the fact that also direct activation of the oxidase with the protein kinase C-agonist phorbol myristate acetate (PMA) was inhibited to the same extent.
Fig. 4.
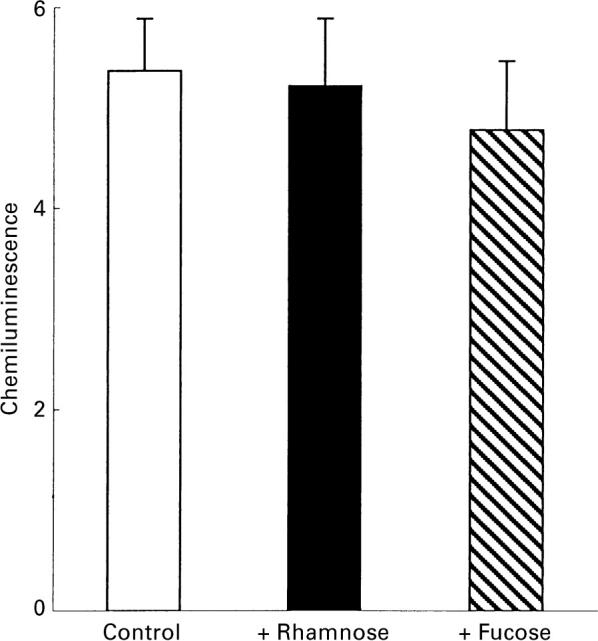
Effect of monosaccharides on the superoxide anion production in human neutrophils induced by M. tuberculosis PGL. The figure shows the mean peak value (+ SD) of the CL responses induced by M. tuberculosis PGL (coating concentration 10 μg/ml) in the absence (□) or presence of 3 mm rhamnose (▪) or fucose (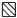 ). The responses were measured in the presence of luminol and HRP. Ordinata, chemiluminescence (arbitrary units).
). The responses were measured in the presence of luminol and HRP. Ordinata, chemiluminescence (arbitrary units).
Effect of CR3 inhibitors
Earlier studies have shown that the complement receptor 3 (CR3; CD11b/CD18, Mac-1) can be involved in the uptake of mycobacteria by phagocytes [28,39]. The influence of three different CR3 inhibitors (α-methyl-d-mannoside, NAG, and the tri-peptide Arg-Gly-Asp in concentrations known to inhibit CR3-mediated functions [31,33]) on the neutrophil–PGL interaction was investigated. None of these compounds could inhibit the PGL induced production of oxygen metabolites (data not shown).
Subcellular localization of the superoxide anion production
Oxygen metabolites can be produced from the NADPH-oxidase in intracellular compartments (e.g. phagosomes) or be released from the cell when produced by the plasma membrane-localized NADPH-oxidase [40–42]. When coated on a surface, M. tuberculosis PGL induced an extracellular release of reactive oxygen metabolites, while much less superoxide anion was detected intracellularly (Fig. 5). When neutrophils were allowed to interact with PGLs coated on latex beads, mimicking a phagocytosable prey, reactive oxygen radicals were produced intracellularly (Fig. 5).
Fig. 5.
Extra- and intracellular NADPH-oxidase activity triggered by PGL from M. tuberculosis. The figure shows the time-course of the CL responses in neutrophils induced by M. tuberculosis PGL coated on a surface as measured by a Luminoskan (a) or coated on latex beads as measured by a six channel Biolumat LB 9505 (b). Coating concentration (a) 10 μg/ml and (b) 50 μg/ml. The intracellular responses to PGL (▪) were measured in the presence of SOD, catalase, and luminol, while the extracellular responses to PGL (□) were measured in the presence of HRP and isoluminol. In (b) the intracellular (♦) and extracellular response (⋄) to control latex beads is included. Abscissa, time (min); ordinata, chemiluminescence. (a) Arbitrary units, (b) Mcpm). The insets show the mean peak value (+ SD) of three experiments.
Role of the cytoskeleton
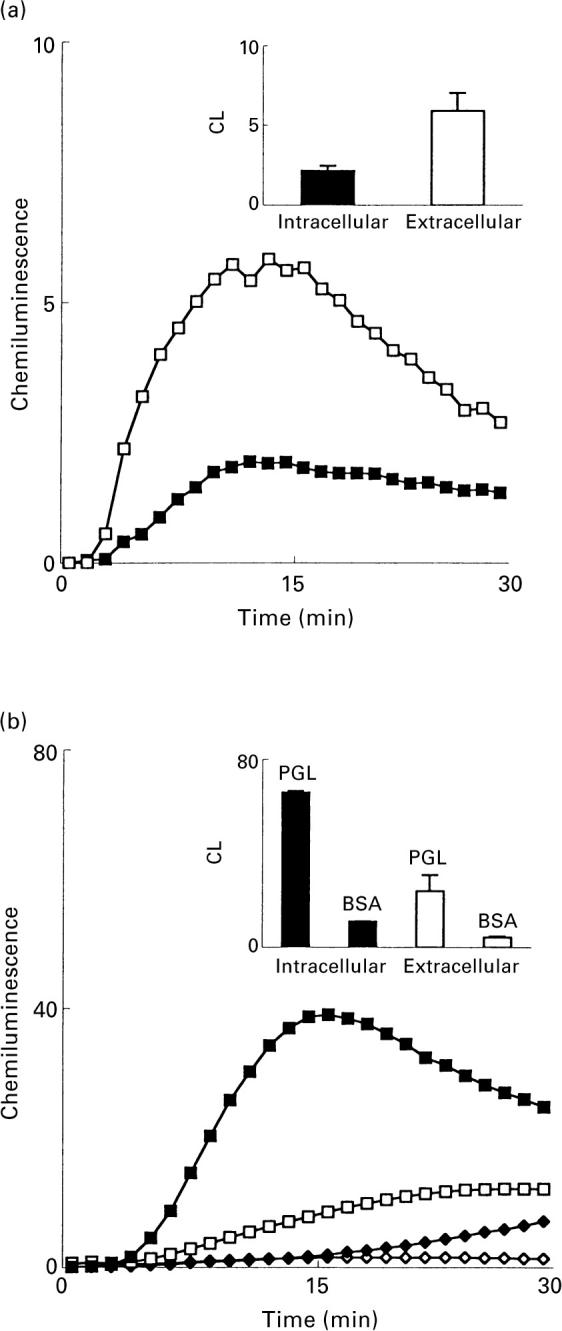
The role of the neutrophil cytoskeleton in the PGL interaction with neutrophils was investigated by adding cytochalasin B (a fungal metabolite that blocks actin filament formation) to the assay system. Cytochalasin B abolished the production of oxygen metabolites (Fig. 6), indicating that an intact cytoskeleton is a prerequisite for the PGL-induced activation of the neutrophil NADPH-oxidase.
Fig. 6.
Effect of cytochalasin B on the superoxide anion production in human neutrophils induced by M. tuberculosis PGL. The time-course of the CL response induced by M. tuberculosis PGL (coating concentration 10 μg/ml) in the absence (▪) or presence (□) of cytochalasin B (1 μg/ml final concentration) is shown. The responses were measured in the presence of luminol and HRP. Abscissa, time (min); ordinata, chemiluminescence (arbitrary units). The inset shows the oxidative response in neutrophils preincubated with (broken line) or without (solid line) 1 μg/ml cytochalasin B and subsequently stimulated with fMLP (10−7m final concentration). Abscissa, time (min); ordinata, CL (Mcpm).
Effect of granule mobilization
Neutrophils with mobilized granules, induced either through a 30-min preincubation at 37°C or by preincubation with the chemoattractant fMLP, were challenged with the M. tuberculosis PGL coated on a surface. We have earlier shown that these protocols results in a primed neutrophil response to other stimuli, including chemotactic peptides and the lectin galectin-3 [34–37]. No effect of the priming procedures was obtained when the cells were challenged with PGL, i.e. the response induced in the primed neutrophils equaled that of ‘unprimed’ control cells (data not shown).
DISCUSSION
The M. tuberculosis PGL and M. kansasii PGL induced an oxidative burst in neutrophils, while PGL from M. marinum and M. bovis BCG did not. Since the lipid core is similar in all four PGLs [3], the carbohydrate part is most likely responsible for the differences in response. To evaluate the importance of the sugar moiety for the PGL activation of the neutrophil NADPH-oxidase, M. tuberculosis PGL were treated with sodium meta-periodate, which degrades the sugar part [4,7,25,26]. Degradation of the carbohydrate resulted in a reduced oxidative response, indicating that the sugar moiety is of crucial importance. The M. kansasii PGL, containing a tetrasaccharide, induced a larger response than the M. tuberculosis PGL, which contains a trisaccharide. The M. tuberculosis trisaccharide is similar to the three internal monosaccharides of the M. kansasii tetrasaccharide, but differs in methylation [2,4,6,7]. The PGLs from M. marinum and M. bovis BCG, which were not able to activate the neutrophil NADPH-oxidase, both contain a monosaccharide (methyl-rhamnose) differing from each other in the position of the methylation [5,7]. The difference in capability of activating neutrophils can thus dependent either on the length of the carbohydrate part of the PGL or on the identity of the sugar. To test this, we attempted to inhibit the production of oxygen metabolites with l-rhamnose or methyl-β-l-fucopyranoside since both of the activating PGLs have rhamnose and fucose as part of their oligosaccharides. These two monosaccharides were, however, not capable to inhibit the oxidative response. Whether this is due to different substitutions on the monosaccharides or an elongated binding site in the neutrophil receptor, needing an oligosaccharide for binding, cannot be determined from these experiments.
Earlier studies have shown that CR3 (CD11b/CD18, Mac-1) may be involved in the binding and uptake of M. tuberculosis [28,39]. The CR3 contains two different binding sites, a peptide binding site that can bind the tripeptide Arg-Gly-Asp and a lectin site that binds carbohydrates. The sugar specificity of this lectin site is broad, allowing it to bind polysaccharides containing mannose or N-acetyl-d-glucosamine, as well as glucose [31]. The interaction between CR3 and a ligand can thus be inhibited either by the peptide sequence Arg-Gly-Asp or by carbohydrates, depending on which of the integrin attachment sites the interaction involves. Opsonized M. tuberculosis binds CR3 at its C3 bi binding site (the peptide site), whereas nonopsonized M. tuberculosis binds with surface polysaccharides to the lectin site near the C-terminus of the CR3 α-chain (CD11b) [43,44]. We investigated whether either of the CR3 binding sites was involved by adding the tri-peptide Arg-Gly-Asp or either of the monosaccharides α-methyl-d-mannoside and NAG, using concentrations known to inhibit CR3-mediated functions [27–33]. No inhibition of oxygen metabolite production was detected, indicating that CR3 is not involved in the opsonin–independent interaction between the mycobacterial PGL and neutrophils.
Many neutrophil receptors for bacteria and other stimulating substances are localized in the membranes of intracellular organelles, granules, in resting neutrophils. Upon activation, e.g. during the extravasation process, these receptors are mobilized to the cell surface by fusion of the granules with the plasma membrane [35–37]. To investigate whether the receptor structures mediating the PGL-induced activation are granule localized, the granules were mobilized prior to stimulation with the lipids. Since no difference was seen between resting and mobilized neutrophils regarding PGL interaction, we conclude that the receptor(s) involved are present on the cell surface only.
The oxygen radicals produced after neutrophil interaction with surface-adhered PGL was to a large extent extracellularly located while much less superoxide anion was detected intracellularly. These data support the concept of ‘frustrated phagocytosis’ [45], i.e. when the PGLs are bound to a nonphagocytosable surface, oxygen radicals leak from the unclosed ‘phagocytic’ compartment. In the present study, the release of superoxide anion was the direct consequence of the assay system, a conclusion based on the fact that PGL coated on a phagocytosable particle induced intracellular (phagosome-localized) production of oxygen metabolites.
The actin filament formation can be inhibited by the fungal metabolite cytochalasin B [46,47]. The consequence of such an inhibition is that phagocytosis is inhibited and oxidase activity and granule secretion is elevated upon stimulation. When cytochalasin B was added together with neutrophils to the PGL-coated surfaces, the NADPH-oxidase activity was completely inhibited, indicating that the cytoskeleton is involved in this interaction and that the event in this aspect resembles a normal phagocytic engulfment of a particulate prey.
The results of the present study indicate that the differences in capability among the tested PGLs to activate neutrophils might be due to the different number of monosaccharides presented. Thus, the PGLs with only a monosaccharide might not bind to the neutrophils efficiently enough. The importance of PGLs in the intracellular survival of mycobacteria can only be speculated upon. From a virulence point of view it may be an advantage for the microbe to expose cell wall structures which are unable to activate neutrophils. The organism might actually be able to conceal itself under such a cover. Both increased and decreased oxygen metabolite production have been shown in phagocytes after interaction with different mycobacterial lipids, including PGLs [16,48]. Extended analysis of PGLs including the one of M. leprae, which contain a trisaccharide, would be of value in elucidating their importance in mycobacterial pathogenesis. It would be particularly interesting to study whether or not the different PGLs have any priming or downregulatory effects on neutrophils and how these capacities are related to the chemical features of the PGLs.
Acknowledgments
This study was supported by Oscar II's Jubilee Fund of Swedish Heart and Lung Foundation (M.R.), the Swedish Medical Research Council (C.D.), the King Gustaf V 80-year Foundation (C.D.), and the Fredrik and Ingrid Thuring Foundation (A.K.). J.F. is supported by a PhD grant from Oscar II's Jubilee Fund of Swedish Heart and Lung Foundation. A.M.S.A. is a PhD-student supported by the Goverment of the United Arab Emirates.
REFERENCES
- 1.Rook GAW, Bloom BR. Mechanism of pathogenesis in tuberculosis. In: Bloom BR, editor. Tuberculosis. Washington, DC: ASM Press; 1994. pp. 485–501. [Google Scholar]
- 2.Rivière M, Fourniè JJ, Puzo G. A novel mannose containing phenolic glycolipid from Mycobacterium kansasii. J Biol Chem. 1987;262:14879–84. [PubMed] [Google Scholar]
- 3.Hartmann S, Minnikin DE. Mycobacterial phenolic glycolipids. In: Tyman JHP, editor. Surfactants in lipid chemistry. Cambridge: Royal Society of Chemistry; 1992. pp. 135–58. [Google Scholar]
- 4.Daffé M, Lacave C, Laneelle MA, Laneelle G. Structure of the major triglycosyl phenol-phthiocerol of Mycobacterium tuberculosis(strain Canetti) Eur J Biochem. 1987;167:155–60. doi: 10.1111/j.1432-1033.1987.tb13317.x. [DOI] [PubMed] [Google Scholar]
- 5.Minnikin DE, Dobson G, Parlett JH, Goodfellow M, Magnusson M. Analysis of dimycocerosates of glycosyl phenolphthiocerols in the identification of some clinically significant mycobacteria. Eur J Clin Microbiol. 1987;6:703–7. doi: 10.1007/BF02013082. [DOI] [PubMed] [Google Scholar]
- 6.Fournie′ JJ, Rivie'Re M, Puzo G. Absolute configuration of the unique 2,6-dideoxy-4-O-methyl-arabino-hexopyranose of the major phenolic glycolipid antigen from Mycobacterium kansasii. Eur J Biochem. 1987;168:181–3. doi: 10.1111/j.1432-1033.1987.tb13402.x. [DOI] [PubMed] [Google Scholar]
- 7.Daffé M, Lanéelle MA, Lacave C, Lanéelle G. Monoglycosyldiacylphenol-phthiocerol of Mycobacterium tuberculosis and Mycobacterium bovis. Biochim Biophys Acta. 1988;958:443–9. doi: 10.1016/0005-2760(88)90230-5. [DOI] [PubMed] [Google Scholar]
- 8.Ridell M, Wallerström G, Minnikin DE, Bolton RC, Magnusson M. A comparative serological study of antigenic glycolipids from Mycobacterium tuberculosis. Tuber Lung Dis. 1992;73:101–5. doi: 10.1016/0962-8479(92)90063-P. [DOI] [PubMed] [Google Scholar]
- 9.Adams DO, Hamilton TA. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- 10.Brown AE, Holzer TJ, Andersen BR. Capacity of human neutrophils to kill Mycobacterium tuberculosis. J Infect Dis. 1987;156:985–91. doi: 10.1093/infdis/156.6.985. [DOI] [PubMed] [Google Scholar]
- 11.Riedel DD, Kaufmann SHE. Chemokine secretion by human polymorphonuclear granulocytes after stimulation with Mycobacterium tuberculosis and lipoarabinomannan. Infect Immun. 1997;65:4620–3. doi: 10.1128/iai.65.11.4620-4623.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva MT, Nazare′ M, Silva T, Appelberg R. Neutrophil-macrophage cooperation in the host defence against mycobacterial infections. Microb Pathogen. 1989;6:369–80. doi: 10.1016/0882-4010(89)90079-x. [DOI] [PubMed] [Google Scholar]
- 13.Gallin JI, Goldstein IM, Snyderman R. Inflammation basic principles and clinical correlates. New York: Raven Press; 1992. [Google Scholar]
- 14.Zhang L, Goren MB, Holzer TJ, Andersen BR. Effect of Mycobacterium tuberculosis-derived sulfolipid I on human phagocytic cells. Infect Immun. 1988;56:2876–83. doi: 10.1128/iai.56.11.2876-2883.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, English D, Andersen BR. Activation of human neutrophils by Mycobacterium tuberculosis derived sulfolipid-1. J Immunol. 1991;146:2730–6. [PubMed] [Google Scholar]
- 16.Zhang L, Gay JC, English D, Andersen BR. Neutrophil priming mechanisms of sulfolipid-I and N-formyl-methionyl-leucyl-phenylalanine. J Biomed Sci. 1994;1:253–62. doi: 10.1007/BF02253310. [DOI] [PubMed] [Google Scholar]
- 17.Geertsma MF, Nibbering PH, Pos O, Van Furth R. Interferon-γ-activated human granulocytes kill ingested Mycobacterium fortuitum more efficiently than normal granulocytes. Eur J Immunol. 1990;20:869–73. doi: 10.1002/eji.1830200423. [DOI] [PubMed] [Google Scholar]
- 18.Dobson G, Minnikin DE, Besra GS, Mallet AI, Magnusson M. Characterisation of phenolic glycolipids from Mycobacterium marinum. Biochim Biophys Acta. 1990;1042:176–81. doi: 10.1016/0005-2760(90)90004-h. [DOI] [PubMed] [Google Scholar]
- 19.Dobson G, Minnikin DE, Minnikin SM, Parlett JH, Goodfellow M, Ridell M, Magnusson M. Chem Meth Bact Syst. London: Academic Press; 1985. Systematic analysis of complex mycobacterial lipids; pp. 237–65. [Google Scholar]
- 20.Bøyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest. 1968;97:77–89. [PubMed] [Google Scholar]
- 21.Allen RC, Loose LD. Phagocytic activation of a luminol-dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun. 1976;69:245–52. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- 22.Dahlgren C, Follin P, Johansson A, Lock R, Orselius K. Localization of the luminol-dependent chemiluminoscence reaction in human granulocytes. J Biochem Chemilum. 1989;4:263–6. doi: 10.1002/bio.1170040137. [DOI] [PubMed] [Google Scholar]
- 23.Dahlgren C, Follin P, Johansson A, Lock R, Lundqvist H, Walan Å. Chemiluminescence as a means of following the function of phagocytic cells. Trends Photochem Photobiol. 1991;2:427–43. [Google Scholar]
- 24.Lundqvist H, Dahlgren C. Isoluminol-enhanced chemiluminescence: a sensitive method to study the release of superoxide anion from human neutrophils. Free Radic Biol Med. 1996;20:785–92. doi: 10.1016/0891-5849(95)02189-2. [DOI] [PubMed] [Google Scholar]
- 25.Vann WF, Jann K. Structure and serological specificity of the. K13-antigenic polysaccharide (K13 antigen) of urinary tract-infective Escherichia coli. Infect Immun. 1979;25:85–92. doi: 10.1128/iai.25.1.85-92.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt MA, Jann K. Structure of the 2-keto-3-deoxy-D-manno-octonic-acid-containing capsular polysaccharide (K12 antigen) of the urinary-tract-infective Escherichia coli O4:K12:H−. Eur J Biochem. 1983;131:509–17. doi: 10.1111/j.1432-1033.1983.tb07291.x. [DOI] [PubMed] [Google Scholar]
- 27.Sehgal G, Zhang K, Todd RF III, Boxer LA, Petty HR. Lectin-like inhibition of immune complex receptor-mediated stimulation of neutrophils. J Immunol. 1993;150:4571–80. [PubMed] [Google Scholar]
- 28.Schlesinger LS. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–30. [PubMed] [Google Scholar]
- 29.Schlesinger LS, Hull SR, Kaufman TM. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J Immunol. 1994;152:4070–9. [PubMed] [Google Scholar]
- 30.Schlesinger LS, Kaufman TM, Iyver S, Hull SR, Marchiando LK. Differences in mannose receptor-mediated uptake of lipoarabinomannan from virulent and attenuated strains of Mycobacterium tuberculosis by human macrophages. J Immunol. 1996;157:4568–75. [PubMed] [Google Scholar]
- 31.Thornton BP, Vetvicka V, Pitman M, Goldman RC, Ross GD. Analysis of the suger specificity and molecular location of the β-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18) J Immunol. 1996;156:1235–46. [PubMed] [Google Scholar]
- 32.Wright SD, Levin SM, Jong MT, Chad Z, Kabbash LGCR. 3 (CD11b/CD18): expresses one binding site for Arg-Gly-Asp-containing peptides and a second site for bacterial lipopolysaccharide. J Exp Med. 1989;169:175–83. doi: 10.1084/jem.169.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herrmann M, Jaconi MEE, Dahlgren C, Waldvogel FA, Stendahl O, Lew DP. Neutrophil bactericidal activity aganist Staphylococcus aureus adherent on biological surfaces. J Clin Invest. 1990;86:942–51. doi: 10.1172/JCI114796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tauber AI, Karnad AB, Hartshorn KL, Myers JB, Schwartz JH. Parameters of neutrophil activation: models of priming and deactivation. Prog Clin Biol Res. 1989;297:297–309. [PubMed] [Google Scholar]
- 35.Sengeløv H, Follin P, Kjeldsen K, Lollike K, Dahlgren C, Borregaard N. Mobilization of granules and secretory vesicles during in vivo exudation of human neutrophils. J Immunol. 1995;154:4157–65. [PubMed] [Google Scholar]
- 36.Andersson T, Dahlgren C, Lew PD, Stendahl O. Cell surface expression of fMet-Leu-Phe receptors on human neutrophils. Correlation to changes in cytosolic free Ca2+ level and action of phorbol myristate acetate. J Clin Invest. 1987;79:1226–33. doi: 10.1172/JCI112941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karlsson A, Follin P, Leffler H, Dahlgren C. Galectin-3 activates the NADPH-oxidase in exudated but not peripheral blood neutrophils. Blood. 1998;91:3430–8. [PubMed] [Google Scholar]
- 38.Lundqvist H, Gustafsson M, Johansson A, Särndahl E, Dahlgren C. Neutrophil control of formylmethionyl-leucyl-phenylalanine induced mobilization of secretory vesicles and NADPH-oxidase activation: effect of an association of the ligand-receptor complex to the cytoskeleton. Biochim Biophys Acta. 1994;1224:43–50. doi: 10.1016/0167-4889(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 39.Hirsch CS, Ellner JJ, Russell DG, Rich EA. Complement receptor-mediated uptake and tumor necrosis factor-α-mediated growth inhibition of Mycobacterium tuberculosis by human alveolar macrophages. J Immunol. 1994;152:743–53. [PubMed] [Google Scholar]
- 40.Rosen GM, Pou S, Ramos CL, Cohen MS, Britigan BE. Free radicals and phagocytic cells. FASEB J. 1995;9:200–9. doi: 10.1096/fasebj.9.2.7540156. [DOI] [PubMed] [Google Scholar]
- 41.Babior BM. The respiratory burst oxidase and the molecular basis of chronic granulomatous disease. Am J Hematol. 1991;37:263–6. doi: 10.1002/ajh.2830370410. [DOI] [PubMed] [Google Scholar]
- 42.Dahlgren C, Johansson A, Lundqvist H, Bjerrum OW, Borregaard N. Activation of the oxygen-radical-generating system in granules of intact human neutrophils by a calcium ionophore (ionomycin) Biochim Biophys Acta. 1992;1137:182–8. doi: 10.1016/0167-4889(92)90200-u. [DOI] [PubMed] [Google Scholar]
- 43.Cywes C, Godenir NL, Hoppe HC, Scholle RR, Steyn LM, Kirsch RE, Ehlers MR. Nonopsonic binding of Mycobacterium tuberculosis to human complement receptor type 3 expressed in chinese hamster ovary cells. Infect Immun. 1996;64:5373–883. doi: 10.1128/iai.64.12.5373-5383.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cywes C, Hoppe HC, Daffe′ M, Ehlers MRW. Nonopsinic binding of Mycobacterium tuberculosis to complement receptor type 3 is mediated by capsular polysaccharides and is strain dependent. Infect Immun. 1997;65:4258–66. doi: 10.1128/iai.65.10.4258-4266.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu L, Elwing H, Karlsson A, Nimeri G, Dahlgren C. Surface-related triggering of the neutrophil respiratory burst. Characterization of the response induced by IgG adsorbed to hydrophilic and hydrophobic glass surfaces. Clin Exp Immunol. 1997;109:204–10. doi: 10.1046/j.1365-2249.1997.4311329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheterline P, Rickard JE. Hallet MB. The neutrophil: cellular biochemistry and physiology boca raton. Florida, USA: CRC Press; 1989. The cortical actin filament network of neutrophil leucocytes during phagocytosis and chemotaxis; pp. 141–65. [Google Scholar]
- 47.Takemura R, Stenberg P, Bainton D, Werb Z. Rapid distribution of clathrin onto macrophage plasma mambranes in response to Fc receptor–ligand interaction during frustrated phagocytosis. J Cell Biol. 1986;102:55–59. doi: 10.1083/jcb.102.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moura ACN, Modolell M, Mariano M. Down-regulatory effect of Mycobacterium leprae cell wall lipids on phagocytosis, oxidative respiratory burst and tumour cell killing by mouse bone marrow derived macrophages. Scand J Immunol. 1997;46:500–5. doi: 10.1046/j.1365-3083.1997.d01-158.x. [DOI] [PubMed] [Google Scholar]



