Abstract
Benznidazole (BZL) is a nitroheterocyclic drug employed in the chemotherapy of Chagas' disease, a protozoan disease caused by Trypanosoma cruzi. Because this parasite mostly replicates in macrophages, we investigated whether BZL was likely to modify the synthesis of macrophage mediators such as nitrite, tumour necrosis factor-alpha (TNF-α), IL-1β, IL-6 and IL-10. Control and stimulated murine macrophages (lipopolysaccharide (LPS) and/or interferon-gamma (IFN-γ)) were treated with BZL and measurements were carried out in culture supernatants collected 24 h later. Synthesis of nitrite, IL-6 and IL-10 was maximal upon combined stimulation with LPS + IFN-γ, whereas lower amounts of the three mediators were detected when both stimuli were given alone. BZL treatment significantly reduced nitrite, IL-6 and IL-10 production, to undetectable levels in some cases, particularly IL-6 and IL-10. LPS was the most potent stimulus of IL-1β and TNF-α production, followed by LPS + IFN-γ and IFN-γ in decreasing order. BZL partly inhibited TNF-α synthesis, but this effect was smaller than that observed for nitrite, IL-6 and IL-10. LPS-induced production of IL-1β was also affected by BZL. Semiquantification of gene expression for inducible nitric oxide synthase (iNOS) showed that BZL completely inhibited iNOS gene induction by IFN-γ, and resulted in respective inhibitions of 30% and 50% with LPS- and LPS + IFN-γ-stimulated cells. BZL was not cytotoxic on macrophage cultures, as shown by the lactate dehydrogenase activity. Besides its trypanocidal activity, BZL may also alter the balance between pro- and anti-inflammatory mediators with important consequences for the course of T. cruzi infection.
Keywords: benznidazole, nitrite, inducible nitric oxide synthase, cytokines, down-regulation
INTRODUCTION
Benznidazole (BZL) is a nitroheterocyclic drug usually employed in the chemotherapy of Chagas' disease, a protozoan disease caused by the haemoflagellate Trypanosoma cruzi which remains a major health problem in Latin America [1,2]. Although the biochemical basis for the trypanocidal activity of BZL is still not properly understood, experimental studies suggest that BZL could interfere directly in the synthesis of macromolecules via covalent binding or other types of interaction between nitroreduction intermediates and various cellular components, like DNA, lipids and proteins of T. cruzi [3,4].
As BZL is also toxic to the mammalian host [5], the question arises whether it induces any metabolic changes in cells serving as sites of habitation by T. cruzi, such as macrophages. We therefore investigated the in vitro effects of BZL on the functional activity of cultured macrophages. We studied the synthesis of various macrophage-synthesized soluble mediators known to be present during T. cruzi infection: nitrite, a measurement of nitric oxide (NO) production, tumour necrosis factor-alpha (TNF-α), IL-1β, IL-6 and IL-10 [6–12]. Some of these probably play a role in the immunopathological mechanisms accompanying this trypanosomiasis.
Synthesis of NO occurs through the conversion of l-arginine to citrulline by different isoforms of the NO synthases (NOS): two constitutive enzymes (NOS-I and NOS-III) detected in the central nervous system and endothelial cells, and an inducible NO synthase (iNOS or NOS-II) that is present in many cells, including macrophages [13,14]. In these cells, the enzyme is transcriptionally induced by interferon-gamma (IFN-γ) alone or synergistically with lipopolysaccharide (LPS) or TNF-α [15,16]. Several studies indicate that NO production by activated macrophages plays an essential role in in vitro and in vivo resistance against T. cruzi infection [6,17–19], while excessive amounts of NO released by these cells may have harmful effects because of its proinflammatory activity. Given this background, we were also interested in ascertaining whether BZL could affect iNOS gene expression in stimulated macrophages.
MATERIALS AND METHODS
Reagents
Recombinant murine IFN-γ (specific activity 2 × 107 U/mg) was a gift from Dr G. Adolf (Boehringer-Ingelheim, Vienna, Austria), and BZL was a gift from Roche Labs. (Buenos Aires, Argentina). LPS from Escherichia coli, serotype 0111:B4, was from Sigma (St Louis, MO).
Cell cultures and stimulation
The macrophage cell line RAW 264.7 (macrophage, Abelson leukaemia virus transformed) was obtained from the American Type Culture Collection (Rockville, MD). Cells were adjusted to a concentration of 250 000 cells/well (24-well Nunc plates) and grown in Dulbecco's modified Eagle's minimal essential medium (DMEM) supplemented with 5% heat-inactivated fetal calf serum (FCS) shown to be endotoxin-free, 2 mml-glutamine, non-essential amino acids, 2 mm HEPES and 20 μg gentamycin in 5% CO2. Cells were cultured overnight. Subsequently, culture medium was replaced by 1 ml of fresh medium with or without LPS (100 ng/ml), in presence or absence of IFN-γ (500 U/ml), and of BZL (final concentrations 0.1 mm, 0.5 mm, or 1 mm). BZL was dissolved in 2-methoxiethanol as described by Moreno et al. [20]. Cultures containing medium alone and those in which LPS and IFN-γ were added with similar amounts of 2-methoxiethanol were also performed for comparison purposes. After 24 h of stimulation, culture supernatants were collected and stored at −70°C until use.
Nitrite determination
Nitrite accumulation in supernatants from cultured cells was measured by the Griess method [13]. The Griess reagent was prepared by mixing equal volumes of 1% sulfanilamide in 30% acetic acid and 0.1% naphthylethylene diamine dihydrochloride in 60% acetic acid. Equal amounts of culture supernatant from the macrophages and Griess reagent were combined, and incubated for 10 min at room temperature. Absorbance was measured at 543 nm. Nitrite concentration was quantified using various NaNO2 concentrations in culture medium as standard and data were expressed in μm.
Cytokine immunoassays
Murine cytokines were measured by specific two-site ELISA according to the manufacturer's specifications with reference standard curves, using known amounts of the respective murine recombinant cytokines. The antibody pairs (capture antibody and horseradish peroxidase (HRP)-conjugated antibodies) for the detection of TNF-α (detection limit 23.4 pg/ml), IL-6 (detection limit 15.6 pg/ml), IL-10 (detection limit 15.6 pg/ml) and IL-1β (detection limit 3 pg/ml) were from R&D Systems (Minneapolis, MN). All samples were assayed in duplicate and plates were read on an ELISA reader.
RNA extraction, cDNA synthesis and polymerase chain reaction amplification
Cells from parallel cultures were lysed in Trizol reagent, and total RNAs was extracted as described by the manufacturer (Life Technologies, Gibco BRL, Cergy Pontoise, France). Successful isolation of undegraded RNA was checked by minigel electrophoresis. Specific mRNA was detected by Northern blot analysis [21]. To this end, 20 μg of total RNA were denatured with 6% formaldehyde, submitted to denaturing 1% agarose gel electrophoresis in MOPS buffer, and transferred to a nylon membrane (Pall Biodyne B; 0.46 μm). The blot was hybridized for 24 h at 42°C with approximately 1.5 × 106 ct/min per ml of α-32P-radiolabelled cDNA probe, washed twice for 15 min with 0.1× SSPE/0.1% SDS at 65°C and exposed for phosphorImager analysis (Molecular Dynamics). The iNOS cDNA probe was a 781-base pair BamHI fragment excised from whole murine cDNA for iNOS of 2.9 kb, kindly provided by Dr Lowenstein [22]. Single-stranded cDNA was synthesized from total RNA using Moloney's murine leukaemia virus reverse transcriptase and random hexamers as primers. The reaction mixture was composed of 10 μl of cDNA template (diluted 1:500) obtained from 1 μg of extracted RNA, 50 pmol for iNOS primers and 40 pmol for β-actin primers, 25 nmol of each dNTP (Pharmacia, Uppsala, Sweden), 1.25 U of Taq DNA polymerase (Roche, Meylan, France), 10 μl of 10× PCR buffer (Promega, Madison, WI), in a final volume of 100 μl. Amplifications were performed in a thermocycler (Perkin-Elmer) according to the following programme: initial denaturation at 94°C for 5 min (one cycle) followed by denaturation for 1 min at 94°C, annealing for 1 min at 58°C and extension at 72°C for 72 s (35 cycles). Final extension was done at 72°C for 10 min. The primer used for iNOS was designed to generate a fragment of 598 bp: sense, 5′-AAGCTGCATGTGACATCGACCCGT-3′, anti-sense, 5′-GCATCTGGTAGCCAGCGTACCGG-3′. The primer sequence used to generate a fragment of 644 bp for β-actin was: sense, 5′-ATCATGTTTGAGACCTTCAA-3′, anti-sense, 5′-TTGCGCTCAGGAGGAGCAAT-3′. Semiquantification of gene expression for iNOS was performed after co-amplification and normalization with the β-actin internal control. The expression levels for the iNOS transcript were evaluated after ethidium bromide staining by densitometric scanning using a video camera (Imager, Appligene, Illkirch, France) and analysed using Image 1.44 b11 MacIntosh software.
Studies of cell viability
Cell cytotoxicity was determined by measuring the release of lactate dehydrogenase (LDH) activity (Sigma assay) in culture supernatants.
Statistical analysis
Four sets of independent experiments were conducted under identical conditions, unless otherwise stated. Results are presented as the mean ± s.e.m. of triplicate determinations from one set of experiments. The significance of the observed differences was calculated by analysis of variance and Student's t-test. P < 0.05 was considered significant.
RESULTS
Effects of BZL on the in vitro synthesis of nitrite and cytokines by stimulated macrophages
Differences in nitrite production and cytokine synthesis were analysed according to stimulation procedures and BZL concentrations added to culture media. Data wherein LPS, LPS + IFN-γ or IFN-γ were added with 2-methoxiethanol are omitted as they did not differ from their counterparts without solvent.
Nitrite production in unstimulated cells was low and was unaffected by the addition of BZL (Fig. 1). In contrast, synthesis of TNF-α by unstimulated cells appeared to be significantly lowered in cultures containing 0.5 mm and 1 mm BZL. IL-1β, IL-6 and IL-10 were not found in any culture supernatants.
Fig. 1.
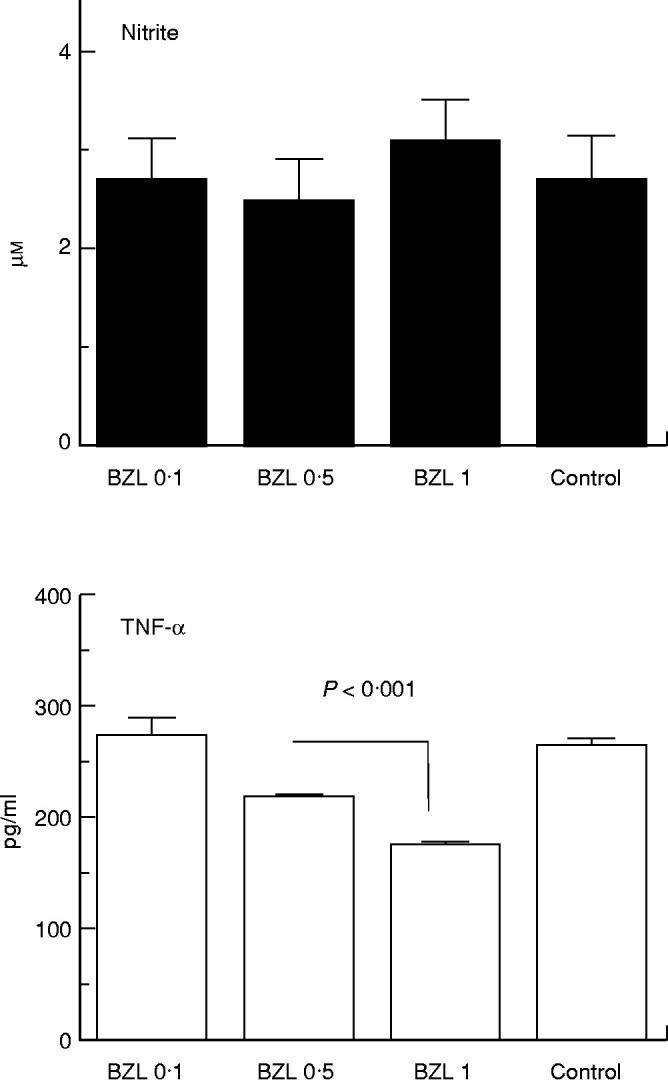
Nitrite and tumour necrosis factor-alpha (TNF-α) production by RAW 264.7 murine macrophages subjected to benznidazole (BZL) treatment. Macrophages (2.5 × 105 cells/well) were cultured in Dulbecco's modified Eagle's medium (DMEM) plus 5% heat-inactivated fetal calf serum, l-glutamine, non-essential amino acids, HEPES and gentamycin. After 24 h, culture medium was replaced by 1 ml of fresh medium with or without BZL (final concentrations 0.1 mm, 0.5 mm, or 1 mm, dissolved in 2-methoxiethanol). Nitrite and TNF-α measurements were performed in culture supernatants collected 24 h later. Data correspond to a representative experiment of four independent experiments performed with similar results. Bars and columns represent the mean of triplicate determinations + 1 s.e.m. TNF-α levels in control and BZL 0.1 significantly different from BZL 0.5 and BZL 1, P < 0.005.
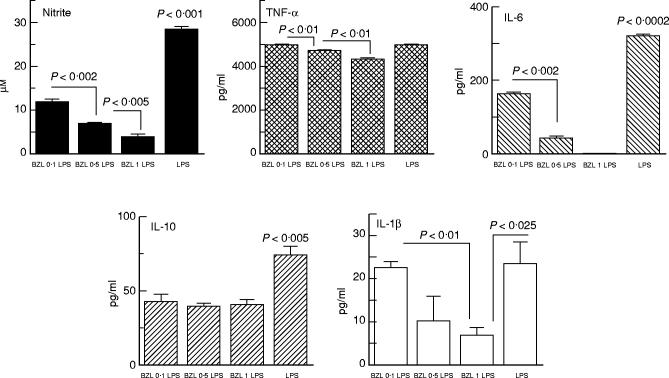
LPS stimulation of cell cultures increased TNF-α and nitrite production, and also induced IL-1β, IL-6 and IL-10 synthesis (Fig. 2). Measurements in supernatants from BZL-treated cells showed that BZL at any of the employed concentrations significantly lowered nitrite, IL-6, and IL-10 levels, dose-relatedly in the case of the first two. IL-1β levels were also lowered by BZL, but only when it was added at 1 mm. Although TNF-α values in cultures containing 0.5 mm and 1 mm BZL appeared to be significantly below those from LPS-stimulated control cultures, such a decrease was much less important in biological terms.
Fig. 2.
Effect of benznidazole (BZL) on nitrite, tumour necrosis factor-alpha (TNF-α), IL-6, IL-10 and IL-1β production by lipopolysaccharide (LPS)-stimulated RAW 264.7 murine macrophages. Cells were cultured overnight as described for Fig. 1. Culture medium was then discarded and cells were grown for 24 h in fresh medium containing LPS (100 ng/ml) with or without BZL. Data correspond to a representative experiment of four independent experiments performed with similar results. Bars and columns represent the mean of triplicate determinations + 1 s.e.m. TNF-α levels of LPS significantly different from BZL 0.5 and BZL 1, P < 0.01.
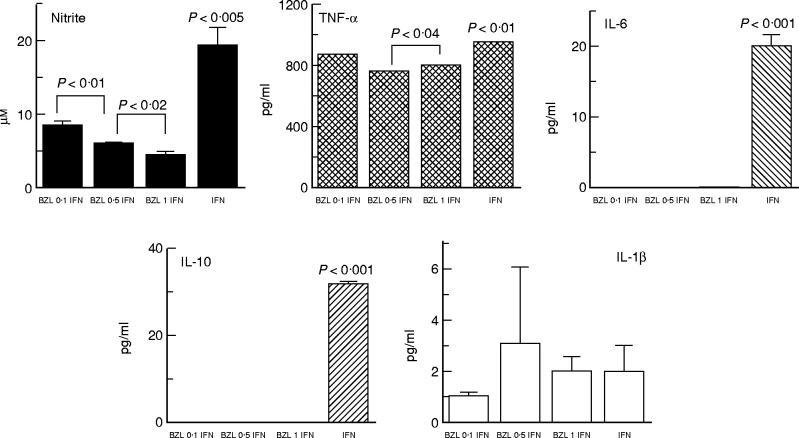
BZL caused a marked decrease of nitrite production in IFN-γ-stimulated cells, and completely blocked IL-6 and IL-10 synthesis even at its lowest concentration (Fig. 3). TNF-α levels were slightly reduced in BZL-treated cultures, the effect being more pronounced at 0.5 mm and 1 mm. Nevertheless, it should be emphasized that the down-regulatory effects of BZL on TNF-α synthesis by IFN-γ-stimulated cells did not reach the inhibition seen in the case of nitrite, IL-6 and IL-10 production. Synthesis of IL-1β by IFN-γ-stimulated cells was low irrespective of whether or not cultures contained BZL.
Fig. 3.
IFN-γ stimulation of RAW 264.7 murine macrophages and the effect of benznidazole (BZL) on the synthesis of nitrite, tumour necrosis factor-alpha (TNF-α), IL-6, IL-10 and IL-1β. Following overnight incubation with supplemented Dulbecco's modified Eagle's medium (DMEM) medium (see Fig. 1 legend), culture medium was replaced by fresh medium containing IFN-γ (500 U/ml) with or without BZL. Culture supernatants were collected 24 h later for nitrite and cytokine measurements. Data correspond to a representative experiment of four independent experiments performed with similar results. Bars and columns represent the mean of triplicate determinations + 1 s.e.m.
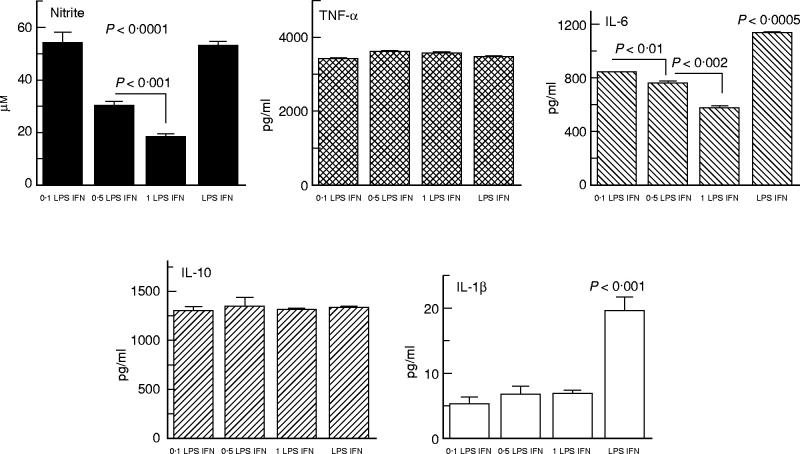
Data analysis from cultures stimulated with LPS plus IFN-γ showed that synthesis of nitrite, IL-1β and IL-6 was affected by in vitro BZL treatment (Fig. 4). Decreased nitrite production was observed at 0.5 mm and 1 mm BZL, whereas IL-1β and IL-6 synthesis was reduced at all three BZL concentrations. In contrast, addition of BZL to cultures did not affect synthesis of TNF-α or IL-10, levels of which were increased in all culture supernatants, with statistical comparisons yielding non-significant differences.
Fig. 4.
Levels of nitrite, tumour necrosis factor-alpha (TNF-α), IL-6, IL-10 and IL-1β in culture supernatants from lipopolysaccharide (LPS) + IFN-γ-stimulated RAW 264.7 murine macrophages in the presence or absence of benznidazole (BZL). Experimental conditions were similar to those employed in LPS-stimulated cultures except for the fact that IFN-γ (500 U/ml) was also present in the medium with which cells were cultured during the last 24 h. Data correspond to a representative experiment of four independent experiments performed with similar results. Bars and columns represent the mean of triplicate determinations + 1 s.e.m.
iNOS gene expression
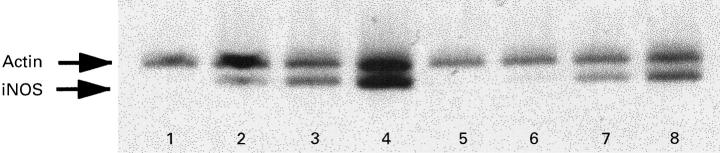
Given the differences observed in nitrite production when adding BZL to cultures, we investigated iNOS gene expression in such cells. Northern blot analysis indicated that iNOS mRNA induction could be detected after 6 h of stimulation, being highest in cells stimulated with LPS + IFN-γ, with presence of BZL (1 mm) leading to a marked inhibition of iNOS mRNA induction regardless of the inducing agent (data not shown). In order to quantify the reduction in iNOS mRNA expression by BZL treatment, the same preparation of total RNA was submitted to semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR). BZL treatment markedly reduced iNOS mRNA levels (Fig. 5). In agreement with findings from Northern blot analysis, densitometric quantification by using β-actin as an internal control indicated that BZL completely inhibited iNOS gene induction by IFN-γ, and led to respective reductions of 30% and 50% with LPS- or IFN-γ + LPS-stimulated cells (same figure). Similar results were obtained in three independent experiments.
Fig. 5.
Induction of inducible nitric oxide synthase (iNOS) gene expression in RAW 264.7 murine macrophages stimulated in the presence of benznidazole (BZL). Cells were stimulated in the absence or presence of lipopolysaccharide (LPS; 100 ng/ml) and/or IFN-γ (500 U/ml), with or without BZL. Total RNA of cells stimulated for 6 h was reverse-transcribed and polymerase chain reaction (PCR) co-amplification was performed for iNOS and the β-actin gene, as described in Materials and Methods. The expression levels for the iNOS transcript were evaluated after ethidium bromide staining by densitometric scanning using a video camera and analysed by using Image 1.44 b11 MacIntosh software. Lanes 1 and 5, untreated cells; lanes 2 and 6, IFN-γ-treated cells; lanes 3 and 7, LPS-treated cells; lanes 4 and 8, LPS + IFN-γ-treated cells. Cells were untreated (lanes 1–4) or treated with 1 mm BZL (lanes 5–8).
To rule out changes in cell viability due to BZL treatment, LDH levels in supernatants from stimulated cells were measured. Data analysis revealed no significant differences in LDH concentrations in culture supernatants, irrespective of whether or not cultures were BZL-treated. In all cases, LDH values did not exceed 0.5% of the total LDH activity obtained from the corresponding cell extracts (data not shown).
DISCUSSION
Activated macrophages have long been recognized as the effector arm of cell-mediated immunity. With regard to experimental infections, such macrophages have been shown to exert an anti-microbial effect against a broad range of intracellular pathogens, mainly through the synthesis of NO. In murine cells, synthesis of NO is easily inducible by IFN-γ in combination with a second stimulus like LPS, which constitutes a major component of the outer membrane of Gram-negative bacteria. Furthermore, LPS has been implicated as the primary initiating agent in Gram-negative sepsis because of the activation of inflammatory cells, particularly the mononuclear phagocyte, which releases a series of additional mediators including TNF-α, IL-1 and IL-6 [23].
In the studies reported here, we have demonstrated that in vitro BZL treatment of mouse activated macrophages down-regulated the synthesis of nitrite, IL-6, and to a lesser extent IL-1β and IL-10. In fact, BZL-induced inhibition of nitrite and IL-6 production was observed regardless of whether or not cells were stimulated with LPS and/or IFN-γ. In contrast, BZL-related blocking of the synthesis of IL-1β and IL-10 was more dependent on the way that the cells were stimulated. More specifically, decreased synthesis of IL-10 was demonstrated when BZL was added to cultures stimulated with LPS or IFN-γ alone, whereas in the case of IL-1β the BZL-induced inhibition became evident upon using a powerful stimulus of IL-1β production, like LPS, and when employing the highest BZL concentration for cultures stimulated with LPS alone.
The mechanism underlying BZL-induced changes in the activity of macrophages, in terms of their immunologically active secreted mediators, is presently unknown. Some experimental evidence indicates that BZL-reactive metabolites resulting from the enzymatic reduction of BZL are able to bind covalently to DNA, nuclear proteins and lipids [24–26], which in turn might be responsible for the deleterious effects that BZL is known to have in mammalian cells [24–26]. If decreased macromolecular synthesis resulting from covalent binding of drug-reactive metabolites to cellular components is to account for our results, a rather unspecific inhibition of the synthesis of all macrophage products should have occurred. Yet when TNF-α measurements were compared it was evident that BZL-related inhibition of TNF-α synthesis was minimal. Furthermore, BZL failed to down-regulate the synthesis of IL-10 by LPS + IFN-γ-stimulated cultures, as well as the synthesis of IL-1β under some experimental conditions. Taken together, our results appear to suggest a selective effect of BZL on macrophage-secreted products. In the case of nitrite production, studies of iNOS gene expression further demonstrated that BZL down-regulated iNOS gene expression induced by IFN-γ, LPS or LPS + IFN-γ. Anti-fungal imidazoles were also found to inhibit the induction of nitrite synthesis in macrophages, but in this case at a stage after the production of mRNA [27].
As mentioned above, inducible NO synthase is expressed in many different cell types and produces high levels of NO, a compound essential for the bactericidal action of macrophages and for a series of physiological functions including control of vascular tone, neurotransmission and platelet function [13,14]. However, under pathological conditions high output of NO would have profound pathological consequences, as observed in septic shock [13,14]. Hence, it is worth exploring the possibility that BZL may ameliorate the tissue damage resulting from excessive NO formation, i.e. experimental sepsis.
In addition to the participation of IL-6 in the generation of B and T cell-mediated responses, it should be recalled that this cytokine is primarily involved in the elicitation of the acute-phase response to injury [28,29]. It follows that BZL-induced blocking effects of IL-6 production might also be important in vivo. The same may be true for BZL-induced inhibition of IL-1β production. In fact, IL-1β is a cytokine produced primarily by monocytes and activated macrophages which exerts multiple biologic effects such as proinflammatory activity, and a regulation of host response to injury and infection [30,31].
Cytokines facilitate communication within the immune system and play a key role in host resistance against pathogens. Some cytokines are proinflammatory, such as IL-1β and IL-6, while others behave as anti-inflammatory proteins like IL-10. In fact, IL-10 is known to inhibit the synthesis of NO, TNF-α, and IL-12 by macrophages and IL-2 and IFN-γ by lymphocytes [32]. The relevance of endogenous IL-10 synthesis is further exemplified by experiments performed in IL-10 knock out mice which are highly susceptible to LPS-induced endotoxaemia because of their increased TNF-α production [33]. With regard to IL-10 effects on NO production, divergent results were recently reported by Jacobs et al. [34] demonstrating that IL-10 acted to increase NO production by LPS-activated macrophages with an improved control of T. cruzi infection in vitro. In view of such cross-regulatory cytokine influences one may assume our findings of decreased nitrite synthesis are due partly to an indirect mechanism, i.e. the presently inhibitory effects of BZL on IL-10 production. While this possibility cannot be discarded, it must be remembered that nitrite production in LPS + IFN-γ-stimulated cells continued to be decreased by BZL treatment, despite IL-10 production remaining unaffected by such a compound.
Whatever the proper mechanism, it may be speculated that in addition to its trypanocidal activity BZL may also modify the balance between pro- and anti-inflammatory mediators, with important consequences for the course of trypanosomal infection. Our unpublished results from a rat model of experimental T. cruzi infection indicate that nitrite production is significantly decreased when rats are given BZL, even if they are also given recombinant rat IFN-γ.
Acknowledgments
This work was supported by FONCYT (BID 802/OC-AR) and by a grant from the INSERM-CONICET co-operative programme. We are grateful for the help rendered by Josianne Sanceau in the standardization of the RT-PCR technique. We also wish to thank Catherine Silvestri for supplying the cell line employed in the studies.
References
- 1.Kirchhof LV. American trypanosomiasis (Chagas' disease). A tropical disease now in the United States. N Engl J Med. 1993;329:639–44. doi: 10.1056/NEJM199308263290909. [DOI] [PubMed] [Google Scholar]
- 2.Schmun~is GA. Trypanosoma cruzi, the etiologic agent of Chagas' disease: status in the blood supply in endemic and nonendemic countries. Transfusion. 1991;31:547–57. doi: 10.1046/j.1537-2995.1991.31691306255.x. [DOI] [PubMed] [Google Scholar]
- 3.Diaz de Toranzo EG, Castro JA, Franke de Cazzulo BM, Cazzulo JJ. Interaction of benznidazole reactive metabolites with nuclear and kinetoplastic DNA, proteins and lipids from Trypanosoma cruzi. Experientia. 1988;44:880–1. doi: 10.1007/BF01941187. [DOI] [PubMed] [Google Scholar]
- 4.Do Campo R. Sensitivity of parasites to free radical damage by antiparasitic drugs. Chem Biol Interact. 1990;73:1–27. doi: 10.1016/0009-2797(90)90106-w. [DOI] [PubMed] [Google Scholar]
- 5.Marr JJ, Docampo R. Chemotherapy for Chagas' disease: a perspective of current therapy and considerations for future research. Rev Infect Dis. 1986;8:884–903. doi: 10.1093/clinids/8.6.884. [DOI] [PubMed] [Google Scholar]
- 6.Gazzinelli RT, Oswald IP, Hieny S, James SL, Sher A. The microbicidal activity of interferon-γ-treated macrophages against Trypanosoma cruzi involves an L-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-β. Eur J Immunol. 1992;22:2501–6. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- 7.Starobinas N, Russo M, Minoprio P, Honteyberie-Joskowicz M. Is TNFα involved in early susceptibility of Trypanosoma cruzi-infected C3H/He mice? Res Immunol. 1991;142:117–22. doi: 10.1016/0923-2494(91)90019-f. [DOI] [PubMed] [Google Scholar]
- 8.Black CM, Israelski DM, Suzuki Y, Remington JS. Effect of recombinant tumor necrosis factor on acute infection in mice with Toxoplasma gondii or Trypanosoma cruzi. Immunology. 1989;68:570–4. [PMC free article] [PubMed] [Google Scholar]
- 9.Truyens C, Angelo-Barrios A, Torrico F, Van Damme J, Heremans H, Carlier Y. Interleukin-6 (IL-6) production in mice infected with Trypanosoma cruzi: effect of its paradoxical increase by anti-IL-6 monoclonal antibody treatment on infection and acute-phase and humoral immune responses. Infect Immun. 1994;62:692–6. doi: 10.1128/iai.62.2.692-696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva JS, Morrisey PJ, Grabstein KH, Mohler KM, Anderson D, Reed SG. Interleukin 10 and interferon gamma regulation in experimental Trypanosoma cruzi infection. J Exp Med. 1992;175:169–74. doi: 10.1084/jem.175.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Revelli S, Didoli G, Roggero E, Moreno H, Bernabo J, Wietzerbin J, Bottasso O. Macrophage activity, IL-6 levels, antibody response and heart histology in rats undergoing an attenuated Trypanosoma cruzi acute infection upon treatment with recombinant interferon gamma. Cytokines, Cellular Mol Therapy. 1998;4:153–9. [PubMed] [Google Scholar]
- 12.Tarleton RL. Trypanosoma cruzi-induced suppression of IL-2 production. I. Evidence for the presence of IL-2 producing cells. J Immunol. 1988;140:2763–8. [PubMed] [Google Scholar]
- 13.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:119–25. [PubMed] [Google Scholar]
- 14.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–64. [PubMed] [Google Scholar]
- 15.Pellat-Deceunynck C, Wietzerbin J, Drapier JC. Nicotinamide inhibits nitric oxide synthase mRNA induction in activated macrophages. Biochem J. 1994;297:53–58. doi: 10.1042/bj2970053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drapier JC, Wietzerbin J, Hibbs JB. IFN-γ and TNF-α induce the L-arginine-dependent cytotoxic effector mechanism in murine macrophages. Eur J Immunol. 1988;18:1587–92. doi: 10.1002/eji.1830181018. [DOI] [PubMed] [Google Scholar]
- 17.Vespa GNR, Cunha FQ, Silva JS. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect Immun. 1994;62:5177–82. doi: 10.1128/iai.62.11.5177-5182.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petray P, Castan~os-Velez E, Grinstein S, Örn A, Rottenberg ME. Role of nitric oxide in resistance and histopathology during experimental infection with Trypanosoma cruzi. Immunol Letters. 1995;47:121–6. doi: 10.1016/0165-2478(95)00083-h. [DOI] [PubMed] [Google Scholar]
- 19.Holscher C, Kohler G, Muller U, Mossman H, Schaub GU, Brombacher F. Defective nitric oxide effect or functions lead to extreme susceptibility of Trypanosoma cruzi-infected mice deficient in gamma interferon receptor or inducible nitric oxide synthase. Infect Immun. 1998;66:1208–15. doi: 10.1128/iai.66.3.1208-1215.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno SNJ, Docampo R, Mason RP, Leon W, Stoppani AOM. Different behaviors of benznidazole as free radical generator with mammalian and Trypanosoma cruzi preparations. Arch Biochem Biophys. 1982;218:585–91. doi: 10.1016/0003-9861(82)90383-6. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2. Cold Spring Harbor: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 22.Lowenstein CJ, Alley ZX, Raval P, Snowman AM, Snyder SH, Russel SW, Murphy WJ. Macrophage NO synthase gene: two upstream regions mediate induction by IFN-γ and LPS. Proc Natl Acad Sci USA. 1993;90:9730–4. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrison DC, Ryan JL. Endotoxin and disease mechanisms. Ann Rev Med. 1987;38:417–32. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- 24.Masana M, de Toranzo EGD, Castro JA. Reductive metabolism and activation of benznidazole. Biochem Pharmacol. 1984;33:1041–5. doi: 10.1016/0006-2952(84)90511-2. [DOI] [PubMed] [Google Scholar]
- 25.Masana MI, de Toranzo EGD, Rubio MC, Castro JA. Effect of benznidazole on the mixed function oxygenase system from rat liver microsomes. Arch Int Pharmaco Ther. 1985;276:4–11. [PubMed] [Google Scholar]
- 26.Gorla NB, Diaz Gomez MI, Castro JA. Interaction of benznidazole with DNA and nuclear proteins from rat liver. Arch Int Pharmacodyn Ther. 1986;280:22–31. [PubMed] [Google Scholar]
- 27.Bogle RG, Whitley G St J, Soo S-C, Johnstone AP, Vallance P. Effect of anti-fungal imidazoles on mRNA levels and enzyme activity of inducible nitric oxide synthase. Br J Pharmacol. 1994;111:1257–61. doi: 10.1111/j.1476-5381.1994.tb14881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snick J. Interleukin-6: an overview. Ann Rev Immunol. 1990;8:253–78. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 29.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 30.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–147. [PubMed] [Google Scholar]
- 31.Arend WP. Interleukin-1 receptor antagonist. Adv Immunol. 1993;54:167–227. doi: 10.1016/s0065-2776(08)60535-0. [DOI] [PubMed] [Google Scholar]
- 32.Mosmann TR. Properties and functions of interleukin-10. Adv Immunol. 1994;56:1–26. [PubMed] [Google Scholar]
- 33.Berg DJ, Kuhn R, Rajewsky K, Muller W, Menon S, Davidson N, Grunig G, Rennick D. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Schwartzman reaction but not endotoxin tolerance. J Clin Invest. 1995;96:2339–47. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobs F, Chaussabel D, Truyens C, Leclerq V, Carlier Y, Goldman M, Vray B. IL-10 up-regulates nitric oxide (NO) synthesis by lipopolysaccharide (LPS)-activated macrophages: improved control of Trypanosoma cruzi infection. Clin Exp Immunol. 1998;113:59–64. doi: 10.1046/j.1365-2249.1998.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]