Abstract
Loss of the T cell receptor-associated CD3 ζ chain has been proposed as a possible mechanism of the acquired immunosuppression in both tumour-bearing hosts, and in symptomatic patients with HIV infection. However, other reports suggest that the ζ-chain loss may in part be caused by protease activity of contaminating phagocytes ex vivo. Using flow cytometry and Western blot analysis on highly purified T cells, and ensuring adequate addition of protease inhibitors, we have studied the expression of CD3ζ on peripheral blood T cells from patients with colorectal carcinoma, and compared these with normal controls, and pregnant donors, as a further example of an immunocompromised state. Immunohistochemistry was performed on tumour sections from patients with colorectal carcinoma to measure CD3ζ expression in tumour infiltrating T cells, and compared with normal mucosa and tonsil. Using these three approaches, our data provide no evidence for downregulation of CD3ζ chain expression either in colorectal carcinoma or pregnancy and suggest that this explanation is unlikely to fully account for the reduced T cell function associated with these conditions in all patients.
Keywords: CD3, zeta, carcinoma, pregnancy, immunosuppression
INTRODUCTION
There is widespread evidence that escape from the host immune system plays an important role in tumour occurrence and progression [1–3]. It has been demonstrated in mice bearing a colon carcinoma tumour (MCA-38) that downregulation of two important T cell signal transduction molecules, CD3ζ and p56lck could be the basis of immune dysfunction [4]. These observations have been confirmed in splenic T cells from mice bearing a CMS5 sarcoma [5] and in T cells from humans bearing a range of tumour types [6–9]. The degree of CD3ζ downregulation may be dependent on proximity to the tumour, such that lower expression is seen in tumour infiltrating lymphocytes (TIL) than peripheral blood lymphocytes (PBL) of tumour patients. Also, the reduction in ζ is most apparent in colonic T cells adjacent to the tumour compared to T cells isolated from a distal, non-cancerous colonic site [8]. Furthermore, the decreased ζ levels may correlate with increased Dukes stage of disease [8].
The observation that CD3ζ is downregulated in tumour bearing hosts is not without controversy. Using highly purified T cell preparations, levels of CD3ζ were normal in splenic T cells isolated from three different murine tumour models as measured by Western blotting [10]. Furthermore, variable expression of CD3ζ chain was measured in TIL from patients with renal cell carcinoma [11,12], with no decrease detected in PBL of patients compared to PBL of healthy controls [12]. In addition, normal CD3ζ expression was observed in patients with non-Hodgkins B cell lymphoma [13]. Importantly, the downregulation of CD3ζ may not be uniquely associated with tumour bearing hosts: PBL from patients with symptomatic but not asymptomatic HIV infection also demonstrate reduced CD3ζ expression [14], which may suggest a functional role for CD3ζ downregulation in other immunocompromised states.
Accurate determination of CD3ζ-chain downregulation in cancer patients may be important for efficacy of future therapies. For example, cancer vaccines may be less effective in CD3ζ-downregulated patients. In addition, reversal of CD3ζ-chain downregulation might represent a novel mechanism for drug intervention. The aim of the current study was to examine further CD3ζ expression in T cells of patients with colorectal carcinoma, and to compare these to normals. Pregnant donors were also compared as an example of an immunocompromised state [15].
It has been suggested that the apparent loss of CD3ζ may be attributed, in part, to proteolytic degradation by enzymes derived from granulocytes or macrophages, which may contaminate some T cell preparations [16,17]. Flow cytometry and Western blotting were performed on highly purified T cell preparations ensuring adequate addition of protease inhibitors. In addition, immunohistochemistry was performed to detect CD3ζ and ε expression on tumour sections from patients with colorectal carcinoma and compared with normal colon and tonsillar control tissue. Using these three techniques, our data show no demonstrable downregulation of CD3ζ chain expression either in colorectal carcinoma or pregnancy, relative to controls.
MATERIALS AND METHODS
Measurement of CD3ζ and ε in peripheral blood
Venous peripheral blood (20 ml) was collected into heparinized tubes. Blood was diluted 1:2 with PBS and layered onto a Ficoll gradient (Lymphoprep; Nycomed Pharma, Oslo, Norway). Following centrifugation (1200 r.p.m., 30 min), the PBMC layer was removed and washed twice with PBS. Aliquots of PBMC were taken for flow cytometry or Western blot analysis.
Flow cytometry
PBMC (4 × 105, 100 μl) were fixed (2 ml lysis solution; Becton Dickinson, Oxford, UK) for 10 min before centrifugation (1500 r.p.m., 7 min) and the cell pellet resuspended in permeabilization solution (0.5 ml; Becton Dickinson, UK). Following a 10 min incubation, cells were washed in 2 ml, 0.5% BSA/PBS and centrifuged at 1500rpm for 7 min. Intracellular lymphocyte ζ chain was detected using TIA-2 (10 μg ml−1; Coulter, Luton, UK) using MOPC21 (Sigma, Poole, UK) as an isotype matched antibody control. A fluorescein isothiocyanate (FITC) conjugated goat antimouse F(ab′)Ig monomer (Dako Ltd, Cambridge, UK) was used to detect bound Ig. Cells were incubated with 2% mouse serum (0.5 ml) for 30 min, prior to staining for CD3ε (0.6 μg ml−1, PE-labelled; Becton Dickinson, UK) using a PE-conjugated isotype matched antibody as control. Cells were analysed using a Coulter (XL) flow cytometer, and 5000 events were analysed within a lymphocyte gate, defined by forward/side scatter, and confirmed by positive staining for CD3ε.
Western blotting
CD3+ cells were purified from PBMC using T cell enrichment columns (R&D Systems, Oxford, UK, HTCC-500) to give a purity of > 94%. CD3 cells were washed twice with PBS, and resuspended at 2 × 105 cells/μl in lysis buffer containing protease inhibitors (Tris pH 7.4 (50 mm); EDTA (5 mm); NaCl (300 mm); sodium orthovanadate (1 mm); aprotinin (10 μg/ml); PMSF (2 mm); leupeptin (10 μg/ml); chymostatin (100 μg/ml); trypsin chymotrypsin inhibitor (100 μg/ml); Triton-X 100 (0.5%) (all from Sigma) for 15 min at 4°C. The lysate was then centrifuged (10000 r.p.m., 5 min 4°C) to remove the nuclear pellet. An aliquot of cell lysate was mixed 1:1 with sample load buffer (bromophenol blue, Tris (0.1 m); glycerol (20%), SDS (4%); 2-mercaptoethanol (10%) (Sigma) and heated for 3 min at 100°C. Samples containing 5 and 10 μg protein/lane were separated by SDS-PAGE on a 14% gel [18]. The protein bands were then electrophoretically transfered to polyvinylidene difluoride membrane (Immobilon P, 0.4 μm; Millipore, Watford, UK). Following blocking by 3% nonfat milk protein in PBS (overnight, 4°C) the membranes were blotted with optimal concentrations of rabbit anti-CD3ζ polyclonal antiserum (1:150, Glaxo Wellcome, Stevenage, UK) and rabbit anti-CD3ε polyclonal antibody (1:800, Dako Ltd) for 1 h at room temperature. After washing (PBS/0.1–0.2% Tween-20), the membrane was incubated with antirabbit immunoglobulin-horseradish peroxidase (1:10000; Sigma) for 1 h. After further washing, protein bands were detected by enhanced chemiluminescence (ECL, Amersham, Aylesbury, UK). Densiometry was carried out using Phoretix software with a band intensity calibration and results expressed as integrated optical density units. Under these blot conditions, a decrease in the amount of protein loaded is clearly seen as a decrease in band intensity. It was necessary to use a polyclonal antibody for the detection of CD3ε, therefore to allow ζ and ε to be visualized on the same blot a polyclonal antiserum was used to detect ζ. In T cell lysates, the ζ antisera used detects a single band of the same molecular weight as that detected by TIA-2 (data not shown).
Preparation of tissue samples
Primary adenocarcinoma tumour specimens and nonmalignant tonsil were obtained at time of surgery from St George's Hospital, London. The tissue was immediately snap-frozen in isopentane on dry ice, embedded in OCT-Compound (Tissue Tex® Miles Scientific, Elkhart, IN) and stored in the vapour phase of liquid nitrogen.
Immunohistochemistry
Frozen human colon adenocarcinoma or control tonsil blocks were sectioned (6–8 μm) onto VectabondTM (Vector Labs, Peterborough, UK) coated microscope slides and air-dried before fixing with ice-cold acetone for 2 min. Endogenous alkaline phosphatase was blocked by incubation with 15% acetic acid for 20 s. Sections were washed (Tween 20/PBS) and stained using optimal concentrations of anti-CD3ε (UCHT1, Dako Ltd), anti-CD3ζ (TIA-2, Coulter) or an isotype control (mouse IgG1, Vector Labs) for 45 min in 1% BSA/PBS at room temperature. After washing, the sections were incubated with a biotinylated horse antimouse IgG (1 : 200, Vector Labs) for 30 min at room temperature. After further washing, sections were developed with avidin-alkaline phosphatase (ABC-AP Kit, Vector Labs) for 30 min and washed, before application of substrate solution (Vector Labs) for 20 min. Sections were given a final wash before counterstaining in Mayers haematoxylin and viewed by light microscopy.
RESULTS
Colorectal carcinoma
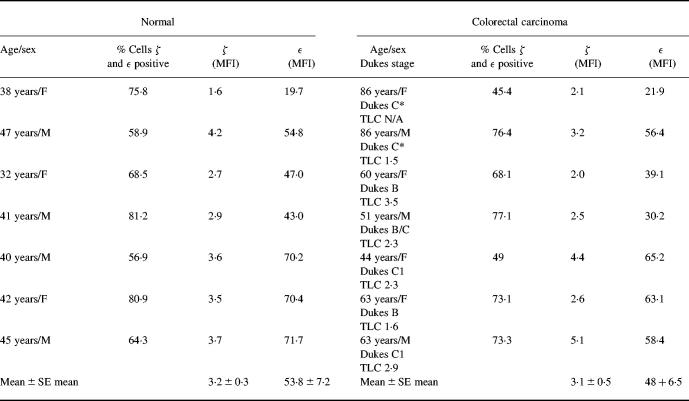
The total lymphocyte count for patients with colorectal carcinoma was within the normal range (1.5–3.5 × 109/l). Flow cytometric analysis of PBL did not reveal any significant difference in the expression of CD3ε or ζ between patients with colorectal carcinoma compared to healthy controls either in terms of percentage positive cells or MFI. (Table 1,Fig. 1). The MFI of ζ specific staining (minus MFI of isotyped matched controls) was 3.2 ± 0.3 in controls compared to 3.1 ± 0.5 for PBL isolated from patients with colorectal carcinoma. As a positive control for flow cytometric analysis the effect of hydrogen peroxide was tested, as this is reported to downregulate ζ expression in vitro [19,20]. Hydrogen peroxide (100 μm) reduced ζ expression by 40 ± 5% (n = 3 experiments), clearly demonstrating that the method used to stain and analyse for ζ expression can detect a decrease, when present.
Table 1.
CD3 ε and ζ expression on PBMC from patients with colorectal carcinoma measured by flow cytometry
The mean fluorescence intensity (MFI) of staining for ε and ζ on a CD3+ population is shown. Blood was taken from patients with colorectal carcinoma prior to surgery. The Dukes stage of disease is noted for each patient; two patients (*) had inoperable tumours and were estimated as Dukes stage C. The total lymphocyte count (TLC expressed as cells × 109/l) for the colorectal carcinoma patients was within the normal range.
Fig. 1.
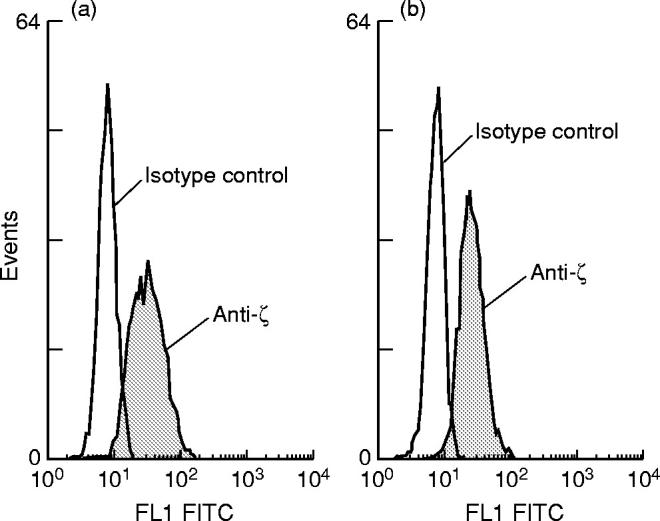
Example of flow cytometric analysis of CD3ζ expression of PBL from (a) healthy control (male, 41 years) compared with (b) a patient with colorectal carcinoma Dukes B/C (male, 51 years).
The normal expression of CD3ε and ζ in patients with colorectal carcinoma compared to controls was confirmed by Western blot analysis on highly purified CD3 cells (Fig. 2). Densitometric analysis of bands confirmed no difference in ζ band intensity between groups (10 μg/lane blot, mean units: controls 1463, carcinoma patients 1544). Western blots were also performed at a lower protein concentration of 5 μg/lane, again no difference was seen between the controls and carcinoma patients (data not shown).
Fig. 2.
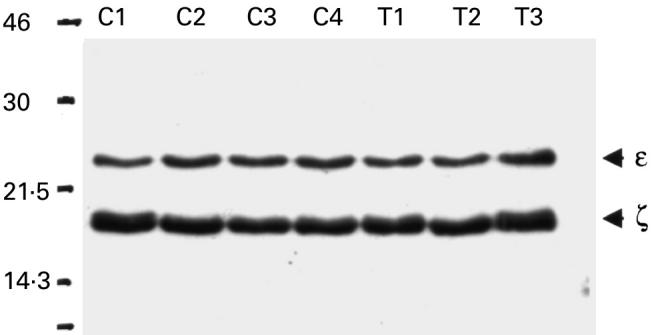
Levels of CD3ζ and ε in purified CD3 purified cells from the peripheral blood of patients with colorectal carcinoma (T1–T3, all Dukes C) and healthy controls by Western blot (C1–C4). Samples were loaded at 10 μg of total protein per lane.
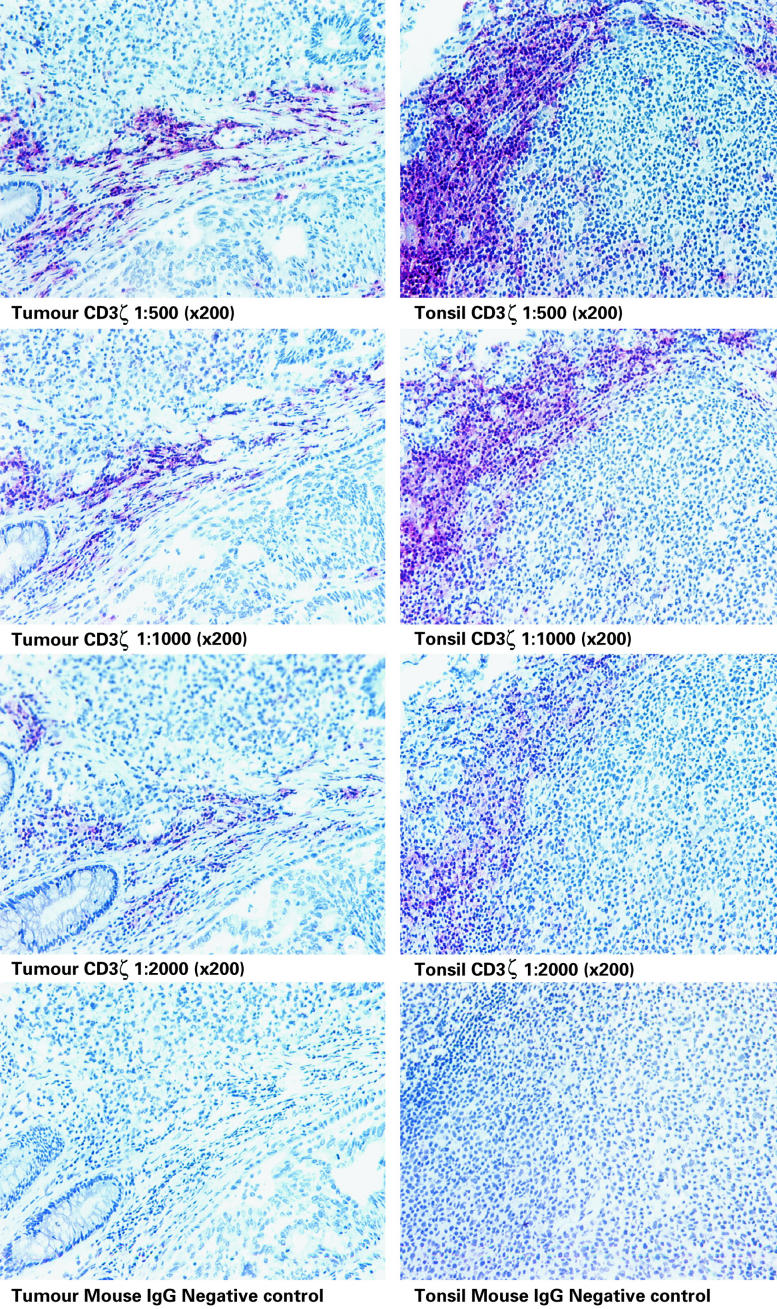
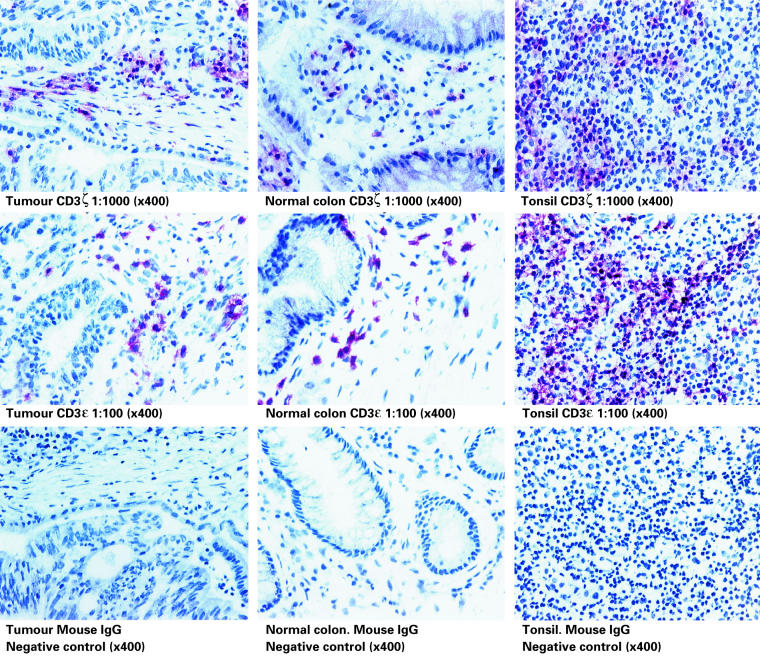
Immunohistochemistry was used to show that the ratio of CD3ε to CD3ζ staining intensity of tumour-infiltrating T cells was indistinguishable to that of both normal tonsil and colon. Tumours from seven patients were studied (one Dukes A, four Dukes B and two Dukes C). Figure 3 shows a direct comparison of staining on a representative tumour and tonsil, using optimal (1:1000), supra-optimal [1:500]: and suboptimal (1:2000) concentrations of primary antibodies. Staining of both tissues was performed in parallel. Visual analysis revealed no difference in staining intensity of expression between tonsil and tumour at any given antibody concentration. These findings were confirmed using image analysis of a section stained with TIA-2, increasing the dilution range to 1:8000, but without counterstain (one Dukes C, two Dukes B, a representative experiment is shown in Fig. 4). A further comparison was performed by staining colorectal tumour, normal colon and tonsil for CD3 and expression, and observing at both low and high magnification. Figure 5 shows no discernible difference in either ζ or ε expression between tissues, using optimal concentrations of both antibodies.
Fig. 3.
CD3ζ chain expression in colorectal carcinoma (Dukes stage B) compared with nonmalignant tonsil. Staining was performed on sequential tissue sections at the dilutions shown and visualized using alkaline phosphatase. An appropriate murine IgG antibody was included as a negative control.
Fig. 4.
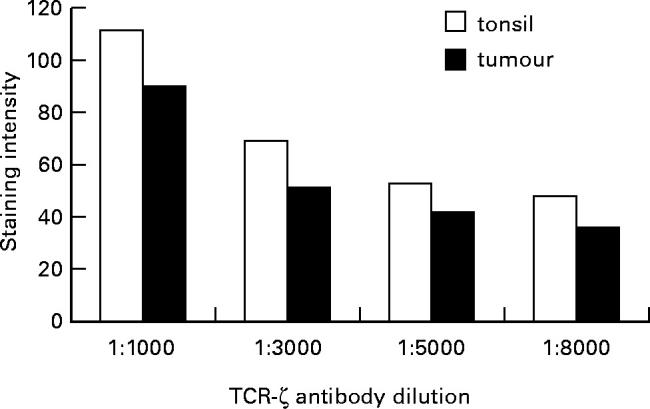
Image analysis of CD3ζ expression in colorectal carcinoma (Dukes stage B) compared with nonmalignant tonsil. Staining was peformed on sequential tissue sections at the dilutions shown and visualized using alkaline phosphatase, without counterstain. The sections were colour (green channel) imaged on a Leica Q600 Image Analyser with a × 10 objective lens. The mean intensity of the staining is measured in arbitrary units. There was no difference in CD3ζ staining intensity observed between the tumour and tonsil sections at a range of antibody dilutions.
Fig. 5.
CD3ζ and ε-chain expression in colorectal carcinoma (Dukes stage B) compared with nonmalignant colon and tonsil. Staining was performed on sequential tissue sections at the optimal dilutions shown and visualized using alkaline phosphatase. An appropriate murine IgG antibody was included as a negative control.
Pregnancy
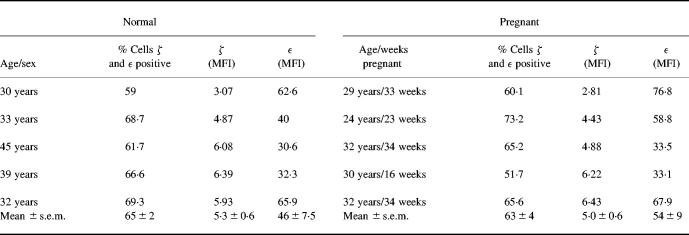
The expression of CD3ζ was investigated by flow cytometry using an antibody directed against the intracellular portion of the ζ chain (TIA-2) on lymphocyte-gated cells. The results from five pregnant donors are compared to five, female, age-matched, nonpregnant controls in Table 2. CD3ε or ζ expression did not differ between the two groups in terms of percentage positive cells or mean fluorescent intensity (MFI). The MFI for ζ specific staining (minus MFI for isotype matched control) was 5.3 ± 0.6 in control PBL compared to 5.0 ± 0.6 for PBL from pregnant donors (n = 5). Furthermore, Western blot analysis on highly purified CD3 cells, using rabbit polyclonal antibodies also showed that total levels of CD3ε and ζ detected were similar in the pregnant and nonpregnant donors (Fig. 6). Densiometric analysis of bands confirmed no difference in ζ band intensity between groups (mean units: controls 2488, pregnant donors 2412). Western blots were also performed at a lower protein concentration of 5 μg/lane; again no difference was seen between the control and pregnant donors (data not shown).
Table 2.
CD3 ε and ζ expression on PBMC from pregnant donors measured by flow cytometry
The mean fluorescence intensity (MFI) of staining for ε and ζ (minus the MFI of the isotype matched control) on a CD3+ population is shown. The controls were all female and were not taking any oral contraceptives.
Fig. 6.
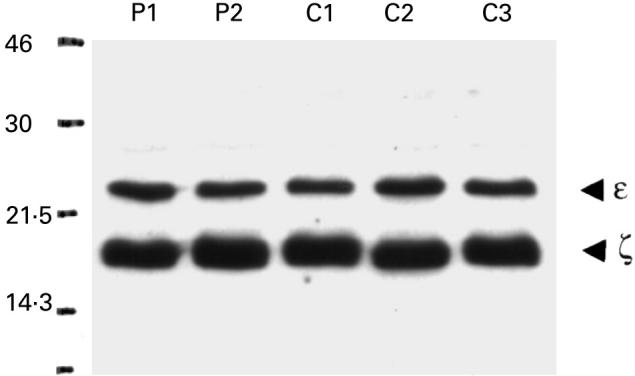
Levels of CD3ζ and ε in CD3 purified cells from the peripheral blood of pregnant (P1–P2) and nonpregnant female donors (C1–C3) detected by Western blot. Samples were loaded at 10 μg of total protein per lane.
DISCUSSION
The initial aim of this study was to confirm and extend previous findings of downregulated CD3ζ chain expression in colorectal carcinoma patients, by study of PBL and tumour tissue. However, we have shown that the expression of CD3ζ is neither uniformly, nor demonstrably downregulated in the PBL of patients with colorectal carcinoma as detected by flow cytometry and highly sensitive, optimized Western blot analysis. Furthermore, immunohistochemical examination of CD3ζ in TIL showed no reduction compared with nonmalignant tonsil tissue, analysed both visually and using sensitive image analysis equipment.
In addition to reduced CD3ζ chain expression, immunosuppression in colorectal carcinoma [21,22] and symptomatic HIV infection [14] has been linked to a skewed Th2 cytokine profile. We therefore studied pregnant women as another example of an immunocompromised state associated with a dominant Th2 profile [22]. Similar to our findings in colorectal cancer patients, we did not detect a decrease in ζ chain expression in pregnancy compared with normal controls.
The current study has used a commercially available monoclonal antibody (TIA-2) to detect ζ by flow cytometry and immunohistochemical analysis. It may be possible that tumour associated factors modify ζ at an epitope not recognized by this antibody. Indeed, the presence of a clipped ζ-chain (p14) has been described in activated T cells [23]. However, most other studies reporting a decrease in ζ-chain expression in patients with colorectal carcinoma have used the same antibody (TIA-2) either for flow cytometry on PBL [7,8] or immunohistochemistry [24]. We believe therefore that these disparate results cannot be directly attributed to differences in the antibody used to detect ζ-chain. Importantly, the method we used for analysis of PBMC can detect a reduction in CD3ζ expression: incubation of purified peripheral blood CD3 cells with hydrogen peroxide, which has been shown to downregulate CD3ζ expression [19,20], produced a substantial decrease in CD3ζ expression as determined by flow cytometry (data not shown). Furthermore, the failure to measure a downregulation of ζ cannot be attributed to disease severity. The present study includes patients of Dukes stage B and C and PBL from patients with similar disease severity have previously been shown to express decreased CD3ζ chain [8].
The use of permeabilization techniques for the flow cytometry and immunohistochemical analysis may enable the detecting antibody to bind to ζ in the intracellular compartment and not only that present in the TCR complex. Although some previous studies have performed two-dimensional gel analysis following immunoprecipitation of CD3ε [7,8], and are therefore measuring a decrease in ζ within the TCR complex, the Western blot analysis in the present study measures the whole cell reservoir of CD3ζ. While others have measured a decrease in ζ using whole cell lysates in Western blots [6,9] our study does not confirm these findings.
Reports that CD3ζ is downregulated not only in tumour specific lymphocytes but in the entire lymphocyte repertoire of cancer-bearing hosts appears to be inconsistent with the specific immunological suppression observed in tumour patients [25]. Indeed, the original observation that ζ chain was downregulated in splenic T cells from tumour bearing mice [4,5] has since been questioned [16,17]. The downregulation of CD3ζ measured in splenic cells from tumour bearing mice was not observed when Mac-1+ cells were removed [17], or when highly purified T cells were analysed [10]. The downregulation of CD3ζ may therefore be attributed to proteolytic degradation by contaminating phagocytes as no downregulation was observed following removal of contaminating granulocytes or addition of protease inhibitors to cell lysates [16]. However, the latter study did demonstrate that CD3ζ expression in PBL from cancer patients was downregulated even in the presence of a cocktail of protease inhibitors [16]. Nevertheless, the possibility of proteolytic degradation of signalling molecules during enzymatic treatment is greater for TIL than for preparations of PBL, which do not require such preparation. In a further study, the level of CD3ζ expression was measured by Western blot analysis on a tumour-derived mononuclear cell fraction compared with PBL from healthy volunteers [26]. However, the tumour derived sample contained 53–75% CD3+ cells whereas the PBL control was greater than 96% CD3+. This may account for the observed reduction in CD3ζ levels in the tumour-derived samples [26]. The present study used highly purified (> 94%) T cell preparations, and a combination of protease inhibitors shown to inhibit macrophage/granulocyte proteases [16]. Under these stringent conditions, we repeatedly failed to see a decrease in CD3ζ expression by Western blot.
The present study demonstrates a lack of evidence for down-modulation of CD3ζ expression in colorectal carcinoma using multiple detection methods. Similarly, other groups have also failed to demonstrate a decrease in CD3ζ chain expression in cancer patients with B cell lymphoma [13] or have reported minimal or variable decreases from patients with renal cell carcinoma [11,12,25,27]. The demonstration that both PBL and TIL from patients with colorectal carcinoma appear to have normal expression of CD3ζ and CD3ε is encouraging for the successful development of cancer vaccines.
Acknowledgments
We are grateful to Dr John Tite for providing anti-ζ chain polyclonal antiserum and for useful discussions during the course of this work.
REFERENCES
- 1.Maccubbin DL, Mace KF, Ehrke MJ, Mihich E. Modification of host antitumor defense mechanisms in mice by progressively growing tumor. Cancer Res. 1989;49:4216–24. [PubMed] [Google Scholar]
- 2.Miescher S, Whiteside TL, Carrel S, Von Fliedner V. Functional properties of tumor-infiltrating and blood lymphocytes in patients with solid tumors: effects of tumor cells and their supernatants on proliferative responses of lymphocytes. J Immunol. 1986;136:1899–907. [PubMed] [Google Scholar]
- 3.Oliver RT, Nouri A. T-cell immune response to cancer in humans and its relevance for immunodiagnosis and therapy. Cancer Surv. 1992;13:173–204. [PubMed] [Google Scholar]
- 4.Mizoguchi H, O'Shea JJ, Longo DL, Loeffler CM, McVicar DW, Ochoa AC. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science. 1992;258:1795–8. doi: 10.1126/science.1465616. [DOI] [PubMed] [Google Scholar]
- 5.Salvadori S, Gansbacher B, Pizziment AM, Ziers KS. Abnormal signal transduction by T cells of mice with parental tumours is not seen in mice bearing IL-2-secreting tumors. J Immunol. 1994;153:5176–81. [PubMed] [Google Scholar]
- 6.Finke JH, Zea AH, Stanley J, Longo DL, et al. Loss of T-cell receptor ζ chain and p56lck in T-cells infiltrating human renal cell carcinoma. Cancer Res. 1993;53:5613–6. [PubMed] [Google Scholar]
- 7.Nakagomi H, Petersson M, Magnusson I, et al. Decreased expression of the signal-transducing ζ chains in tumour-infiltrating T-cells and NK cells of patients with colorectal carcinoma. Cancer Res. 1993;53:5610–2. [PubMed] [Google Scholar]
- 8.Matsuda M, Pettersson M, Lenkei R, et al. Alterations in the signal-transducing molecules of T cells and NK cells in colorectal tumor-infiltrating gut mucosal and peripheral lymphocytes: correlation with the stage of disease. Int J Cancer. 1995;61:765–72. doi: 10.1002/ijc.2910610605. [DOI] [PubMed] [Google Scholar]
- 9.Zea AH, Curti BD, Longo DL, et al. Alterations in T cell receptor and signal transduction molecules in melanoma patients. Clin Cancer Res. 1995;1:1327–35. [PubMed] [Google Scholar]
- 10.Levey DL, Srivastava PK. T cells from late tumor-bearing mice express normal levels of p56lck, p59fyn, ZAP-70, and CD3ζ despite suppressed cytolytic activity. J Exp Med. 1995;182:1029–36. doi: 10.1084/jem.182.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tartour E, Latour S, Mathiot C, et al. Variable expression of CD3ζ chain in tumor-infiltrating lymphocytes (TIL) derived from renal-cell carcinoma: relationship with TIL phenotype and function. Int J Cancer. 1995;63:205–12. doi: 10.1002/ijc.2910630210. [DOI] [PubMed] [Google Scholar]
- 12.Cardi G, Haeney JA, Schned AR, Phillips DM, Branda MT, Ernstoff MS. T-cell receptor ζ-chain expression on tumour infiltrating lymphocytes from renal cell carcinoma. Cancer Res. 1997;57:3517–9. [PubMed] [Google Scholar]
- 13.Wang Q, Stanley J, Kudoh S, et al. T cells infiltrating non-Hodgkins lymphoma show altered tyrosine phosporylation pattern even though T cell receptor/CD3-associated kinases are present. J Immunol. 1995;155:1382–92. [PubMed] [Google Scholar]
- 14.Stefanova I, Saville MW, Peters C, et al. HIV infection-induced posttranslational modification of T cell signalling molecules associated with disease progression. J Clin Invest. 1996;98:1290–7. doi: 10.1172/JCI118915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpson E. Immunology: why baby isn't thrown out. Curr Biol. 1996;6:43–4. doi: 10.1016/s0960-9822(02)00418-9. [DOI] [PubMed] [Google Scholar]
- 16.Franco JL, Ghosh P, Wiltrout RH, et al. Partial degradation of T-cell signal transduction molecules by contaminating granulocytes during protein extraction of splenic T cells from tumour bearing mice. Cancer Res. 1995;55:3840–6. [PubMed] [Google Scholar]
- 17.Noda S, Nagata-Narumija T, Kosugi A, Narumija S, Ra C, Fujiwara H, Hamaoka T. Do structural changes of T cell receptor complex occur in tumor-bearing state? Jpn J Cancer Res. 1995;86:383–94. doi: 10.1111/j.1349-7006.1995.tb03068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Kono K, Salazar-Onfray F, Petersson M, et al. Hydrogen peroxide secreted by tumor-derived macrophages down-modulates signal- transducing zeta molecules and inhibits tumor-specific T cell and natural killer cell-mediated cytotoxicity. Eur J Immunol. 1996;26:1308–13. doi: 10.1002/eji.1830260620. [DOI] [PubMed] [Google Scholar]
- 20.Otsuji M, Kimura Y, Aoe T, Okamoto Y, Saito T. Oxidative stress by tumor-derived macrophages suppresses the expression of CD3 ζ chain of T cell receptor complex and antigen-specific T-cell responses. Proc Natl Acad Sci USA. 1996;93:13119–24. doi: 10.1073/pnas.93.23.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pellegrini P, Berghella AM, Del Beato T, Cicia S, Adorno D, Casciani CU. Disregulation in TH1 and TH2 subsets of CD4+ T cells in periperheral blood of colorectal cancer patients and involvement in cancer establishment and progression. Cancer Immunol Immunother. 1996;42:1–8. doi: 10.1007/s002620050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosmann TR, Sad S. The expanding universe of T-cell subsets. Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 23.Taupin JL, Anderson P. Activation-induced proteolysis of the cytoplasmic domain of ζ in T cell receptors and Fc receptors. Eur J Immunol. 1994;24:3000–4. doi: 10.1002/eji.1830241212. [DOI] [PubMed] [Google Scholar]
- 24.Mulder WMC, Bloemena E, Stukart MJ, Kummer JA, Wagstaff J, Scheper RJ. T cell receptor-ζ and granzyme B expression in mononuclear cell infiltrates in normal colon mucosa and colon carcinoma. Gut. 1997;40:113–9. doi: 10.1136/gut.40.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey DL, Srivastava PK. Alterations in T cells of cancer-bearers: whence specificity? Immunol Today. 1996;17:365–8. doi: 10.1016/0167-5699(96)10013-X. [DOI] [PubMed] [Google Scholar]
- 26.Choi SH, Chung EJ, Wang DY, Lee SS, Jang Y-S, Kim CW. Alterations of signal-transducing molecules in tumour-infiltrating lymphocytes and peripheral blood T lymphocytes from human colorectal carcinoma patients. Cancer Immunol Immunother. 1998;45:229–305. doi: 10.1007/s002620050446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolenko V, Wang Q, Riedy MC, et al. Tumour-induced suppression of T-lymphocyte proliferation coincides with inhibition of Jak3 expression and IL-2 receptor signalling. Role of soluble products from human renal cell carcinoma. J Immunol. 1997;159:3057–67. [PubMed] [Google Scholar]