Abstract
The contribution of serotype-specific IgG concentration, subclasses, and avidity to opsonophagocytic activity (OPA) against Streptococcus pneumoniae (Pnc) was evaluated in sera of adults and infants immunized with different pneumococcal vaccines. Antibody concentrations and avidities were measured by enzymeimmunoassay (EIA) and OPAs by killing assay of Pnc. The most important factor contributing positively to OPA was the specific IgG level. In infants, a tendency to negative correlation was found between the concentration needed for killing of bacteria and avidity, suggesting that less antibodies of high rather than low avidity were required for killing. No such correlation was seen in adults. However, in adults the avidity was high already before vaccination and the variation was narrow. Thus, avidity was probably not a limiting factor influencing OPA. The effect of IgG2/IgG1 ratio on OPA was mostly negative but insignificant.
Keywords: opsonophagocytic activity, Streptococcus pneumoniae, conjugate vaccines
INTRODUCTION
Polysaccharide (PS) protein conjugate vaccines against Streptococcus pneumoniae (Pnc) have proved to be immunogenic in infants [1–6]. Moreover, they are able to induce immunological memory [2,4–6], production of different isotypes [7,8], as well as affinity maturation of antibodies [9,10]. In addition to humoral response, they elicit mucosal response [11,12] and reduce nasopharyngeal carriage of vaccine serotypes [13,14]. A lot of immunogenicity data of pneumococcal conjugate vaccines have been reported [1–6], and efficacy trials are now underway.
No data are available on the correlates or surrogates of protective immune response in humans to conjugate vaccines against Pnc. The possibility of correlating serological data with protection would have great practical value in permitting vaccine efficacy to be predicted on the basis of serological studies. Efficacy trials, in which pneumococcal conjugate vaccines are tested for their protective efficacy against invasive infections, pneumonia, acute otitis media, and carriage in infants, are ongoing. One of the objectives of these trials is to determine the most reliable laboratory correlates of protection against pneumococcal diseases.
Host protection against Pnc is mainly mediated by opsonin-dependent phagocytosis [15]. Therefore, opsonophagocytic activity (OPA) of antibodies to pneumococcal capsular polysaccharides (PS) is believed to measure their functional activity and thus may represent a better surrogate of in vivo protection than the commonly used antibody concentration. Components that contribute to OPA are the quantitative and qualitative characteristics of antibodies, such as antibody concentration, isotype, and avidity. In this study, the contribution of serotype-specific IgG concentration, subclasses, and avidity to OPA against Pnc types 6B, 19F, and 23F was evaluated in sera of adults and infants immunized with different pneumococcal vaccines.
SUBJECTS AND METHODS
Vaccines
PncD (Pasteur-Mèrieux Connaught, Swiftwater, PA) and PncT (Pasteur Mèrieux Connaught, Marcy l'Etoile, France) were tetravalent pneumococcal conjugate vaccines containing 10 μg of type 6B, 14, 19F, and 23F capsular PS conjugated to either diphtheria or tetanus toxoid. PncCRMos and PncCRMps (Wyeth Lederle Vaccines and Paediatrics, West Henrietta, NY) were, respectively, penta- and heptavalent conjugate vaccines, the former containing 10 μg of type 6B, 14, 18C, 19F and 23F oligosaccharides (OS) and the latter containing 2 μg of type 4, 9V, 14, 19F and 23F capsular PS, 2 μg of 18C OS, and 4 μg of type 6B PS conjugated to non-toxic variant of diphtheria toxin CRM197. Pneumovax (Pasteur-Mèrieux Connaught) and PNU-IMMUNE (Wyeth Lederle Vaccines and Paediatrics) were commercial 23-valent pneumococcal PS vaccines (PncPS) containing 25 μg of each capsular PS.
Vaccinees and sampling
Healthy adults were immunized in consecutive, clinical trials [8,11] with one of the three different pneumococcal conjugate vaccines: PncD (n = 12), PncT (n = 10), and PncCRMos (n = 10). Blood samples were obtained before (day 0) and 1 month after vaccination (day 28). Sera were stored at −20°C until testing. The sera obtained from adults immunized with Pneumovax (n = 10) were provided by Dr D. Goldblatt (Institute of Child Health, University of London, UK). Blood samples were taken before and 4–8 weeks after vaccination. Sera were lyophilized and stored at 4°C. After dissolving, the sera were stored at −70°C until testing. For analyses, data obtained from adults immunized with different pneumococcal vaccines were combined (n = 42). Before combination it was assured that the relationship between different serological parameters was similar in different vaccine groups.
Infants (n = 16) were immunized at 2, 4 and 6 months of age with PncCRMps and boosted at 15 months with the homologous conjugate or a PS vaccine (PNU-IMMUNE) [10]. Blood samples were obtained at 7, 15 and 16 months of age. Infants boosted either way were retained as one group. Although the avidities between the infants boosted by conjugate or PS vaccine differed, as shown previously [10], the contributions of various factors on OPA were alike. Therefore, combination of the groups was assumed not to interfere with the interpretation of the results.
Enzymeimmunoassay for total anti-Pnc PS IgG
Concentrations of IgG antibodies to pneumococcal polysaccharides were measured by enzymeimmunoassay (EIA) method described previously [2]. The results are given as μg/ml calculated on the basis of the officially assigned IgG values of the 89-SF reference serum [16].
EIA for anti-Pnc PS IgG1 and IgG2 antibodies
Concentrations of anti-Pnc PS IgG1 and IgG2 antibodies (μg/ml) were measured by EIA method described by Soininen et al. using assigned IgG1 and IgG2 values of 89-SF standard [17]. IgG subclasses were detected by mouse MoAbs to human IgG1 (clone HP6070) and IgG2 (clone HP6002; Zymed Labs, San Francisco, CA) followed by alkaline phosphatase-conjugated rabbit anti-mouse antibody (Jackson ImmunoResearch Labs, West Grove, PA). Reactions were developed by p-nitrophenyl phosphate (Sigma, St Louis, MO), and optical densities (OD) were read at 405 nm.
EIA for the avidity of anti-Pnc PS antibodies
The relative avidity of IgG antibodies to capsular PS of Pnc was determined by EIA method described by Anttila et al. [9]. The assay was based on dissociation of antibody–antigen complexes by 0.5 m sodium thiocyanate (NaSCN). Of each sample, series with and without NaSCN treatment were made. Results were expressed as avidity index (AI), assigned as percentage of antibodies that remained bound to the antigens after thiocyanate treatment. AI was calculated by dividing end-point titre (OD 0.5 as the cut-off value) of the serum sample with NaSCN treatment by end-point titre of the sample without NaSCN treatment and by multiplying this by 100. The reproducibility of the assay was checked by including a control serum into each plate.
Opsonophagocytosis assay
Opsonophagocytic activity of serum antibodies was determined by measuring the killing of live pneumococci by fresh human polymorphonuclear leucocytes (PMNL) in the presence of antibody and complement. A modification of the method described by Romero-Steiner et al. was used [18]. Instead of differentiated HL-60 cells, PMNL were used. The cells were isolated from the peripheral blood of healthy adult volunteers by dextran sedimentation and Ficoll–Paque density gradient centrifugation. OPAs were expressed as titres: OPA is the reciprocal of the serum dilution with 50% killing compared with the bacterial growth in the controls which contain no serum. Sera with undetectable OPAs were reported as a titre 4, titre of 8 being the detection limit. The reproducibility of the assay was checked by including a control serum into each plate. To accept a run, the opsonophagocytic titre of the control serum had to be within ± 1 dilution of the mean titre, dilution factor being 1:2 [18]. When the exact titres of the control were calculated for the accepted runs performed during the years 1997–98 (n = 55, 16, or 10 for 6B, 19F, and 23F, respectively), the coefficient of variance was ≤ 30% for all the serotypes.
Streptococcus pneumoniae serotypes 6B, 19F and 23F (reference strains received from CDC, Atlanta, GA) were grown in Todd–Hewitt broth (THB) supplemented with 0.5% yeast extract and kept frozen (–70°C) in aliquots in THB with 15% glycerol. Highly encapsulated strains were used. The encapsulation was judged by the quellung test [18], and by preincubating the sera with pneumococcal cell wall PS (CPS) to neutralize the antibodies to CPS. The encapsulation of the strains seemed to be thick enough to prevent the access of anti-CPS to CPS because, in these preliminary tests, no difference was found in OPAs of the sera whether or not neutralized by CPS.
Statistical analysis
The relationship between different factors was evaluated by correlation analysis and contribution of IgG concentration, IgG2/IgG1 ratio, and avidity to OPA was determined by multiple regression analysis. While performing the analyses, pre- and post-vaccination sera of adults and sera of infants taken at different time points were retained separately due to their dependency on each other. The minimum concentration needed for 50% killing of bacteria was calculated by dividing the total IgG concentration of a sample by the opsonophagocytic titre. This was done only for the samples with detectable opsonic activity. Samples obtained at 15 months were excluded from the comparison of concentration required for killing versus avidity because very few of them had detectable OPAs (e.g. three out of 16 for 6B). Student's t-test was used to compare the respective concentrations needed for killing in post-vaccination sera of adults and in 7- and 16-month sera of infants stratified into two groups by low or high antibody avidity (AI < 60 and AI > 60). AI 60 was used as a limit for division to obtain as similar a number of subjects as possible into the stratified groups. Again, prevaccination sera of adults and 15-month sera of infants were excluded due to the small number of active sera. In all statistical analyses log transformed data of concentrations and OPAs were used.
RESULTS
Adults immunized with pneumococcal vaccines responded with rises in total IgG, IgG1 and IgG2 levels, as well as in opsonophagocytic activities of antibodies to pneumococcal capsular PS (Table 1). The subtype dominating the response was IgG2. No significant increases were found in mean antibody avidities, which were already high in prevaccination sera of adults. In infants, antibody levels and OPAs obeyed similar kinetics: a decrease between 7 and 15 months and an increase after booster vaccination (Table 1). IgG1 was the dominating subclass but most infants started to produce IgG2 as well after booster vaccination. The kinetics of antibody avidity was different from that of antibody concentrations: the mean AI increased significantly from 7 to 15 months and booster vaccination either increased it further or caused no change, depending on the serotype and on vaccine given as a booster [10]. For serotypes 6B and 23F, the avidity of antibodies was lower in the 7-month sera of infants than in the prevaccination sera of adults, even though infants had received three doses of conjugate vaccine. However, by age and by booster vaccination infants achieved or exceeded the adult level of antibody avidity.
Table 1.
Geometric mean concentrations (GMC), mean avidities (MAI), and geometric mean opsonophagocytic activities (GMOPA) of antibodies to pneumococcal type 6B, 19F and 23F polysaccharides in pre- and post-vaccination sera of adults (n = 42) and in sera of infants (n = 16) obtained at 7, 15 and 16 months of age

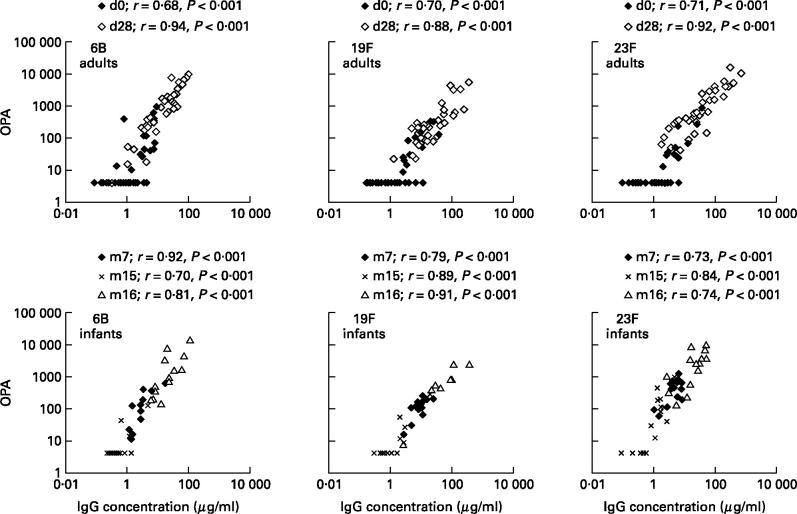
The total concentration of IgG antibodies to all serotypes strongly correlated with the OPA in sera of both adults and infants (Fig. 1). In adults, the correlation was better in post- than in pre-vaccination sera. In infants, the correlation coefficients were high throughout and no differences between age groups were seen. Instead, the sera of adults and infants differed in the IgG concentration required to produce detectable OPA of antibodies: generally, there were more adult than infant sera with moderate or high IgG concentrations but no opsonic activity (Fig. 1).
Fig. 1.
Relationship between total IgG concentration and opsonophagocytic activity (OPA) in pre- and post-vaccination sera of adults (n = 42) and in sera of infants (n = 16) taken at 7, 15 and 16 months of age. d, Day of sampling; m, month of sampling.
As total IgG, both IgG1 and IgG2 anti-Pnc PS concentrations correlated significantly with OPA in adults and in infants. Again, the correlation in adults was better in post- than in pre-vaccination sera and for IgG2 than for IgG1 (post: r = 0.63–0.80 for IgG1 and r = 0.79–0.86 for IgG2; pre: r = 0.32–0.54 for IgG1 and r = 0.53–0.59 for IgG2). By contrast, in infants IgG1 correlated better with OPA than IgG2, with type 23F as an exception (6B and 19F: r = 0.80–0.87 for IgG1 and r = 0.43–0.83 for IgG2; 23F: r = 0.25–0.59 for IgG1 and r = 0.57–0.81 for IgG2). Both in adults and in infants, the sum of IgG1 and IgG2 concentrations correlated well with the total IgG concentration for all the serotypes (adults: r = 0.78–0.99, P < 0.001; infants: r = 0.76–0.98, P < 0.001), even if the sum of IgG1 and IgG2 geometric mean concentration (GMC) did not add up exactly to the GMC of total IgG (Table 1).
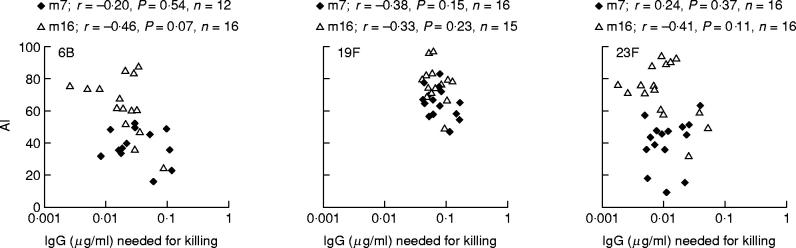
Although no direct correlation between relative avidity of antibodies and opsonophagocytic activity was found, there was a tendency to negative correlation between IgG concentration required for 50% killing of bacteria and antibody avidity in infants (Fig. 2). Furthermore, when infants were stratified into groups of AI lower or higher than 60, the concentration needed for killing was lower in the groups of high rather than low avidity (Table 2). At 16 months, the difference was significant for serotypes 6B and 23F. Nevertheless, for serum samples with comparable antibody avidity, there was a wide range in the concentrations of antibodies required for killing (Fig. 2). In adults, neither such a correlation nor difference was seen for any serotype (Table 2).
Fig. 2.
Relationship between total IgG concentration required for killing of bacteria and antibody avidity in sera of infants obtained after primary series of immunizations at 7 months of age and after booster vaccination at 16 months of age. Only sera with detectable opsonophagocytic activity (OPA) were included in the comparison. m, Month of sampling.
Table 2.
Antibody concentrations needed for 50% killing of Streptococcuspneumoniae in post-vaccination sera of adults and in 7- and 16-month sera (m7 and m16) of infants stratified by low- or high-avidity antibodies (avidity index (AI) < 60 and AI > 60). Student's t-test was used to compare the stratified groups with each other. At 7 months, none of the infants had anti-6B avidity higher than 60, therefore the group is missing from the Table

When the contributions of total IgG level, IgG2/IgG1 ratio, and relative antibody avidity on OPA were analysed by multiple regression analysis, only IgG concentration was found to have a significant positive influence on OPA for all serotypes in sera of both adults and infants (regression coefficient β = 0.65–1.59, P < 0.01–0.001). The effect of IgG2/IgG1 ratio on OPA was mostly negative but not significant. Likewise, in this analysis no significant influence of antibody avidity on OPA was found in adults or in infants. The models fit the data well (high coefficient of determination or r2 values: 0.65–0.88), except for the adults' prevaccination models, for which the fit was only good (r2 = 0.5–0.55). Analysis of the residuals showed no evidence of problems, instead a good fit of the models was further emphasized. Generally, in adults the regression coefficients for IgG concentrations increased after vaccination and fit of the model improved. In infants no significant changes were seen in the regression coefficients between the time points, but fit of the model tended to improve, except for 6B.
DISCUSSION
In both adults and infants, the most important factor contributing positively to OPA was the total IgG concentration of specific antibodies to 6B, 19F and 23F. Likewise, the concentration of IgG1 and IgG2 subclasses correlated significantly with OPA. Similar orders of correlation between OPA and levels of different antibody isotypes to type 6B have been presented by Vidarsson et al. [7]. Moreover, we found that some adult sera with quite high concentrations of specific antibodies did not have detectable OPA. Most of these OPA-negative sera were obtained before the vaccination of the adults. No such discrepancies were seen with the sera of infants. Thus, the difference between the sera may be due to the polyreactive, unspecific antibodies with low functional activity present in the prevaccination sera of adults but not in the sera of infants [19–21], or alternatively, different dominance of IgG subclasses between adults and infants. Namely, most antibodies in infant sera were of IgG1 subclass, which is generally known to serve as an opsonin and to activate classical pathway of complement more efficiently than IgG2, the dominating subclass in adults. The finding that the IgG2/IgG1 ratio usually had a negative influence on OPA may support the latter assumption. However, this should be confirmed by isolating IgG1 and IgG2 subclasses from the sera and by analysing the OPAs of the fractions.
In agreement with previous studies, we found a good correlation between the sum of IgG1 and IgG2 concentrations and the total IgG concentration, even if the sum of IgG1 and IgG2 GMC did not add up exactly to the GMC of total IgG [17,22]. The inconsistency may be explained mostly by the methodological differences in measuring the concentration of total IgG or IgG subclasses. Other IgG subtypes besides IgG1 and IgG2 may have a minor influence on total IgG concentration [22].
Although no significant contribution of antibody avidity was found by multiple regression analysis, there was a tendency to negative correlation in infants between concentration needed for killing of bacteria and relative avidity. Probably due to the low number of infants (n = 16), the correlation was not statistically significant. However, this suggests that less antibodies of high avidity than of low avidity were needed for killing of bacteria. Furthermore, for all serotypes, lower levels of antibodies with AI > 60, compared with antibodies with AI < 60, were required for killing. Similarly, Schlesinger et al. found a negative relationship between antibody avidity and serum bactericidal concentration in a study of infants immunized by Haemophilusinfluenzae type b (Hib) conjugate vaccines [23]. Surprisingly, no such tendency to correlations, nor difference between classified groups seen in infants, were found for adults. This may be due to the fact that antibody avidity was already rather high in adult sera before vaccination and generally little variation in avidity was found among adults. Therefore, avidity was probably not the limiting factor contributing to OPA in adults.
To improve further the fit of the models, especially of adults' prevaccination models, other factors possibly influencing OPA should be included in the regression analysis. These additional factors could be IgM antibodies to pneumococcal PS, polyreactive antibodies, and antibodies to pneumococcal surface proteins. Although antibodies of IgM isotype are not able to serve as opsonins, they may enhance phagocytosis by activating classical pathway of complement. Polyreactive antibodies and antibodies to pneumococcal surface proteins may, in turn, have an influence on OPA because the sera of which OPA was analysed were not purified, but normal polyclonal sera of adults and infants, containing a wide variety of antibodies to different antigens. Thus, if there were proteins breaking through the capsule in the surface of Pnc, the above-mentioned antibodies may have had access to them. By contrast, unexplained activity was unlikely to be due to the antibodies to cell wall PS because neutralization of them had no influence on the OPA of the sera. Thus, the encapsulation of the bacteria was probably thick enough to prevent access of the anti-CPS to CPS. Besides, anti-CPS antibodies have been found to have very little opsonic activity [24].
Altogether these results suggest that total IgG concentration is by far the most important factor contributing OPA to Pnc in adults and infants. However, there are other factors, such as relative antibody avidity and isotype or subclass of the antibodies, which seem to have some, though minor, influence on the functional activity. Probably the effect of these factors becomes especially important at the low concentrations of the antibodies and in subjects with an immature immune system. The influence of all these factors on the actual protection against pneumococcal diseases will be evaluated in the efficacy trials of pneumococcal conjugate vaccines.
Acknowledgments
The study was supported partly by World Health Organization, GVP/VRD contract V23/181/76. We thank Hannele Lehtonen, Teija Jakkola, Arja Vuorela, Sirkka-Liisa Wahlman, and Jukka Nenonen for technical assistance, Tea Nieminen for adult samples, Anu Soininen for subclass results of adults, and Heidi Åhman for IgG concentration results of infants. We also wish to thank personnel of study centres for their help in the clinical part of the study. In the study the European guidelines for Good Clinical Practice (based on directive no. 91/507/EEC) were followed. The protocol of the studies was approved by Ethics Committee of the National Public Health Institute. Written informed consent was obtained from the parents prior to enrolment of their children.
REFERENCES
- 1.Steinhoff MC, Edwards K, Keyserling H, Thoms ML, Johnson C, Madore D, Hogerman D. A randomized comparison of three bivalent Streptococcus pneumoniae glycoprotein conjugate vaccines in young children: effect of polysaccharide size and linkage characteristics. Pediatr Infect Dis J. 1994;13:368–72. doi: 10.1097/00006454-199405000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Käyhty H, Åhman H, Rönnberg P-R, Tillikainen R, Eskola J. Pneumococcal polysaccharide–meningococcal outer membrane protein complex conjugate vaccine is immunogenic in infants and children. J Infect Dis. 1995;172:1273–8. doi: 10.1093/infdis/172.5.1273. [DOI] [PubMed] [Google Scholar]
- 3.Leach A, Ceesay SJ, Banya WA, Greenwood BM. Pilot trial of pentavalent pneumococcal polysaccharide/protein conjugate vaccine in Gambian infants. Pediatr Infect Dis J. 1996;15:333–9. doi: 10.1097/00006454-199604000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Dagan R, Melamed R, Zamir O, Leroy O. Safety and immunogenicity of tetravalent pneumococcal vaccines containing 6B, 14, 19F and 23F polysaccharides conjugated to either tetanus or diphtheria toxoid in young infants and their boosterability by native polysaccharide antigens. Pediatr Infect Dis J. 1997;16:1053–9. doi: 10.1097/00006454-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Åhman H, Käyhty H, Lehtonen H, Leroy O, Froeschle J, Eskola J. Streptococcus pneumoniae capsular polysaccharide–diphtheria toxoid conjugate vaccine is immunogenic in early infancy and able to induce immunologic memory. Pediatr Infect Dis J. 1998;17:211–6. doi: 10.1097/00006454-199803000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Rennels M, Edwards K, Keyserling H, et al. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatrics. 1998;101:604–11. doi: 10.1542/peds.101.4.604. [DOI] [PubMed] [Google Scholar]
- 7.Vidarsson G, Sigurdardottir ST, Gudnason T, et al. Isotypes and opsonophagocytosis of pneumococcus type 6B antibodies elicited in infants and adults by an experimental pneumococcus type 6B-tetanus toxoid vaccine. Infect Immun. 1998;66:2866–70. doi: 10.1128/iai.66.6.2866-2870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soininen A, Seppälä I, Nieminen T, Eskola J, Käyhty H. IgG subclass distribution of antibodies after vaccination of adults with pneumococcal conjugate vaccines. Vaccine. 1999;17:1889–97. doi: 10.1016/s0264-410x(98)00475-7. [DOI] [PubMed] [Google Scholar]
- 9.Anttila M, Eskola J, Åhman H, Käyhty H. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J Infect Dis. 1998;177:1614–21. doi: 10.1086/515298. [DOI] [PubMed] [Google Scholar]
- 10.Anttila M, Eskola J, Åhman H, Käyhty H. Differences in the avidity of antibodies evoked by four different pneumococcal conjugate vaccines in early childhood. Vaccine. 1999;17:1970–7. doi: 10.1016/s0264-410x(98)00458-7. [DOI] [PubMed] [Google Scholar]
- 11.Nieminen T, Eskola J, Käyhty H. Pneumococcal conjugate vaccination in adults: circulating antibody secreting cell response and humoral antibody responses in saliva and in serum. Vaccine. 1998;16:630–6. doi: 10.1016/s0264-410x(97)00235-1. [DOI] [PubMed] [Google Scholar]
- 12.Korkeila M, Lehtonen H, Åhman H, Leroy O, Eskola J, Käyhty H. Salivary anti-capsular antibodies in infants and children immunized with Streptococcus pneumoniae capsular polysaccharide–tetanus or diphtheria toxoid conjugate vaccines. Vaccine. doi: 10.1016/s0264-410x(99)00393-x. in press. [DOI] [PubMed] [Google Scholar]
- 13.Dagan R, Rimma M, Muallem M, et al. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J Infect Dis. 1996;174:1271–8. doi: 10.1093/infdis/174.6.1271. [DOI] [PubMed] [Google Scholar]
- 14.Dagan R, Muallem M, Rimma M, Leroy O, Yagupsky P. Reduction of pneumococcal nasopharyngeal carriage in early infancy after immunization with tetravalent pneumococcal vaccines conjugated to either tetanus toxoid or diphtheria toxoid. Pediatr Infect Dis J. 1997;16:1060–4. doi: 10.1097/00006454-199711000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Tuomanen EI, Austrian R, Masure HR. Pathogenesis of pneumococcal infection. N Engl J Med. 1995;332:1280–4. doi: 10.1056/NEJM199505113321907. [DOI] [PubMed] [Google Scholar]
- 16.Quataert SA, Kirch CS, Wiedl LJ, Phipps DC, Strohmeyer S, Cimino CO, Skuse J, Madore DV. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, Lot 89-S. Clin Diagn Lab Immunol. 1995;2:590–7. doi: 10.1128/cdli.2.5.590-597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soininen A, Seppälä I, Wuorimaa T, Käyhty H. Assignment of immunoglobulin G1 and G2 concentrations to pneumococcal capsular polysaccharides 3, 6B, 14, 19F, and 23F in pneumococcal reference serum 89–SF. Clin Diagn Lab Immunol. 1998;5:561–6. doi: 10.1128/cdli.5.4.561-566.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero-Steiner S, Libutti D, Pais LB, Dykes J, Anderson P, Whitin JC, Keyserling HL, Carlone GM. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol. 1997;4:415–22. doi: 10.1128/cdli.4.4.415-422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soininen A, van den Doppelsteen G, Oomen L, Käyhty H. International Symposium on Pneumococci and Pneumococcal Diseases, June. Helsingor, Denmark: 1998. Are there cross-reactive epitopes in Pnc capsular polysaccharides [Abstract P118] [Google Scholar]
- 20.Coughlin RT, White AC, Anderson CA, Carlone GM, Klein DL, Treanor J. Pneumococcal Vaccines for the World Conference, October. Washington DC, USA: 1998. Characterization of anti-pneumococcal antibodies in healthy unvaccinated adults [Abstract 41] [DOI] [PubMed] [Google Scholar]
- 21.Nahm M, Xinhing Y. Pneumococcal Vaccines for the World Conference, October. Washington DC, USA: 1998. Pneumococcal polysaccharide vaccines may elicit antibodies that lack serotype specificity and opsonophagocytic capacity. [Abstract 36] [Google Scholar]
- 22.Ziembiec NA, Sikkema DJ, Hildreth SW, Quataert SA, Madore DV. 99th General Meeting of American Society for Microbiology, May–June. Chicago, Illinois, USA: 1999. Assignment of IgG1 and IgG2 concentrations to additional pneumococcal capsular polysaccharides in pneumococcal reference serum 89-SF. [Google Scholar]
- 23.Schlesinger Y, Granoff DM. Vaccine Study Group. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. JAMA. 1992;267:1489–94. [PubMed] [Google Scholar]
- 24.Vidarsson G, Jonsdottir I, Jonsson S, Valdimarsson H. Opsonization and antibodies to capsular and cell wall polysaccharides of Streptococcus pneumoniae. J Infect Dis. 1994;170:592–9. doi: 10.1093/infdis/170.3.592. [DOI] [PubMed] [Google Scholar]