Abstract
Most anti-phospholipid antibodies (aPL) associated with the anti-phospholipid syndrome are autoantibodies with specificity towards β2-GPI (anti-β2-GPI) or prothrombin (anti-II). They are mainly screened by ELISA using polyoxygenated plates. However, some authors have claimed that immunoblotting can also be used. Exposure of cryptic epitopes or increase of antigen density on its binding to either phospholipids or suitable plastic surfaces are the two hypotheses proposed for the interaction of β2-GPI or prothrombin with their antibodies. Forty-five patients with aPL were studied: 25 with lupus anti-coagulant (LA) and anti-cardiolipin antibodies (aCL), 10 with LA alone and 10 with aCL but negative LA. All patients with LA and aCL were positive for anti-β2-GPI by ELISA and dot blot, while 15/25 had anti-IIELISA and 14 of them also had anti-II by dot blot assay. No patient with LA alone tested positive for anti-β2-GPI by ELISA or dot blot, whereas 6/10 had anti-IIELISA (five of them were also positive by dot blot). Four out of 10 aCL-positive patients had anti-β2-GPI by ELISA and dot blot, while none of this group had anti-II by ELISA or dot blot. Antibody binding to β2-GPI or prothrombin in both ELISA and dot blot was significantly reduced by phospholipid liposomes mixed together with β2-GPI or prothrombin, whereas liposomal eluants retained it in both assays. Parallel fluid-phase inhibition experiments using increasing concentrations (up to 200 μg/ml) of β2-GPI or prothrombin demonstrated that antibody binding reduction was more evident on dot blot than on ELISA. It was almost completely abolished on dot blot, while on ELISA a moderate inhibition was achieved even at the highest protein concentration. However, antibody binding on ELISA was virtually abolished when diluted sera were incubated with high protein concentrations applied to nitrocellulose membranes. We could infer that ELISA and dot blot detect antibodies with some differences in avidity but directed against native epitopes on β2-GPI and prothrombin.
Keywords: anti-prothrombin antibodies, anti-β2-glycoprotein I antibodies, anti-phospholipid syndrome
INTRODUCTION
Anti-cardiolipin antibodies (aCL) and lupus anti-coagulant (LA) belong to the heterogeneous family of the anti-phospholipid antibodies (aPL). The association of their presence with thrombosis, fetal loss and/or thrombocytopenia is known as the anti-phospholipid syndrome (APS) [1]. It may occur as a primary disorder or may be secondary to autoimmune diseases, such as systemic lupus erythematosus (SLE).
Since the first description of the requirement of certain phospholipid-binding proteins for the interaction between autoimmune aPL and phospholipids [2,3], several reports have studied the role of such proteins in the APS. In particular, β2-GPI and prothrombin (II) have been extensively investigated [4,5]. It is now widely accepted that autoimmune aPL specifically reacting with β2-GPI (anti-β2-GPI) are mainly responsible for the aCL reactivity and some LA activities [6–10], whereas those with specificity for prothrombin (anti-II) display LA properties [10–14]. We and others have shown that anti-β2-GPI and anti-II are associated with the clinical features of the APS, and this association appears even closer than that of aPL themselves [15–21].
However, the exact nature of the epitopes recognized by anti-β2-GPI and anti-II is still matter of discussion. One hypothesis indicates that these antibodies recognize cryptic epitopes expressed on the protein molecules by conformational changes when the proteins interact with phospholipid membranes or with irradiated polystyrene surfaces [22]. On the other hand, there is recent evidence that anti-β2-GPI and also anti-II are directed against the native structure of the proteins and are low-affinity antibodies requiring high density or clustering of the antigen to allow their bivalent attachment [23,24]. Anti-β2-GPI and anti-II are mainly detected by ELISA methods using only suitable plates, but some authors have demonstrated that Western or dot blotting can be used [18,25]. These findings contribute to the discussion about the nature of the interaction between β2-GPI and prothrombin and their antibodies.
In this study, we evaluate the binding of aPL to β2-GPI and prothrombin by means of two different techniques, ELISA and dot blot. In addition, we perform parallel solid- and fluid-phase inhibition experiments in order to investigate if the antibody binding to these proteins in both assays is influenced to the same extent.
PATIENTS AND METHODS
Patients
Forty-five patients with aPL were included in this study. There were 16 males and 29 females (mean age 36.0 years, range 15–61 years). Clinical manifestations were: venous thrombosis (n = 21), arterial thrombosis (n = 5), recurrent fetal loss (n = 10) and thrombocytopenia (n = 3). Ten patients were diagnosed with systemic lupus erythematosus (SLE) and seven of them also had clinical features of APS.
Patients were divided into three groups according to their aPL data. Group A included 25 patients with LA and moderate or high titres of aCL (IgG and/or IgM). All of them had moderate or high levels of anti-protein antibodies measured by ELISA: 25 had anti-β2-GPIELISA and 15 had anti-IIELISA. Group B included 10 patients with LA without aCL. Anti-β2-GPIELISA were found negative but six out of 10 had anti-IIELISA. In group C, 10 patients with aCL but negative LA were included. None of them had anti-IIELISA but four had positive anti-β2-GPIELISA.
Twenty healthy blood donors who did not have a history of autoimmune disease or thrombosis served as normal controls. Blood was collected by clean venepuncture and collected into glass tubes and allowed to clot at 37°C, and then centrifuged at 1500 g for 10 min. All sera were stored at −70°C. Plasma samples for LA studies were collected at the same time as serum samples.
Detection of aPL
The presence of LA activity was investigated by means of screening tests, mixing studies and confirmatory procedures as described in detail before [26]. LA was diagnosed according to previously defined criteria [26,27].
aCL of both isotypes were measured using a standardized ELISA technique [28]. International standards (Louisville APL Diagnostics, Louisville, KY) and our own control sera were used for the standard curve calibration. Results were expressed as standard units (U) for IgG (GPL) or IgM (MPL). Titres higher than 20 U were considered diagnostic for APS.
ELISA for anti-protein antibodies
The home-made ELISA for anti-β2-GPI was performed as previously reported [15,16] using microtitre plates (Nunc MaxiSorp, Kamstrup, Roskilde, Denmark) irradiated by electron beam at 100 kGy and coated with purified human β2-GPI (Diagnostica Stago, Asnières, France) at a concentration of 2 μg/well. The cut-off values (mOD 55 for IgG and 50 for IgM) were previously assessed by the method of percentiles (99th) by using 80 normal sera [15,16].
Anti-II were measured by ELISA as recently described [16] using γ-irradiated plates (Nunc MaxiSorp) coated with purified prothrombin (Diagnostica Stago) at a concentration of 1 μg/well. The cut-off values (mOD) were 67 (IgG) and 55 (IgM) [16].
Values above the cut-off points were considered positive. A mOD between the normal mean + 3–5 s.d. was considered low, between the normal mean + 5–10 s.d. moderate and above the mean + 10 s.d. high titre.
Dot blot assays for anti-protein antibodies
Protein immobilization was performed by using the Bio-Dot Microfiltration apparatus (BioRad Labs, New York, NY). Three micrograms of β2-GPI, prothrombin or bovine serum albumin (BSA) were passively filtered on nitrocellulose membranes prewetted in Tris-buffered saline (TBS: 20 mm Tris–HCl pH 7.4, 120 mm NaCl). The blocking and further incubation steps were carried out in separate containers. TBS–0.05% Tween 20 (TTBS) and 5% non-fat dry milk–TBS were used as washing and blocking solutions, respectively. The strips were transferred to separate tubes and incubated with sera diluted 1:50 in 1% BSA–TTBS or purified IgG samples at 100 μg/ml. The samples were incubated with gentle agitation for 2 h at room temperature. F(ab)′2 fragments of peroxidase-conjugated anti-human IgG or IgM were used for revealing antibody–antigen complexes. Colour development was done with 3,3′-diaminobenzidine. We included in each run a commercial rabbit anti-β2-GPI or anti-II (Diagnostica Stago) as positive controls and a negative (TBS) control.
Isolation of IgG
Whole IgG from serum was isolated by affinity chromatography on Protein A Sepharose CL-4B (Sigma Chemical Co., St Louis, MO). IgG was eluted with 0.1 m glycine–HCl buffer pH 3.0. The eluted fractions were dialysed against 0.02 m Tris–0.15 m NaCl buffer pH 7.5 overnight in the cold. Purity of the IgG preparations was checked by SDS–PAGE.
Absorption experiments
Cardiolipin (CL) liposomes were prepared by evaporating bovine CL in ethanol (Sigma) in a microfuge tube under a stream of nitrogen, and then resuspended in PBS in the presence or absence of purified human β2-GPI, and dispersed by continuous vortexing for 3 min. Appropriate dilutions (in PBS) of sera (1:10) or whole human IgG (250 μg/ml) were added to the liposomes and mixed by agitation for 1 min. Final concentrations were 1 mg/ml and 200 μg/ml for CL and β2-GPI, respectively. After incubation for 2 h at 37°C, the mixtures were centrifuged for 15 min at 15 000 g in a microcentrifuge and the supernatants kept as absorbed samples. Remaining reactivity to β2-GPI was tested by ELISA or dot blot assay, as detailed above.
Liposomes containing 40% phosphatidylserine (PS) and 60% phosphatidylcholine (PC; Sigma) were prepared as previously described [13]. In short, PS/PC liposomes were prepared by vortexing dried phospholipid mixtures in TBS with CaCl2 (5 mm) in the presence or absence of human prothrombin. Appropriate dilutions (in TBS–CaCl2) of sera (1:10) or whole human IgG (250 μg/ml) were incubated with the liposomes for 2 h at 37°C. Final concentrations were 1 mg/ml and 200 μg/ml for phospholipids and prothrombin, respectively. After centrifugation at 15 000 g for 15 min, the supernatants were frozen. Residual reactivity to prothrombin was assayed as detailed above.
The liposomal pellets were washed twice with the corresponding buffer (PBS or TBS–CaCl2) and were then dissolved in a 2% solution of n-octyl-β-d-glucopyranoside (Sigma). The solution was vortexed until the pellet dissolved. The preparations were further mixed with an equal volume of chloroform, vortexed, and microcentrifuged. Lipid extraction was repeated up to three times. The eluted antibodies were then diluted and tested by ELISA and dot blot methods.
For absorption studies with β2-GPI or prothrombin blotted on nitrocellulose membranes, three anti-β2-GPI- and two anti-II-positive sera were selected and diluted to twice the concentration, giving 50% of the maximum binding to the proteins by ELISA. One-hundred micrograms of β2-GPI, prothrombin or BSA were applied to nitrocellulose strips and dried at room temperature. The strips were transferred to separate tubes and incubated with sera diluted in 1% BSA–TTBS. The samples were incubated at room temperature for 2 h and at 4°C overnight, with constant mixing. The strips were then removed and other new strips of nitrocellulose blotted with 100 μg of β2-GPI, prothrombin or BSA were incubated with the same diluted serum samples for 2 h at room temperature and overnight at 4°C. After the two consecutive absorption procedures, the remaining activity to β2-GPI or prothrombin was tested on ELISA.
Fluid-phase inhibition studies
To measure fluid-phase inhibition, 10 anti-β2-GPI- and five anti-II-positive serum samples were first diluted to twice the concentration, giving 50% of the maximum binding to β2-GPI or prothrombin, respectively, by ELISA. Diluted samples were incubated with buffer or different concentrations (up to 200 μg/ml) of β2-GPI or prothrombin for 2 h at 37°C and overnight at 4°C. Samples were then retested on the respective ELISA and dot blot assays.
Statistical analysis
The Wilcoxon matched pairs test was used to compare absorbance readings before and after absorption experiments. Mean mOD (OD × 1000) and 95% confidence intervals (95%CI) were used for expressing anti-protein antibody titres. P < 0.05 was taken to indicate statistical significance. Statistical analysis was performed with the GraphPad Prism software.
RESULTS
Binding to β2-GPI or prothrombin by dot blot analysis
In group A, all positive sera in the ELISA for anti-β2-GPI were also clearly positive on dot blot. Both tests were concordant in the results and the isotype of detected immunoglobulin (IgG or IgM). All but one of the 15 anti-IIELISA-positive sera had positive anti-IIdot blot. The remaining 10 patients of this group had neither anti-IIELISA nor anti-IIdot blot.
None of the 10 patients of group B had anti-β2-GPI by ELISA or dot blot assays. On the contrary, positive anti-IIdot blot were found in five out of six patients having raised levels of anti-IIELISA. Patients with anti-IIELISA-negative results (n = 4) were also negative in the dot blot assay for anti-II (IgG or IgM).
Regarding the 10 aCL-positive patients of group C, β2-GPI binding on dot blot was seen only in the four patients with positive anti-β2-GPIELISA. Anti-II were negative by ELISA and dot blot analysis.
Absorption studies
In all sera having anti-β2-GPI and/or anti-II by both ELISA and dot blot techniques, absorption experiments with phospholipid liposomes were done. There was on average a 70% (s.d. 9.8%) decrease in anti-β2-GPIELISA titres and also a decrease in reactivity to β2-GPI on dot blot. For anti-II-positive sera, there was on average a 75% (s.d. 10.9%) decrease in antibody binding on ELISA. Anti-IIdot blot reactivity was also inhibited.
Figure 1 shows the ELISA data pre- and post-absorption studies obtained with whole IgG purified from 30 selected patients with aPL. Anti-β2-GPIELISA as well as anti-IIELISA binding was significantly decreased (Wilcoxon test, P < 0.0001).
Fig. 1.
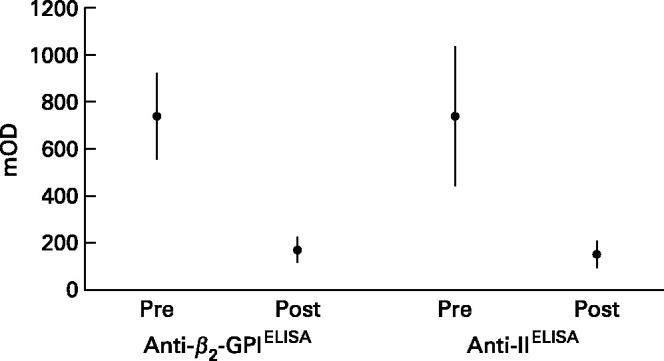
Comparative binding to β2-GPI and prothrombin (II) of whole IgG samples from patients with anti-phospholipid antibodies. The graph shows the absorbance (mean mOD and its 95% confidence interval) obtained by ELISA in the pre- and post-absorption experiments with phospholipid- containing liposomes.
Inhibitory effect of fluid-phase proteins and phospholipid liposomes on antibody binding
Taking into account the above results, we compared the binding profiles of sera diluted in buffer together with the liposomal supernatants and eluants to β2-GPI or prothrombin by both ELISA and dot blot. In addition, fluid-phase inhibition was done in the same selected sera.
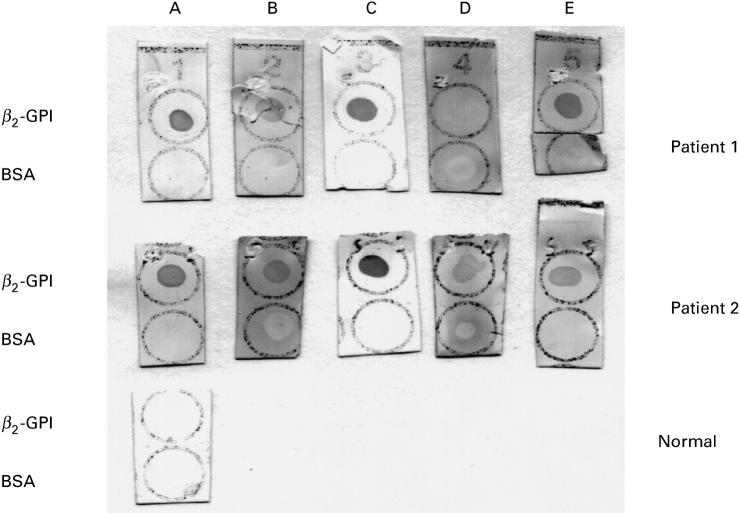
Table 1 shows the results in 10 selected positive sera for β2-GPI. First, the procedure with CL-containing liposomes significantly reduced the reactivity by ELISA in the supernatants. The inhibition ranged from 59% to 88%. Dot blot reactivities were also reduced. On the other hand, the liposomal eluants retained the binding to β2-GPI in both ELISA and dot blot (Fig. 2). Using increasing concentrations of soluble β2-GPI, we found that the inhibition of the β2-GPI-binding activity was more evident on dot blot than on ELISA. In fact, at 200 μg/ml the binding on dot blot was completely abolished in eight out of 10 sera, while on ELISA a moderate inhibition ranging from 32% to 59% was achieved.
Table 1.
Reactivity of 10 selected anti-phospholipid (aPL) sera to β2-GPI by ELISA and dot blot assays before and after inhibition experiments with cardiolipin liposomes or fluid-phase β2-GPI
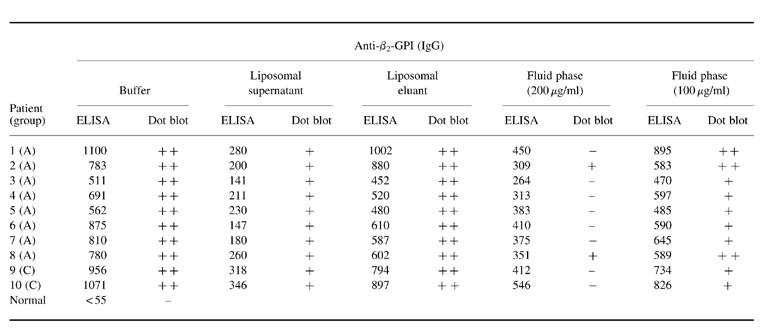
Data by ELISA are shown in mOD and those by dot blot analysis as high positive (++), low positive (+) or negative (−).
Fig. 2.
Dot blot analysis of two representative serum samples from patients having antibodies (IgG) to β2-GPI before and after inhibition experiments. A, Serum diluted in buffer; B, cardiolipin (CL)-liposomal supernatant; C, CL-liposomal eluant; D, fluid-phase inhibition with 200 μg/ml of β2-GPI; E, fluid-phase inhibition with 100 μg/ml of β2-GPI. Strips were dotted with 3 μg of β2-GPI or bovine serum albumin (BSA). A normal serum is shown as control.
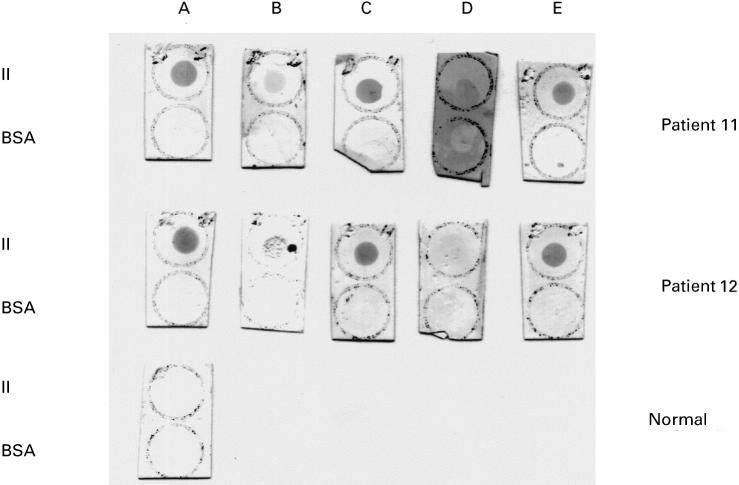
As shown in Table 2, the binding of five sera to prothrombin was greatly inhibited in the ELISA (ranging from 63% to 83%) by PS/PC liposomes in the presence of prothrombin and calcium ions. A low reactivity was also found on dot blot in the supernatants. The activity was retained in the liposomal eluants, as illustrated in Table 2 and Fig. 3. On contrast, with fluid-phase prothrombin at high concentrations a more efficient inhibition of prothrombin-binding activity could be obtained assayed on dot blot than on ELISA. No binding on dot blot was detected in four out of five sera after inhibition with 200 μg/ml, whereas this treatment reduced by 47% the anti-II activity determined on ELISA.
Table 2.
Reactivity of five anti-phospholipid (aPL) sera to prothrombin by ELISA and dot blot assays before and after inhibition experiments with phosphatidylserine/phosphatidylcholine liposomes or fluid-phase prothrombin
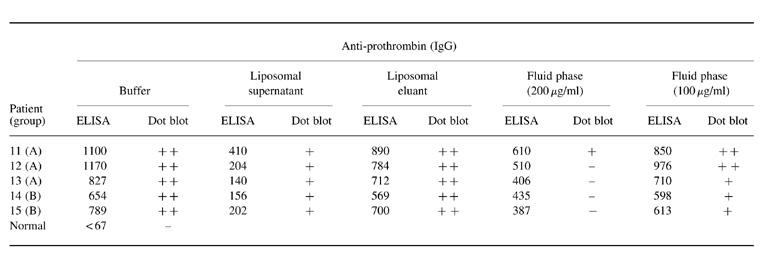
Data by ELISA are shown in mOD and those by dot blot analysis as high positive (++), low positive (+) or negative (−).
Fig. 3.
Dot blot analysis of two representative serum samples from patients having antibodies (IgG) to human prothrombin (II) before and after inhibition experiments. A, Serum diluted in buffer; B, phosphatidylserine (PS)/phosphatidylcholine (PC)-liposomal supernatant; C, PS/PC-liposomal eluant; D, fluid-phase inhibition with 200 μg/ml of II; E, fluid-phase inhibition with 100 μg/ml of II. Strips were dotted with 3 μg of II or bovine serum albumin (BSA). A normal serum is shown as control.
Inhibitory effect of proteins blotted onto nitrocellulose membranes on binding of antibodies in ELISA
Inhibition experiments using consecutive absorption of diluted sera with high concentrations of proteins applied to nitrocellulose membranes virtually abolished the reactivity on ELISA. For anti-β2-GPI-positive sera (n = 3), antibody binding (mean mOD ± s.d.) was decreased from 894 ± 215 to 100 ± 54. Similarly, the prothrombin-binding activity of two serum samples diminished from 1037 ± 195–124 ± 60. When albumin was used as control antigen, no changes in antibody levels were noted.
DISCUSSION
Conflicting data have appeared in the literature regarding the involvement of β2-GPI and prothrombin in aPL binding and the exact nature of the epitopes to which aPL bind [29,30]. Most autoimmune aCL such as those found in SLE and primary APS are directed against epitopes expressed on β2-GPI, not on CL [4,15,17,18]. LA activity has been found to be directed towards either prothrombin [10–12] or β2-GPI [10,11,31]. Despite these findings, the nature of the interaction of anti-β2-GPI and anti-II with their target proteins is not fully understood. Most investigators agree that the mode of presentation of β2-GPI or prothrombin in solid phase greatly influences their recognition by anti-β2-GPI or anti-II, respectively. Little or no binding occurs if these proteins are immobilized on untreated microtitre plates [22,23,32]. Conversely, specific antibody binding to β2-GPI or prothrombin is observed if high binding polystyrene plates are used. It is mainly due to the introduction of oxygen atoms onto the polystyrene surface by γ-ray irradiation. The oxidation process results in the formation of functional groups that can mimic anionic phospholipids to which proteins can cross-link [33]. This is consistent with the new evidence presented by Hörkkö et al. [30], who demonstrated that many aPL bind to adducts of oxidized phospholipids and certain proteins.
Two hypotheses have been proposed to explain the interaction between aPL and protein antigens: (i) antibody recognition of conformational epitopes expressed when the antigen is bound to a suitable surface [22]; (ii) low-affinity antibodies recognizing epitopes on native proteins through a bivalent attachment promoted by the enhanced surface density of the antigens [23]. Several methodological approaches have been used to support both hypotheses. The need for oxidized surfaces in the ELISA assay [13–17,22] and the conformational changes observed in β2-GPI or prothrombin upon interaction with phospholipids [4,5] lend support to the former one. Moreover, the finding that antibody binding is inhibited by preincubation with beads coated with CL and β2-GPI, but not with CL-coated beads, or β2-GPI in solution strengthens the first theory [17,22]. On the other hand, the fact that specific antibody binding is detected on proteins immobilized onto microtitre plates or phospholipids, or immunoblotting suggests that antigen density may be the key factor for the interaction [11,18,23]. The evidence that only very high protein concentrations in fluid phase are able to inhibit antibody binding and that monovalent Fab′ fragments are unable to bind β2-GPI in ELISA emphasizes the importance of the latter hypothesis. Additionally, Sheng et al. [34] using mutants of β2-GPI generated by site-directed mutagenesis demonstrated that autoantibody binding to β2-GPI is intrinsically of low affinity and that the binding is dependent on the density of the antigen. Their results showed that anti-β2-GPI binding to the mutant (which spontaneously dimerizes) coated onto irradiated or standard polystyrene plates, or in solution, was significantly higher when compared with native, wild-type and other mutant (which does not dimerize).
Our present data in a series of patients with aPL show that autoantibodies to human β2-GPI or prothrombin detected by ELISA can also be demonstrated by immunoblotting, such as dot blot. This method involves the immobilization of proteins on synthetic membrane supports. The passive microfiltration procedure allows the binding of proteins on nitrocellulose membranes in such a way as to favour the recognition of proteins in its native structure [35]. These results would suggest that the same antibodies are detected by means of ELISA and dot blot, indicating mainly the recognition of native epitopes on proteins. Our positive findings on dot blot are in agreement with those from Cabral et al. [18] for anti-β2-GPI and from Permpikul et al. [11] for anti-II. Interestingly, none of our aPL patients with negative results for anti-protein antibodies on ELISA showed the presence of such antibodies on dot blot. To investigate further the reactivity pattern of these antibodies, we performed inhibition experiments using phospholipid liposomes. It was found that this treatment was able to absorb efficiently anti-β2-GPI or anti-II detected by means of ELISA or dot blot.
In a further attempt to understand better the nature of the interaction of these antibodies, the sera of some aPL patients were subjected to parallel solid- and fluid-phase inhibition treatments. We mainly assessed whether antibody binding screened by ELISA and dot blot was influenced to the same extent by these inhibition experiments. First of all, clear evidence for the low-avidity nature of anti-β2-GPI and anti-II was the high inhibitor concentrations required to give 50% inhibition. This explains the discordant results obtained by us in a previous report [15] and others [20,21], in which low concentrations of proteins in fluid phase were used. It is important to note that the addition of soluble β2-GPI or prothrombin at increasing concentrations caused different inhibition patterns in ELISA and dot blot. In fact, antibody binding detected on dot blot was almost completely abolished, while that evaluated on ELISA was less influenced. This finding, and the fact that antibody binding on ELISA was virtually abolished performing repeated absorptions of diluted sera with high protein concentrations applied to nitrocellulose membranes, could indicate the presence of antibodies with different degrees of avidity. Thus, anti-protein antibodies of low avidity are detected on ELISA and those showing higher avidity can be evaluated on dot blot or ELISA. Additional information comes from the comparison between the results obtained in the liposomal supernatants and those after treatment with fluid-phase proteins at the highest concentration. In spite of having higher optical readings in ELISA after fluid-phase experiments than after liposomal inhibition, only positive results on dot blot were found after solid-phase experiments. Therefore, we could infer that ELISA and dot blot are suitable methods to detect anti-β2-GPI and anti-II, but those antibodies showing the lowest avidity could only be measured on ELISA.
In a very recent work, George et al. [36] clearly demonstrated that three β2-GPI-binding IgM MoAbs cloned from a single patient with APS recognize non-cross-reactive epitopes on the β2-GPI molecule. One of them was directed to an epitope present on the native structure, while the remaining two recognized those exposed on β2-GPI after conformational changes. The marked heterogeneity of aPL has been extensively studied [10,32,37,38]. The precise epitopes recognized by anti-β2-GPI have been recently investigated. It has been proposed that the fifth domain of β2-GPI possesses both the phospholipid-binding site and the epitope for anti-β2-GPI [39,40]. Other authors using domain-deleted mutants of β2-GPI have concluded that the cryptic epitope for mouse and human monoclonal aCL [41,42] or some affinity-purified anti-β2-GPI [43] is located in the fourth domain. On the other hand, Iverson et al. [44] found that the epitope recognized by 11 of 11 anti-β2-GPI tested was located in domain 1. The epitopes recognized by anti-II have not yet been defined. Some studies indicate that more than a single epitope on prothrombin are targeted by anti-II [25].
In conclusion, both immunoblotting and ELISA techniques can be used in order to detect anti-β2-GPI and anti-II, which are mainly directed against native epitopes. The demonstration that both assays show some differences in the fluid-phase inhibition pattern could be due to the coexistence of populations of antibodies showing different degrees of avidity.
Acknowledgments
This work was supported by FONCyT grant PICT 97 No. 05-00000-01787, Ministry of Culture and Education, Argentina.
REFERENCES
- 1.Harris EN. The antiphospholipid syndrome—an introduction. In: Harris EN, Exner T, Hughes GRV, Asherson RA, editors. Phospholipid-binding antibodies. Boca Raton: CRC Press; 1991. pp. 373–86. [Google Scholar]
- 2.McNeil HP, Simpson RJ, Chesterman CN, et al. Antiphospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: β2-glycoprotein I (apolipoprotein H) Proc Natl Acad Sci USA. 1990;87:4120–4. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galli M, Comfurius P, Maassen C, et al. Anticardiolipin antibodies (ACA) directed not to cardiolipin but to a plasma protein cofactor. Lancet. 1990;335:1544–7. doi: 10.1016/0140-6736(90)91374-j. [DOI] [PubMed] [Google Scholar]
- 4.Borchman D, Harris EN, Pierangeli SS, et al. Interactions and molecular structure of cardiolipin and β2-glycoprotein I (β2GPI) Clin Exp Immunol. 1995;102:373–8. doi: 10.1111/j.1365-2249.1995.tb03792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu JR, Lenz BR. Phospholipid-specific conformational changes in human prothrombin upon binding to procoagulant acid lipid membranes. Thromb Haemost. 1994;71:596–604. [PubMed] [Google Scholar]
- 6.Viard JP, Amoura Z, Bach JF. Association of anti β2-glycoprotein I antibodies with lupus-type circulating anticoagulant and thrombosis in systemic lupus erythematosus. Am J Med. 1992;93:181–6. doi: 10.1016/0002-9343(92)90049-h. [DOI] [PubMed] [Google Scholar]
- 7.Arvieux J, Roussel B, Jacob MC, et al. Measurement of antiphospholipid antibodies by ELISA using β2-glycoprotein I as an antigen. J Immunol Methods. 1991;143:223–9. doi: 10.1016/0022-1759(91)90047-j. [DOI] [PubMed] [Google Scholar]
- 8.Roubey RAS, Eisenberg RA, Harper MF, et al. Anticardiolipin autoantibodies recognize β2 glycoprotein I in the absence of phospholipid. J Immunol. 1995;154:954–60. [PubMed] [Google Scholar]
- 9.Pengo V, Biasiolo A, Brocco T, Tonetto S, Ruffatti A. Autoantibodies to phospholipid-binding plasma proteins in patients with thrombosis and phospholipid-reactive antibodies. Thromb Haemost. 1996;75:721–4. [PubMed] [Google Scholar]
- 10.Kandiah DA, Krilis SA. Anti-β2 glycoprotein I and anti-prothrombin antibodies in patients with the antiphospholipid syndrome: immunological specificity and clotting profiles. Lupus. 1998;7:323–32. doi: 10.1191/096120398678920253. [DOI] [PubMed] [Google Scholar]
- 11.Permpikul P, Rao LVM, Rapaport SI. Functional and binding studies of the roles of prothrombin and β2-glycoprotein I in the expression of lupus anticoagulant activity. Blood. 1994;83:2878–92. [PubMed] [Google Scholar]
- 12.Bevers EM, Galli M, Barbui T, et al. Lupus anticoagulant IgG's (LA) are not directed to phospholipids only, but to a complex of lipid-bound human prothrombin. Thromb Haemost. 1991;66:629–32. [PubMed] [Google Scholar]
- 13.Arvieux J, Darnige L, Caron C, et al. Development of an ELISA for autoantibodies to prothrombin showing their prevalence in patients with lupus anticoagulants. Thromb Haemost. 1995;74:1120–5. [PubMed] [Google Scholar]
- 14.Horbach DA, van Oort E, Derksen RHWM, et al. The contribution of anti-prothrombin-antibodies to lupus anticoagulant activity. Thromb Haemost. 1998;79:790–5. [PubMed] [Google Scholar]
- 15.Martinuzzo ME, Forastiero RR, Carreras LO. Anti β2-glycoprotein I antibodies: detection and association with thrombosis. Br J Haematol. 1995;89:397–402. doi: 10.1111/j.1365-2141.1995.tb03317.x. [DOI] [PubMed] [Google Scholar]
- 16.Forastiero RR, Martinuzzo ME, Cerrato GS, et al. Relationship of anti β2-glycoprotein I and anti prothrombin antibodies to thrombosis and pregnancy loss in patients with antiphospholipid antibodies. Thromb Haemost. 1997;78:1008–15. [PubMed] [Google Scholar]
- 17.Forastiero RR, Martinuzzo ME, Kordich LC, et al. Reactivity to β2 glycoprotein I clearly differentiates anticardiolipin antibodies from antiphospholipid syndrome and syphilis. Thromb Haemost. 1996;75:717–20. [PubMed] [Google Scholar]
- 18.Cabral AR, Cabiedes J, Alarcón-Segovia D. Antibodies to phospholipid-free β2-glycoprotein-I in patients with primary antiphospholipid syndrome. J Rheumatol. 1995;22:1894–8. [PubMed] [Google Scholar]
- 19.Puurunen M, Vaarala O, Julkunen H, et al. Antibodies to phospholipid-binding plasma proteins and occurrence of thrombosis in patients with systemic lupus erythematosus. Clin Immunol Immunopathol. 1996;80:16–22. doi: 10.1006/clin.1996.0089. [DOI] [PubMed] [Google Scholar]
- 20.Guerin J, Smith O, White B, et al. Antibodies to prothrombin in antiphospholipid syndrome and inflammatory disorders. Br J Haematol. 1998;102:896–902. doi: 10.1046/j.1365-2141.1998.00876.x. [DOI] [PubMed] [Google Scholar]
- 21.Guerin J, Feighery C, Sim RB, et al. Antibodies to β2-glycoprotein I—a specific marker for the antiphospholipid syndrome. Clin Exp Immunol. 1997;109:304–9. doi: 10.1046/j.1365-2249.1997.4601357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuura E, Igarashi Y, Yasuda T, et al. Anticardiolipin antibodies recognize β2 glycoprotein I structure altered by interacting with an oxygen modified solid phase surface. J Exp Med. 1994;179:457–62. doi: 10.1084/jem.179.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roubey RAS, Eisenberg RA, Harper MF, et al. Anticardiolipin autoantibodies recognize β2-glycoprotein I in the absence of phospholipid. Importance of antigen density and bivalent binding. J Immunol. 1995;154:954–60. [PubMed] [Google Scholar]
- 24.Tincani A, Spatola L, Prati E, et al. The anti-β2 glycoprotein I activity in human anti-phospholipid syndrome sera is due to monoreactive low-affinity autoantibodies directed to epitopes located on native β2 glycoprotein I and preserved during species' evolution. J Immunol. 1996;157:5732–8. [PubMed] [Google Scholar]
- 25.Rao LVM, Hoang AD, Rapaport SI. Mechanism and effects of the binding of lupus anticoagulant IgG and prothrombin to surface phospholipid. Blood. 1996;88:4173–82. [PubMed] [Google Scholar]
- 26.Forastiero RR, Cerrato GS, Carreras LO. Evaluation of recently described tests for detection of the lupus anticoagulant. Thromb Haemost. 1994;72:728–33. [PubMed] [Google Scholar]
- 27.Brandt JT, Triplett DA, Alving B, et al. Criteria for the diagnosis of lupus anticoagulants: an update. Thromb Haemost. 1995;74:1185–90. [PubMed] [Google Scholar]
- 28.Harris EN. The Second International Anticardiolipin Standardization Workshop/The Kingston Antiphospholipid Antibody (KAPS) Study Group. Am J Clin Pathol. 1990;94:476–84. doi: 10.1093/ajcp/94.4.476. [DOI] [PubMed] [Google Scholar]
- 29.Roubey RAS. Immunology of the antiphospholipid antibody syndrome. Arthritis Rheum. 1996;39:1444–54. doi: 10.1002/art.1780390903. [DOI] [PubMed] [Google Scholar]
- 30.Hörkkö S, Miller E, Branch DW, et al. The epitopes for some antiphospholipid antibodies are adducts of oxidized phospholipid and β2 glycoprotein I (and other proteins) Proc Natl Acad Sci USA. 1997;94:10356–61. doi: 10.1073/pnas.94.19.10356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnout J, Wittevrongel C, Vanrusselt M, et al. Beta-2-glycoprotein I dependent lupus anticoagulants form stable bivalent antibody beta-2-glycoprotein I complexes on phospholipid surfaces. Thromb Haemost. 1998;79:79–86. [PubMed] [Google Scholar]
- 32.Galli M, Beretta G, Daldossi M, et al. Different anticoagulant and immunological properties of anti-prothrombin antibodies in patients with antiphospholipid antibodies. Thromb Haemost. 1997;77:486–91. [PubMed] [Google Scholar]
- 33.Onyriuka EC, Hersh IS, Herti W. Surface modification of polystyrene by gamma-radiation. Appl Spectr. 1990;44:808–11. [Google Scholar]
- 34.Sheng Y, Kandiah DA, Krilis SA. Anti-beta 2-glycoprotein I autoantibodies from patients with the ‘antiphospholipid’ syndrome bind to beta 2-glycoprotein I with low affinity: dimerization of beta 2-glycoprotein I induces a significant increase in anti-beta 2-glycoprotein I antibody affinity. J Immunol. 1998;161:2038–43. [PubMed] [Google Scholar]
- 35.Towbin H, Gordon J. Immunoblotting and dot immunobinding current status and outlook. J Immunol Methods. 1984;72:313–40. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- 36.George J, Blank M, Levy Y, et al. Differential effects of anti-β2 glycoprotein I antibodies on endothelial cells and on the manifestations of experimental antiphospholipid syndrome. Circulation. 1998;97:900–6. doi: 10.1161/01.cir.97.9.900. [DOI] [PubMed] [Google Scholar]
- 37.Arvieux J, Regnault V, Hachulla E, et al. Heterogeneity and immunochemical properties of anti-β2-glycoprotein I autoantibodies. Thromb Haemost. 1998;80:393–8. [PubMed] [Google Scholar]
- 38.Kandiah DA, Krilis SA. Heterogeneity of lupus anticoagulant (LA) antibodies: LA activity in dilute Russell's viper venom time and dilute kaolin clotting time detect different populations of antibodies in patients with the antiphospholipid syndrome. Thromb Haemost. 1998;80:250–7. [PubMed] [Google Scholar]
- 39.Hunt JE, Krilis SA. The fifth domain of β2 glycoprotein I contains a phospholipid-binding site (Cys281-Cys288) and a region recognised by anticardiolipin antibodies. J Immunol. 1994;152:653–9. [PubMed] [Google Scholar]
- 40.Sheng Y, Kandiah DA, Krilis SA. β2 glycoprotein I: target antigen for ‘antiphospholipid’ antibodies. Immunological and molecular aspects. Lupus. 1998;7:S5–9. doi: 10.1177/096120339800700202. [DOI] [PubMed] [Google Scholar]
- 41.Ichikawa K, Khamashta M, Koike T, et al. β2 glycoprotein I reactivity of monoclonal anticardiolipin antibodies from patients with the antiphospholipid syndrome. Arthritis Rheum. 1994;37:1453–61. doi: 10.1002/art.1780371008. [DOI] [PubMed] [Google Scholar]
- 42.Igarashi M, Matsuura E, Igarashi Y, et al. Human β2 glycoprotein I as an anticardiolipin cofactor determined using deleted mutants expressed by a baculovirus system. Blood. 1996;87:3262–70. [PubMed] [Google Scholar]
- 43.George J, Gilburd B, Hojnik M, et al. Target recognition of β2-glycoprotein I (β2GPI)-dependent anticardiolipin antibodies: evidence for involvement of the fourth domain of β2GPI in antibody binding. J Immunol. 1998;160:3917–23. [PubMed] [Google Scholar]
- 44.Iverson GM, Victoria EJ, Marquis DM. Anti-β2 glycoprotein I (β2GPI) autoantibodies recognize an epitope on the first domain of (β2GPI) Proc Natl Acad Sci USA. 1998;95:15542–6. doi: 10.1073/pnas.95.26.15542. [DOI] [PMC free article] [PubMed] [Google Scholar]