Abstract
MN is characterized by the glomerular deposition of IgG4 immune complexes. This suggests that nephritogenic immune responses in MN are of the Th2 T helper cell type; however, the pathogenesis of MN is still unknown. In this study we examined sera from patients with primary MN for antibodies to renal proteins. A 47-kD protein in both human and porcine renal extracts was found by immunoblotting to react specifically with serum IgG from some patients. This protein was purified from porcine kidney and identified as α-enolase on the basis of its partial amino acid sequences. Sera from 87 patients with primary MN, 24 patients with secondary MN (15 rheumatoid arthritis patients, nine systemic lupus erythematosus patients), and 16 healthy subjects were examined by ELISA using purified α-enolase. In 60 (69%) patients with primary MN and 14 (58%) patients with secondary MN, the measured optical density values, and hence serum anti-α-enolase antibody levels, were greater than the mean + 2 s.d. of healthy subjects. Immunoblot analysis showed that IgG1 or IgG3 was the predominant subclass (Th1 T helper cell type subclass) of antibodies against α-enolase in patients with primary and secondary MN. Since circulating antibodies against α-enolase have recently been reported in patients with various autoimmune disorders, our results suggest that a number of patients with presumed primary MN may also have abnormalities in Th1 T helper cell-mediated immune responses.
Keywords: α-enolase, antibodies, IgG subclass, membranous nephropathy
INTRODUCTION
MN is a distinctive clinicopathological entity commonly associated with nephrotic syndrome in adults [1]. Since MN is characterized by the glomerular deposition of immunoglobulin, predominantly of the IgG4 subclass [2], this disease is regarded as an IgG4-mediated disease [3]. It has become apparent that Th1 and Th2 T helper cell subsets affect patterns of injury in glomerular disorders [4]. In humans, Th2-predominant responses promote a higher ratio of IgG4 to IgG1 and IgG3, whereas Th1-predominant response patterns have higher IgG1 and IgG3 to IgG4. Therefore, MN is considered to be directed by Th2-predominant nephritogenic immune responses [4].
Various systemic diseases, such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), or exposure to a variety drugs can evoke secondary forms of MN. In these cases specific glomerular deposits of endogenous or exogenous antigens have been found [1]. Several pathogenic antigens have also been identified in experimental models for MN, but the nephritogenic antigens involved in the development of human primary MN are still elusive [5].
In this study we examined the specificity of circulating antibodies reactive with renal proteins in patients with MN. We report here the identification of IgG1- or IgG3-predominant antibodies against α-enolase in a number of patients with primary and secondary MN. α-Enolase, a highly conserved glycolytic enzyme [6], has recently been described as a target of autoantibodies in patients with various inflammatory diseases [7–14]. We discuss the significance of Th1-type subclass-predominant antibodies against α-enolase in patients with primary MN.
PATIENTS AND METHODS
Patients
We selected patients with MN who were admitted to Akita University Hospital and its affiliated hospitals between 1986 and 1998. Histopathological diagnosis of MN was made according to findings on renal biopsy specimens. The diagnosis of primary MN was defined by the exclusion of known underlying disease or drug exposure [1]. Sera from 87 patients with primary MN, 24 patients with secondary MN (15 RA, nine SLE), and 16 healthy subjects were stored at −80°C until use.
The background of the patients is summarized in Table 1. Sera from all the patients with primary MN were obtained before specific treatment with steroids or immunosuppressive agents. Before serum sampling, all the patients with RA were treated with gold, bucillamine, or actarit, and six patients with SLE were treated with steroids.
Table 1.
Background of patients with MN
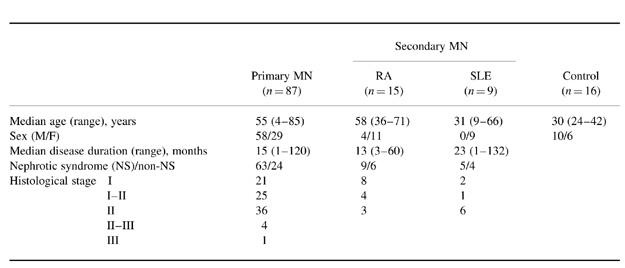
RA, Rheumatoid arthritis; SLE, systemic lupus erythematosus.
Renal tissues of autopsy specimens from a patient with primary MN, who died of myocardial infarction, were used for immunoblots.
Gel electrophoresis and immunoblot
SDS–PAGE was performed by the method of Laemmli [15]. Immunoblot was performed by the method of Towbin et al. [16]. For the first antibody, sera from the subjects were used at a dilution of 1:200. For the second antibody, alkaline phosphatase-conjugated anti-human IgG (BioRad, Richmond, CA) was used at a dilution of 1:500. To determine the subclasses of IgG, alkaline phosphatase-conjugated anti-human IgG1, IgG2, IgG3 and IgG4 (The Binding Site, Birmingham, UK) were also used at a dilution of 1:500.
Preparation of human and porcine renal extracts
Small pieces of human and porcine renal tissues were homogenized with three volumes of 10 mm Tris–HCl pH 7.4, containing 150 mm NaCl. After centrifugation at 20 000 g for 15 min, the supernatants were stored at −80°C until use for immunoblots.
Purification of a 47-kD protein from porcine kidney
All purification steps were performed at 4°C. At each step, immunoblot analysis probed with serum IgG from a patient with primary MN was performed, in order to select the 47-kD immunoreactive protein-rich fractions.
Minced porcine kidney (60 g) was homogenized with 180 ml of buffer A (10 mm Tris–HCl pH 7.4). After centrifugation at 20 000 g for 15 min, the supernatant was fractionated by 50–80% saturated ammonium sulphate. The precipitate was recovered with centrifugation at 20 000 g for 15 min and dissolved in buffer A. After dialysis against buffer A the sample was centrifuged at 20 000 g for 15 min. The supernatant (50 ml) was applied to a DEAE-cellulose (DE52; Whatman, Maidstone, UK) column (37 × 38 cm) pre-equilibrated with buffer A. The column was washed with buffer A, and the 47-kD protein was passed through the column with buffer A. Pooled unbound fractions were dialysed against buffer B (10 mm sodium acetate buffer pH 6.0). After centrifugation at 20 000 g for 15 min, the supernatant (100 ml) was applied to a CM-cellulose (CM52; Whatman) column (37 × 38 cm) pre-equilibrated with buffer B. After the column was washed with buffer B, the column was developed with a linear gradient of 0–500 mm NaCl. The 47-kD protein was eluted in fractions at approx. 320–390 mm NaCl. The eluant was dialysed against buffer C (10 mm potassium phosphate buffer pH 7.0). After centrifugation at 20 000 g for 15 min, the supernatant (35 ml) was applied to a hydroxylapatite (BioRad) column (1.5 × 5.2 cm) pre-equilibrated with buffer C. After washing the column with buffer C, the column was developed with a linear gradient of 10–500 mm potassium phosphate buffer pH 7.0. The 47-kD protein was eluted in fractions at approx. 140–160 mm.
Amino acid sequence
Production and separation of peptides from the purified 47-kD protein were carried out according to the method of Kawasaki et al. [17]. Separated polypeptides were then sequenced.
ELISA
Antibody activities against the 47-kD protein were measured by ELISA. The purified 47-kD protein was coated on wells of microtitration plates (ICN Biomedicals Inc., Aurora, OH). One hundred microlitres of 100-fold diluted sera from the subjects were incubated in the wells for 4 h at room temperature. After washing, the wells were incubated with alkaline phosphatase-conjugated anti-human IgG from an ELISA kit (Kirkegaard & Perry Labs, Gaithersburg, MD) at a 1:500 dilution for 2 h at room temperature. After washing the wells, colour development was performed using the kit. The optical density (OD) was measured in an ELISA reader at 405 nm.
Clinical significance of the antibodies
OD values on ELISA assays exceeding the mean + 2 s.d. of healthy subjects were considered to be positive. We examined clinical data (age, sex, disease duration, development of nephrotic syndrome, and histological stages) in antibody-positive and -negative patients with primary MN.
RESULTS
Detection of circulating antibodies against α-enolase in patients with primary MN
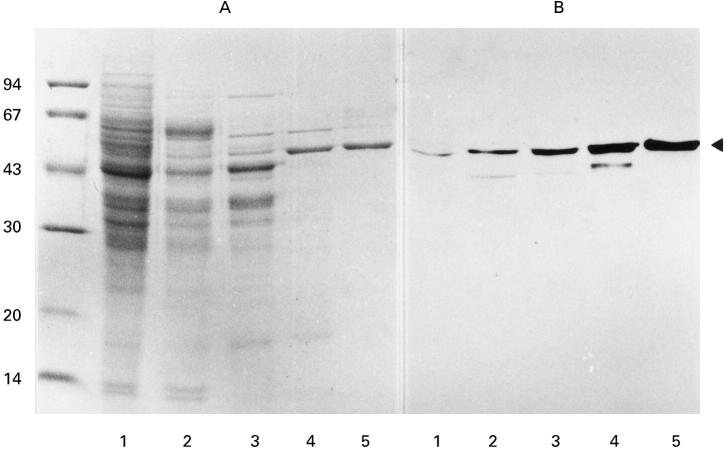
Sera from patients with primary MN were examined for antibodies to renal proteins by immunoblotting. Serum IgG from some patients reacted exclusively with a 47-kD protein in a human renal extract (Fig. 1, C, lane 1). Since the 47-kD immunoreactive protein was also found in a porcine renal extract (Fig. 1, C, lane 2), we purified this protein from porcine kidney, using ammonium sulphate fractionation, ion-exchange column chromatographies, and hydroxylapatite column chromatography (Fig. 2, A). To select fractions containing the 47-kD protein at each step, immunoblots probed with serum IgG from a patient were performed (Fig. 2, B).
Fig. 1.
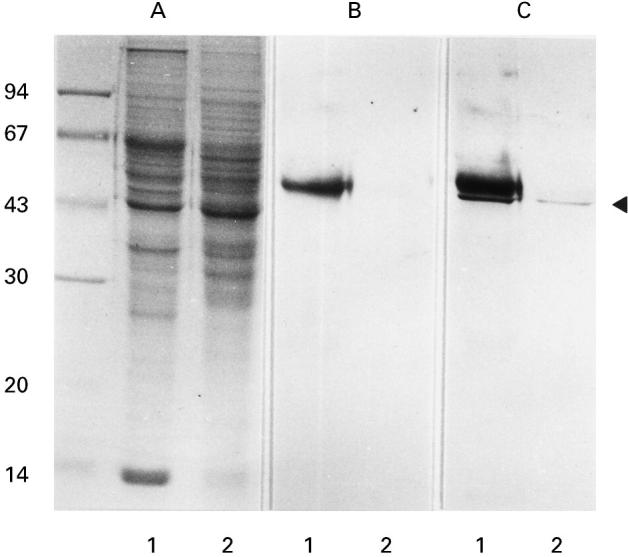
Detection of serum IgG reactive with a 47-kD renal protein in patients with primary MN. A, SDS–PAGE gel stained with coomassie brilliant blue R-250. Numbers on the left indicate molecular mass standards (Pharmacia, Uppsala, Sweden) in kD. Lanes 1 and 2 are isotonic buffer-extractable fractions from human and porcine kidneys, respectively. B, Immunoblot probed only with alkaline phosphatase-conjugated anti-human IgG. A 55-kD immunoreactive protein band in lane 1 is regarded as human IgG heavy chain. C, Immunoblot probed with serum from a patient with primary MN and with alkaline phosphatase-conjugated anti-human IgG. The arrowhead indicates the 47-kD protein bands in the human and porcine renal extracts, which specifically react with the patient's serum IgG.
Fig. 2.
Purification of the 47-kD protein reactive with serum IgG from patients with primary MN from porcine kidney. A, SDS–PAGE gel stained with coomassie brilliant blue R-250. Numbers on the left indicate molecular mass standards as shown in Fig. 1. B, Immunoblot probed with serum from a patient with primary MN and with alkaline phosphatase-conjugated anti-human IgG. Lane 1, Hypotonic buffer-extractable porcine renal fraction; lane 2, pooled fraction after ammonium sulphate fractionation; lane 3, pooled fraction after DEAE-cellulose column chromatography; lane 4, pooled fraction after CM-cellulose column chromatography; lane 5, pooled fraction after hydroxylapatite column chromatography. The arrowhead indicates the 47-kD protein bands reactive with the patient's serum IgG.
Amino acid sequences of two lysylendopeptidase-digested fragments of the purified 47-kD protein were determined. Twenty-one obtained amino acid sequences were completely identical to those deduced from nucleotide sequence of a cDNA encoding human α-enolase (mol. wt = 47 108) [18]: IHAREIFDSRGNPTV (residues 6–20), and YDLDFK (residues 256–261). Thus, the 47-kD protein, which reacted with serum IgG from patients with primary MN, was identified as α-enolase.
Frequency of positive circulating antibodies against α-enolase in patients with MN
To examine the frequency of positive circulating antibodies against α-enolase in patients with MN, a standard ELISA was performed. We tested 87 sera from patients with primary MN, 24 sera from patients with secondary MN (15 RA, nine SLE), and 16 healthy subjects. Results of ELISA assays are summarized in Fig. 3. The measured OD values greater than the mean + 2 s.d. of healthy subjects were considered to be positive. The positive rates were 69% (60/87) in patients with primary MN, 67% (10/15) in RA patients with secondary MN, and 44% (4/9) in SLE patients with secondary MN.
Fig. 3.
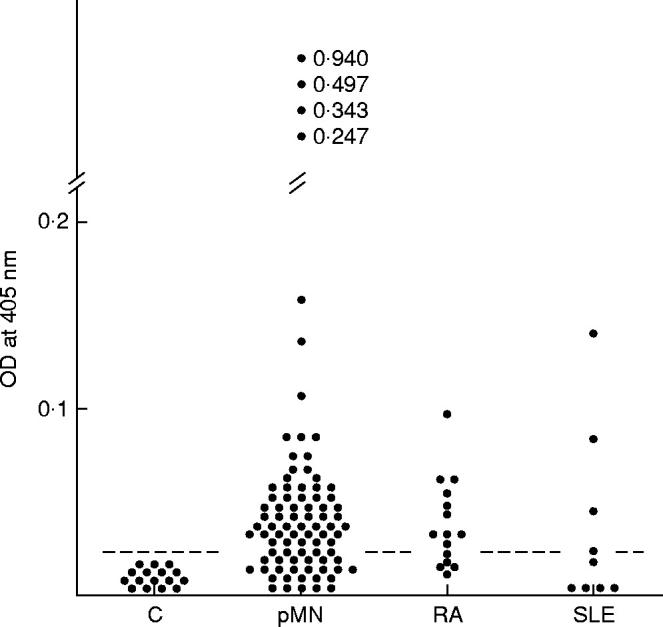
ELISA assays of antibodies against α-enolase in patients with MN. Sera from 87 patients with primary MN (pMN), 15 rheumatoid arthritis (RA) patients with secondary MN, nine systemic lupus erythematosus (SLE) patients with secondary MN, and 16 control subjects (C) were examined. Reactivities are expressed in optical density (OD) values at 405 nm. The dotted lines represent the mean + 2 s.d. of the control subjects.
Determination of IgG subclasses of circulating antibodies against α-enolase in patients with MN
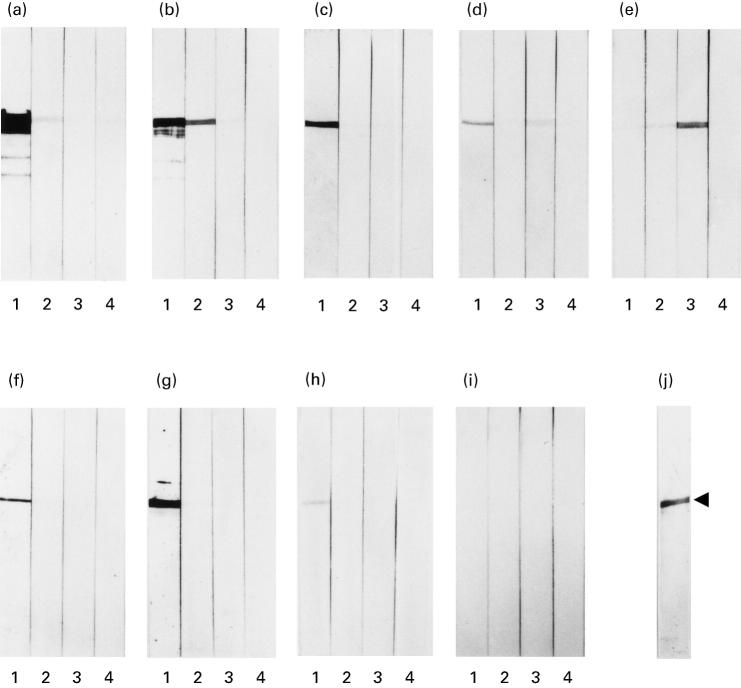
The subclasses of serum IgG from five patients with primary MN, who had high titres of antibodies against α-enolase on ELISA assays, were examined by immunoblots. In four out of five sera from the patients tested, IgG1 was the predominant IgG subclass (Fig. 4a–d). In the remaining patient, IgG3 was predominant (Fig. 4e). No significant IgG4 antibodies against α-enolase were detected in sera from any of these patients (Fig. 4a–e). We also tested three sera from patients with secondary MN, and found IgG1 to be predominant (Fig. 4f–h).
Fig. 4.
Determination of IgG subclasses of antibodies against α-enolase in sera from patients with primary and secondary MN. One microgram of purified α-enolase was run on SDS–PAGE gels, and transferred to each strip. The strips were incubated with sera from patients with primary MN (a–e), patients with secondary MN (f–h), and a healthy individual (i). Thereafter, they were incubated with alkaline phosphatase-conjugated anti-human IgG1 (lane 1), IgG2 (lane 2), IgG3 (lane 3), and IgG4 (lane 4). One of the strips (j) was stained with coomassie brilliant R-250. The arrowhead indicates the purified 47-kD protein band of α-enolase. Immunoreactive protein bands with lower molecular mass on panels (a) and (b) are regarded as fragments of α-enolase.
Clinical significance of antibodies against α-enolase in patients with primary MN
We examined clinical features in anti-α-enolase antibody-positive and -negative patients with primary MN. There was no significant correlation between the presence of antibodies and clinical features (Table 2).
Table 2.
Clinical features in anti-α-enolase antibody-positive and -negative patients with primary MN
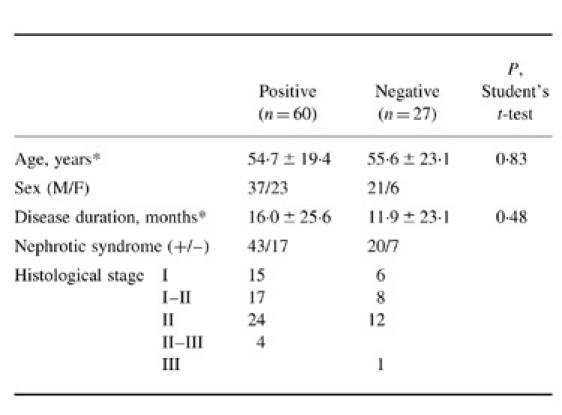
*Data expressed as mean 6s.d.
DISCUSSION
By screening immunoblots of human and porcine renal extracts, we showed that a 47-kD protein specifically reacted with serum IgG from some patients with primary MN. The 47-kD protein was purified from porcine kidney, and identified as α-enolase. By ELISA assays, a number of patients with primary and secondary MN were considered to have circulating antibodies against α-enolase. Immunoblot analysis demonstrated that the predominant subclass of the antibodies was IgG1 or IgG3, but not IgG4. There were no characteristic clinical features in patients who had high titres of the antibodies.
α-Enolase, a highly conserved glycolytic enzyme among species [6], seems to have functions other than an enzymatic one. For example, α-enolase is a member of the family of heat shock proteins and plays a role in thermal tolerance and growth control in Saccharomyces cerevisiae [19]. Candida albicans α-enolase is an abundant immunodominant antigen in cases of disseminated candidiasis [20]. A strong plasmin(ogen) binding protein on the surface of pathogenic streptococci has been identified as α-enolase, which is possibly involved in tissue invasion [21]. Moreover, it has been shown that a rabbit muscle isoenzyme of enolase can accelerate immunoglobulin production in a human hybridoma cell line [22].
α-Enolase has been shown to be a target antigen of anti-neutrophil cytoplasmic antibodies (ANCA) in patients with vasculitis [7], ulcerative colitis and Crohn's disease [13], and primary sclerosing cholangitis [14]. Several recent studies have demonstrated that antibodies against α-enolase are not restricted to ANCA-associated diseases, and that they are found in a wider range of other disorders such as endometriosis [8], cancer-associated retinopathy [9], cryoglobulinaemia with renal involvement [10], discoid lupus erythematosus [11], and primary biliary cirrhosis and autoimmune hepatitis [12]. The subclasses of antibodies against α-enolase have been evaluated in two studies: the predominant antibodies were of the IgG1 subclass in patients with cancer-associated retinopathy [9] and discoid lupus erythematosus [11]. Although the reason for antibody production against α-enolase in patients with such a variety of disorders, including autoimmune diseases, remains to be elucidated, it is possible that the production of antibodies occurs during abnormal immunoregulation in these patients.
The histological pattern of glomerular diseases varies with the deposition pattern of IgG subclasses. We previously showed that MN is characterized by the glomerular deposition of IgG4 [2]. Since the biochemical properties of IgG4 are compatible with a pathogenic mechanism that involves the glomerular deposition of immune complexes, MN is regarded as an IgG4-mediated disease [3]. The current concept that T helper cells differentiate into functionally distinct subsets (Th1 and Th2) has helped to explain the different immunological patterns in diseases. Holdsworth et al. [4] have recently proposed that glomerular diseases can be classified into three groups: Th1-predominant (IgG1- and/or IgG3-predominant), Th2-predominant (IgG4-predominant), and heterogeneous Th1/Th2-predominant groups. According to their proposal, primary MN is considered to be directed by Th2-predominant nephritogenic immune responses, and membranous lupus nephritis (secondary MN) is classified into the heterogeneous group.
Our immunoblot analysis showed that the predominant subclass of circulating antibodies against α-enolase was IgG1 or IgG3, but not IgG4, in patients with primary and secondary MN. There are several possible explanations of this result. First, the inability to detect IgG4 subclass of antibodies against α-enolase may be due to the high levels of the IgG1 subclass. Second, any IgG4 antibodies against α-enolase would be deposited in the kidney, and therefore would not be present in the circulation. Finally, the production of antibodies in our patients may be due to abnormal responses in Th1 T helper cell-mediated immunity, and these antibodies are unlikely to be involved in the pathogenesis of MN. Such assumptions need further studies, however.
In conclusion, our results demonstrate that a number of patients with primary MN have circulating IgG1- or IgG3-predominant antibodies against α-enolase, antibodies which have recently been found in patients having various immunological abnormalities. We therefore suggest that a significant number of patients with presumed primary MN have an abnormal background of Th1 T helper cell-mediated immune responses, in addition to the Th2 T helper cell-mediated nephritogenic immune responses.
Acknowledgments
This work was supported by a grant (to H.I.) from Progressive Renal Disease from the Ministry of Health and Welfare Research Project for Specially Selected Diseases.
REFERENCES
- 1.Glassock RJ, Cohen AH, Adler SG. Brenner BM. The kidney. 5. Philadelphia: W.B. Saunders; 1996. Primary glomerular diseases; pp. 1392–497. [Google Scholar]
- 2.Imai H, Hamai K, Komatsuda A, Ohtani H, Miura AB. IgG subclasses in patients with membranoproliferative glomerulonephritis, membranous nephropathy, and lupus nephritis. Kidney Int. 1997;51:270–6. doi: 10.1038/ki.1997.32. [DOI] [PubMed] [Google Scholar]
- 3.Oliveira DBG. Membranous nephropathy: an IgG4-mediated disease. Lancet. 1998;351:670–1. doi: 10.1016/S0140-6736(97)04122-6. [DOI] [PubMed] [Google Scholar]
- 4.Holdsworth SR, Kitching AR, Tipping PG. Th1 and Th2 T helper cell subsets affect patterns of injury and outcomes in glomerulonephritis. Kidney Int. 1999;55:1198–216. doi: 10.1046/j.1523-1755.1999.00369.x. [DOI] [PubMed] [Google Scholar]
- 5.Allegri L. Antigens in experimental models of membranous nephropathy: are they involved in human disease? Nephrol Dial Transplant. 1997;12:1801–4. doi: 10.1093/ndt/12.9.1801. [DOI] [PubMed] [Google Scholar]
- 6.Verma M, Dutta SK. DNA sequences encoding enolase are remarkably conserved from yeast to mammals. Life Sci. 1994;55:893–9. doi: 10.1016/0024-3205(94)00534-6. [DOI] [PubMed] [Google Scholar]
- 7.Moodie FDL, Leaker B, Cambridge G, Totty NF, Segal AW. Alpha-enolase: a novel cytosolic autoantigen in ANCA positive vasculitis. Kidney Int. 1993;43:675–81. doi: 10.1038/ki.1993.97. [DOI] [PubMed] [Google Scholar]
- 8.Walter M, Berg H, Leidenberger FA, Schweppe KW, Northemann W. Autoreactive epitopes within the human alpha-enolase and their recognition by sera from patients with endometriosis. J Autoimmunol. 1995;8:931–45. doi: 10.1016/s0896-8411(95)80027-1. [DOI] [PubMed] [Google Scholar]
- 9.Adamus G, Aptsiauri N, Guy J, Heckenlively J, Flannery J, Hargrave PA. The occurrence of serum autoantibodies against enolase in cancer-associated retinopathy. Clin Immunol Immunopathol. 1996;78:120–9. doi: 10.1006/clin.1996.0021. [DOI] [PubMed] [Google Scholar]
- 10.Sabbatini A, Dolcher MP, Marchini B, Chimenti D, Moscato S, Pratesi F, Bombardieri S, Migliorini P. Alpha-enolase is a renal-specific antigen associated with kidney involvement in mixed cryoglobulinemia. Clin Exp Rheumatol. 1997;15:655–8. [PubMed] [Google Scholar]
- 11.Gitlits VM, Sentry JW, Matthew MLSM, Smith AI, Toh B-H. Autoantibodies to evolutionarily conserved epitopes of enolase in a patient with discoid lupus erythematosus. Immunology. 1997;92:362–8. doi: 10.1046/j.1365-2567.1997.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akisawa N, Maeda T, Iwasaki S, Onishi S. Identification of an autoantibody against alpha-enolase in primary biliary cirrhosis. J Hepatol. 1997;26:845–51. doi: 10.1016/s0168-8278(97)80251-6. [DOI] [PubMed] [Google Scholar]
- 13.Roozendaal C, Zhao MH, Horst G, Lockwood CM, Kleibeuker JH, Limburg PC, Nelis GF, Kallenberg CGM. Catalase and α-enolase: two novel granulocyte autoantigens in inflammatory bowel disease (IBD) Clin Exp Immunol. 1998;112:10–16. doi: 10.1046/j.1365-2249.1998.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orth T, Kellner R, Diekmann O, Faust J, Meyer Zum Büschenfelde K-H, Mayet W-J. Identification and characterization of autoantibodies against catalase and α-enolase in patients with primary sclerosing cholangitis. Clin Exp Immunol. 1998;112:507–15. doi: 10.1046/j.1365-2249.1998.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedures and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawasaki H, Emori Y, Suzuki K. Production and separation of peptides from proteins stained with Coomassie brilliant blue R-250 after separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal Biochem. 1990;191:332–6. doi: 10.1016/0003-2697(90)90227-z. [DOI] [PubMed] [Google Scholar]
- 18.Giallongo A, Feo S, Moore R, Croce CM, Showe LC. Molecular cloning and nucleotide sequence of a full-length cDNA for human α enolase. Proc Natl Acad Sci USA. 1986;83:6741–5. doi: 10.1073/pnas.83.18.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iida H, Yahara I. Yeast heat-shock protein of Mr 48,000 is an isoprotein of enolase. Nature. 1985;315:688–90. [Google Scholar]
- 20.Sundstrom P, Aliaga GR. Molecular cloning of cDNA and analysis of protein secondary structure of Candida albicans enolase, an abundant, immunodominant glycolytic enzyme. J Bacteriol. 1992;174:6789–99. doi: 10.1128/jb.174.21.6789-6799.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pancholi V, Fischetti VA. α-enolase, a novel strong plasmin(ogen) binding protein on the surface of pathogenic streptococci. J Biol Chem. 1998;273:14503–15. doi: 10.1074/jbc.273.23.14503. [DOI] [PubMed] [Google Scholar]
- 22.Sugahara T, Shimizu S, Abiru M, Matsuoka S, Sasaki T. A novel function of enolase from rabbit muscle; an immunoglobulin production stimulating factor. Biochim Biophys Acta. 1998;1380:163–76. doi: 10.1016/s0304-4165(97)00137-2. [DOI] [PubMed] [Google Scholar]