Abstract
Anti-neutrophil cytoplasmic antibodies directed against PR3 (PR3-ANCA) in patients with Wegener's granulomatosis are supposedly involved in the pathophysiology of this disease as different functional characteristics of the autoantibodies correlate with disease activity. However, little is known about the epitopes of PR3 that are recognized by PR3-ANCA and how epitope specificity may relate to functional characteristics of PR3-ANCA. As candidate tools for epitope mapping we studied 13 anti-PR3 MoAbs, including nine widely used and four newly raised MoAbs, for their mutual binding characteristics to PR3 using biosensor technology. Antigen specificity was confirmed by indirect immunofluorescence, immunoblotting, FACS analysis and antigen-specific ELISA. Competition between anti-PR3 MoAbs in binding to PR3 was investigated in a capture system set up in a BIAcore. In this system grouping of 12 of the 13 anti-PR3 MoAbs based on their mutual recognition patterns was achieved. Four MoAbs, from different research groups, namely 12.8, PR3G-2, 6A6 and Hz1F12, recognized comparable epitopes (group 1). Group 2 MoAbs including PR3G-4 and PR3G-6 bound to overlapping regions on PR3. The MoAbs PR3G-3, 4A5 and WGM2 recognized similar epitopes as they inhibited binding of each other (group 3). The fourth group of related MoAbs consisted of MC-PR3-2, 4A3 and WGM3. Because of its binding characteristics MoAb WGM1 could not be grouped. These results demonstrate that eight well-established anti-PR3 MoAbs produced by different research groups and four newly produced anti-PR3 MoAbs recognize four separate epitope areas on PR3, including one area detected with newly raised MoAbs only.
Keywords: ANCA, proteinase 3, epitope mapping, biosensor
INTRODUCTION
Autoantibodies directed against cytoplasmic proteins of neutrophilic granulocytes and monocytes (so called anti-neutrophil cytoplasmic autoantibodies (ANCA)) are a highly sensitive and specific marker for Wegener's granulomatosis (WG) [1,2].
The major antigen recognized by ANCA in WG has been identified as PR3 [3–5], an elastinolytic enzyme originally described by Baggliolini in 1978 [6]. PR3 is a neutral serine proteinase, which is localized in the azurophilic granules of neutrophils and in granules of monocytes [7]. In vitro, PR3 degrades a number of extracellular matrix proteins such as elastin [8,9] and inactivates human C1 inhibitor [10]. Furthermore, upon intratracheal administration PR3 induces emphysema in hamsters [9].
The involvement of ANCA in inflammatory tissue injury in ANCA-associated vasculitis is further supported by the observation that relapses of WG are frequently preceded by a significant rise in ANCA titre [2,11], although the strength of this association has been questioned [12]. Although the pathogenic role of ANCA has not been fully elucidated yet, in vitro data suggest functional activities of the autoantibodies in vivo. Three functional characteristics of ANCA have been implicated in the pathogenesis of WG. First, ANCA are able to activate primed neutrophils to produce oxygen radicals and release lytic enzymes, including PR3 [13–15]. Second, PR3-ANCA can interfere with the binding of PR3 to its physiological inhibitor α1-antitrypsin (α1-AT) [16–18]. Third, PR3-ANCA can interfere with the proteolytic activity of PR3 [16,17]. These interfering antibodies may thus act as alternative inhibitors. However, at the site of inflammation PR3 can cleave the complex between these inhibiting ANCA and PR3 itself, leaving active PR3 [19]. The latter two functional characteristics of ANCA, that is interference of ANCA with the binding of PR3 to α1-AT and interference with the proteolytic activity of PR3, have been shown to correlate with disease activity of WG [17,18]. Changes in these functional characteristics of ANCA have been suggested to follow changes in disease activity more accurately than the previously mentioned changes in ANCA titres alone [18,20,21]. This may indicate that changes in epitope specificity of these ANCA resulting in changes in functionality occur during the course of the disease [22].
Little is known about the epitopes on PR3 recognized by ANCA. Epitope analysis of PR3-ANCA has been hampered by the fact that the majority of PR3-ANCA recognizes conformational epitopes on PR3 [23]. Some groups have tried to elucidate these epitopes recognized by PR3-ANCA by means of overlapping linear peptides of the whole sequence of PR3. Williams et al. [24] identified various antigenic sites on PR3 that were surface-accessible. However, background binding of sera was high and control antibodies also bound some of the peptides. In a comparable test system Chang et al. could not reproduce these results [25]. Griffith et al. found that PR3-ANCA from different patients with active vasculitis bound to linear peptides of PR3 in a highly restricted manner. The bound peptides were surface-accessible and one even coincided with the catalytic site [26]. However, these peptides did not correspond to those identified by Williams.
To further define epitopes recognized by PR3-ANCA well-characterized MoAbs to PR3 can serve as tools, as has been shown for antibodies to myeloperoxidase (MPO), another major ANCA antigen in systemic vasculitis [27]. These well-characterized MoAbs can be used in inhibition studies with a large panel of PR3-ANCA sera in order to assess possible epitope shifts in relation to disease activity. In addition, many established anti-PR3 MoAbs, from different research groups, are used for the detection of PR3-ANCA in capture ELISA systems in clinical practice [28–31]. Establishing the epitopes on PR3 that are recognized by those MoAbs is therefore also important in order to exclude possible inaccurate results due to interference of the MoAbs with the binding of patient sera to PR3.
The purpose of this study was to determine the epitope areas recognized by a large number of established and newly developed MoAbs to PR3. Specificity of the MoAbs for PR3 was analysed. Epitope restriction of eight established and four newly developed MoAbs to PR3 was determined by biosensor technology. The data presented suggest that those 12 anti-PR3 MoAbs recognize a restricted number of four epitope areas on PR3, including one area detected with newly raised MoAbs only.
MATERIALS AND METHODS
MoAbs
All commonly used MoAbs to PR3 from different research groups were used. In this study MoAb 12.8 has been produced by Goldschmeding et al. [4] and is now available at the Central Laboratory of Blood Transfusion Services (CLB, Amsterdam, The Netherlands). MoAbs WGM1–3 were provided by Dr E. Csernok and Professor W. Gross (Rheumaklinik, Bad Bremsted, Germany), MoAbs 4A3, 4A5 and 6A6 were provided by Dr J. Wieslander (Wieslab, Lund, Sweden). MoAbs Hz1F12 and MC-PR3-2 were obtained from Dr C. M. Lockwood (Cambridge, UK) and Dr U. Specks (Rochester, NY), respectively. Table 1 describes the antigens used for immunization, the isolation procedure of those antigens, the material containing the MoAb used in this study, and the immunoglobulin (sub)class of the MoAbs.
Table 1.
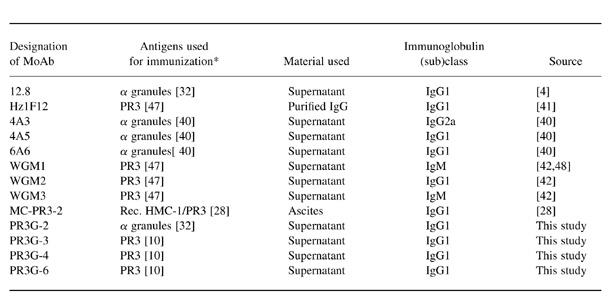
*Reference of the PR3 purification method used for production of MoAb in parentheses.
Rec. HMC-1/PR3, Recombinant PR3 expressed in human mast cells.
MoAbs directed against MPO (4.15; CLB), lactoferrin (produced in our laboratory), and human leucocyte elastase (NP57; Dako, Glostrup, Denmark) were used in Western blot analysis. Furthermore, a rabbit polyclonal antibody against PR3 was used in a capture ELISA.
Generation of MoAbs
In order to have a significant number of MoAbs to PR3 available, new MoAbs to PR3 were made. A crude extract of granules derived from neutrophil granulocytes was obtained according to the isolation procedure of Borregaard et al. [32]. PR3 was purified according to the isolation procedure of Leid et al. [10]. MoAbs to PR3 were produced according to standard procedures. Four hybridomas designated PR3G-2, PR3G-3, PR3G-4 and PR3G-6 produced antibodies to PR3. These MoAbs were selected for further characterization. PR3G-2 was produced by immunization of mice with a crude granule extract, and PR3G-3, PR3G-4 and PR3G-6 by immunization with 100 μg purified PR3. The antigen specificity of these MoAbs was established by antigen-specific direct ELISA, indirect immunofluorescence (IIF) on ethanol-fixed granulocytes, and by Western blotting on a crude granule extract.
Subclass determination
To determine the subclass of each MoAb to PR3 a direct PR3 ELISA was performed as described below. Bound anti-PR3 MoAbs were incubated with subclass-specific antibodies, goat anti-mouse IgG1, IgG2a, IgG2b or total immunoglobulin (Southern Biotech Associates Inc., Birmingham, AL), 1:100 diluted, followed by rabbit anti-goat FITC-labelled conjugate. The optical density (OD) values were detected in a FL500 Fluorescence reader (Titertek Multiscan, Helsinki, Finland) at extinction 585 nm and emission 538 nm.
Indirect immunofluorescence
MoAbs were tested for their binding pattern on neutrophils by IIF. Briefly, isolated neutrophils were fixed on glass slides by means of ethanol and incubated with MoAbs to PR3. Bound MoAbs were detected by FITC-conjugated rabbit anti-mouse immunoglobulin (Dako) according to a standard protocol [1,33] with minor modifications [34].
Western blots
To verify further the antigenic specificity of four newly raised anti-PR3 MoAbs, binding of these MoAbs was tested on Western blots containing a crude granule extract obtained from neutrophil granulocytes according to a standard extraction procedure [32]. The granule extract was applied to a 12.5% SDS–PAGE gel under denaturing and non-reducing conditions. SDS–PAGE was performed according to the method of Laemmli [35]. Next, Western blotting was performed according to standard procedures, followed by immunodetection. Blots were blocked in 4% non-fat milk powder in PBS containing 0.1% Tween-20 (PBS–T). MoAbs to PR3, human leucocyte elastase and lactoferrin, and, as a negative control, an irrelevant IgG1 MoAb, were added. Established MoAbs to PR3 served as positive controls. Anti-PR3 MoAbs were tested at a concentration of 10 ng/ml except for WGM1–3, PR3G-4 and -6, which were tested at 200 ng/ml. After washing in PBS–T, rabbit anti-mouse immunoglobulin peroxidase labelled (Dako; 1:2500) was added. Bound conjugate was detected by enhanced chemiluminescense (ECL). In brief, the blot was incubated in 10 ml 1.25 mm sodium-luminol (Sigma, Zwÿndrecht, The Netherlands) in 0.1 m Tris–HCl pH 8.0, as a substrate with 100 μl 6.7 mm para-hydroxy-coumaric acid (Sigma) in dimethylsulphoxide (DMSO) as enhancer, for 1 min. Finally, the blots were exposed to Kodak Film (X-OMAT AR) for 30 s to 3 min and films were developed in a Kodak RP X-OMAT processor.
FACS analysis
To characterize further the anti-PR3 MoAbs, MoAbs were tested for their recognition of PR3 expressed on the surface of granulocytes. Freshly isolated granulocytes of a healthy volunteer were primed by 2 ng/ml tumour necrosis factor-alpha (TNF-α) for 15 min at 37°C, spun down, washed, and resuspended in Hanks' balanced salt solution (HBSS; Gibco Life Technologies, Paisley, UK), without Ca2+ and Mg2+, containing 1% bovine serum albumin (BSA) and 5% normal goat serum (NGS). Next, MoAbs to PR3, MPO (4.15) and human leucocyte elastase (NP57), or an irrelevant IgG1 MoAb, as a negative control, were added for 1 h at 4°C. Granulocytes were then washed and incubated with FITC-labelled goat antibodies to mouse IgM, IgG2a and IgG1 (Dako) for WGM1 and -3, 4A3 and the other MoAbs, respectively, in HBSS with 1% BSA and 5% NGS for 1 h at 4°C. Finally, granulocytes were washed, fixed in 1% paraformaldehyde, washed, and resuspended in HBSS containing BSA and NGS. Cells were analysed by flow cytometry on a Coulter Epics ELITE (Coulter, Hialeah, FL). Gates were set on granulocytes by forward and sideways scatter and the fluorescence intensity was measured.
ELISA
The antigenic specificity of the MoAbs to PR3 was also verified by antigen-specific direct [29] and capture ELISA, as previously described [4]. For the direct ELISA microtitre plates (Nunc Maxisorp, Roskilde, Denmark) were coated in 0.1 m sodium carbonate pH 9.6, with 1 μg/ml PR3 inactivated by 0.5 mm PMSF (Sigma), as enzymatically active PR3 is able to cleave antibodies attached to PR3 itself [19]. After washing, MoAbs to PR3 were added starting at antibody concentration 2 μg/ml and further two-fold diluted in 0.1 m Tris, 0.3 m NaCl, 1% BSA, 0.05% Tween-20. As a positive control rabbit serum raised to PR3 was added. Finally, affinity-purified F(ab′)2 goat anti-mouse IgG or goat anti-rabbit IgG alkaline phosphatase-conjugated (Sigma; 1:500) was added. MoAbs WGM1 and WGM3, both of IgM class, were added to PR3-coated wells in buffer containing 0.03 m NaCl. Both IgM antibodies were detected with alkaline phosphatase-conjugated rabbit anti-mouse IgM (Southern Biotech Associates; 1:100).
For the capture ELISA 96-well microtitre plates (Greiner F; Greiner BV, Alphen a/d Rÿn, The Netherlands) were coated with 3.2 μg/ml goat anti-mouse IgG (Jackson, West Grove, PA) in 0.1 m carbonate buffer pH 9.6. After washing, the plates were incubated with different dilutions of MoAbs to PR3 starting at antibody concentration 2 μg/ml in 0.1 m Tris, 0.3 m NaCl, 1% BSA, 0.05% Tween-20, 1% NGS. Purified PR3 (0.5 μg/ml) inactivated by 0.5 mm PMSF (Sigma) was added. Then bound PR3 was detected by adding rabbit serum raised against PR3, 1:2000 diluted, which gave optimal detection of PR3 captured by anti-PR3 MoAbs. Finally, affinity-purified F(ab′)2 goat anti-rabbit IgG conjugated with alkaline phosphatase (Sigma; 1:500) was added. The substrate used in both ELISAs was p-nitrophenyl-phosphate disodium.
The OD values were measured at 405 nm. As a negative control an irrelevant IgG1 MoAb was used. The OD values obtained by this antibody were subtracted from the OD values obtained by the MoAbs to PR3. To compare the binding of different anti-PR3 MoAbs in both ELISA systems dose–response curves at different dilutions were fitted using four-parameter analysis and the 50% of maximal binding was calculated for each MoAb.
BIAcore
The possible competition between anti-PR3 MoAbs in binding to PR3 was investigated by biosensor technology using the BIAcore. The BIAcore (Pharmacia, Uppsala, Sweden) uses Surface Plasmon Resonance technology to study macromolecular interaction [36]. In the BIAcore one of the macromolecules, the ligand, is immobilized on a sensor chip. The sensor chip is composed of a carboxy-methylated dextran coating on a thin gold film covering a glass plate. Other macromolecules, the analytes, flow continuously over the sensor chip surface and are allowed to interact with immobilized ligand. The amount of material bound to the sensor chip surface in any experiment was expressed in arbitrary units as Resonance Units (RU). One RU represents approximately 1 pg of protein per mm2 of the sensor chip surface [37].
Coupling antibody to sensor chip surface
In order to test all anti-PR3 MoAbs on one sensor chip rabbit anti-mouse IgG1 or IgG2a was coupled on a sensor chip instead of coupling all MoAbs to PR3 separately. In all experiments PBS pH 7.4 containing 3.4 mm EDTA and 0.05% surfactant P20 (Pharmacia) was used as buffer solution at a flow rate of 5 μl/min and at a thermostatic temperature of 25°C. First, affinity-purified rabbit anti-mouse (RAM)-IgG1 or -IgG2a (Pharmacia) was immobilized on a CM5 sensor chip (research grade) using standard procedures [37]. In brief, the dextran layer of the sensor chip was activated by injection of 35 μl of a mixture of 0.2 m N-ethyl-N′-(dimethylaminopropyl) carbodiimide (EDC; Pharmacia) and 0.05 m N-hydroxysuccinimide (NHS; Pharmacia). Then, 50 μl RAM-IgG1 or -IgG2a (30 μg/ml) diluted in 200 mm sodium acetate pH 4.0 were injected. Next, the uncoupled activated dextran of the sensor chip was blocked by injection of 50 μl 1 m ethanolamine–HCl pH 8.5 (Pharmacia). Finally, the sensor chip surface was regenerated by injection of 15 μl 100 mm phosphoric acid.
Pairwise binding of MoAbs
To determine whether MoAbs directed against PR3 compete in their binding to PR3, all MoAbs (Table 1) were tested pairwise in the BIAcore system. The anti-PR3 MoAbs 12.8, PR3-G2, PR3G-3, PR3G-4 and PR3G-6, WGM1–3, 4A3, 4A5 and 6A6 were used as hybridoma supernatant. The hybridoma supernatants were diluted two-fold in PBS pH 7.4 containing 3.4 mm EDTA and 0.05% surfactant P20. Purified IgG of MC-PR3-2 and Hz1F12 was diluted in PBS pH 7.4 containing 3.4 mm EDTA and 0.05% surfactant P20 to 25 μg/ml and 12 μg/ml, respectively. First, approximately 4600 RU RAM-IgG1 or 4500 RU RAM-IgG2a were immobilized on a CM5 sensor chip. Then, as first MoAb, 25 μl of MoAbs 12.8, PR3G-2, 3, 4 or 6, WGM-2, 4A5, 6A6, MC-PR3-2 or Hz1F12, respectively, were injected and allowed to interact with RAM-IgG1; 25 μl of 4A3 were allowed to interact with RAM-IgG2a. Next, the unbound RAM-IgG1 or -IgG2a was saturated by injection of 35 μl of an irrelevant MoAb of the IgG1 or IgG2a subclass. Then 25 μl of PR3 (30 μg/ml) inactivated by PMSF were injected and allowed to bind to the first anti-PR3 MoAb. As second MoAb, 25 μl of an anti-PR3 MoAb were injected and allowed to bind to PR3 presented by the first anti-PR3 MoAb. Finally, the sensor chip was regenerated by 100 mm phosphoric acid. Sensor chip immobilized with RAM-IgM (Dako) for binding of WGM-1 and WGM-3 as first MoAb, could not generate interpretable results. Therefore, WGM1 and WGM3 were not used as first MoAb in this study.
For every MoAb the percentage of binding to a specified amount of PR3 was determined. The percentage of binding of a certain MoAb to PR3 was expressed relative to the maximal binding as obtained with any of the presenting MoAbs according to the following equation:
A percentage of binding of the second MoAb, in relation to the PR3 presenting first MoAb, of < 10% of the maximal binding was defined as inhibition in binding. Binding of the second MoAb of < 50% and > 10% of the maximal binding was arbitrarily defined as partial inhibition in binding. A binding of > 50% of the maximal binding was defined as concurrent binding.
RESULTS
Characterization of MoAbs
In order to have a significant number of different MoAbs to PR3 available, four new MoAbs, designated PR3G-2, PR3G-3, PR3G-4 and PR3G-6, were raised against human PR3. The antibodies were selected on their ability to bind purified PR3 in a direct ELISA. Culture supernatant was obtained and the MoAbs were identified as IgG1 subclass by subclass antigen-specific ELISA (data not shown). The antigenic specificity of all MoAbs to PR3, including the four newly raised MoAbs, was verified by four different methods: IIF on ethanol-fixed granulocytes, Western blotting on a crude granule extract, FACS analysis on isolated primed granulocytes, and antigen-specific direct and capture ELISA.
All anti-PR3 MoAbs produced the typical c-ANCA pattern on ethanol-fixed neutrophils by IIF, except for PR3G-3, which consistently produced a more diffuse cytoplasmic staining (data not shown).
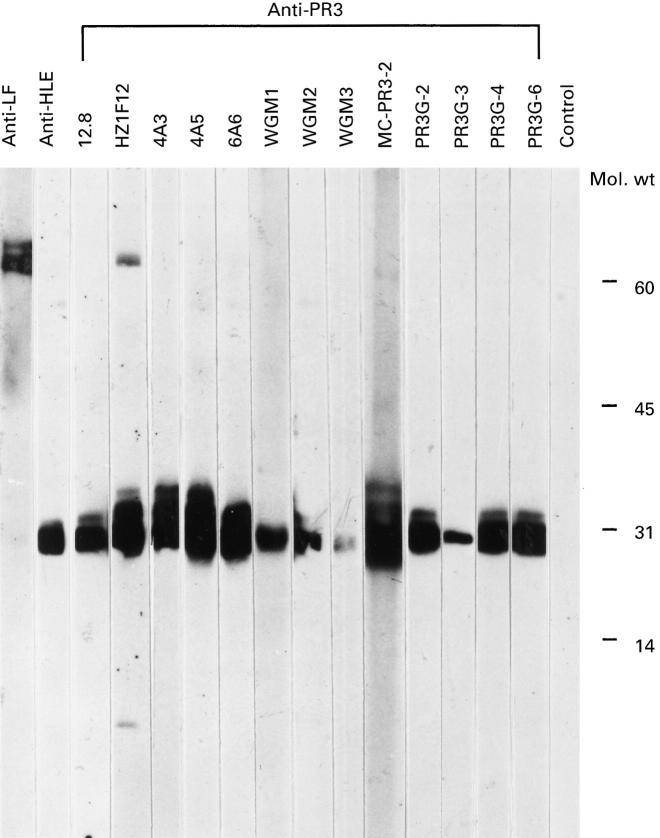
In Western blot analysis on a crude granule extract of neutrophil granulocytes all MoAbs recognized a band at 30 kD, which is identical to PR3 (Fig. 1). No binding to human leucocyte elastase, MPO, lactoferrin, cathepsin G or other granule proteins was seen. This was confirmed by direct ELISA using MPO, human leucocyte elastase, cathepsin G and lactoferrin (data not shown). The additional bands seen on blot at approximately 30 kD were probably due to the intensity of the staining of some MoAbs, which were not seen at higher dilutions. MoAb Hz1F12 gave an additional band at approximately 80 kD and MoAb WGM3 bound PR3 on SDS–PAGE only weakly even at a higher antibody concentration. The MoAbs directed against lactoferrin and human leucocyte elastase gave clear bands at 80 kD and 29 kD, respectively, as expected.
Fig. 1.
Western blot analysis of crude granule extract incubated with anti-PR3 MoAbs. A crude granule extract was applied to a 12.5% polyacrylamide gel under non-reducing conditions. The gel was blotted and incubated with MoAbs to PR3 (lanes 3–15). As controls MoAbs to human leucocyte elastase (lane 2) and lactoferrin (lane 1) and an isotype-matched irrelevant MoAb (lane 16) were used. All MoAbs were used as culture supernatant except for Hz1F12, which is purified IgG, and MC-PR3-2, which is ascites. Blot was exposed for 30 s.
Next, we examined whether anti-PR3 MoAbs could recognize PR3 expressed on the surface of isolated and primed neutrophils. Isolated neutrophils were primed by TNF-α, washed, and incubated with the 13 available MoAbs directed against PR3. All anti-PR3 MoAbs bound to PR3 expressed by primed neutrophils. Representative data are shown in Fig. 2. Also MoAbs directed against human leucocyte elastase and MPO bound to primed neutrophils.
Fig. 2.
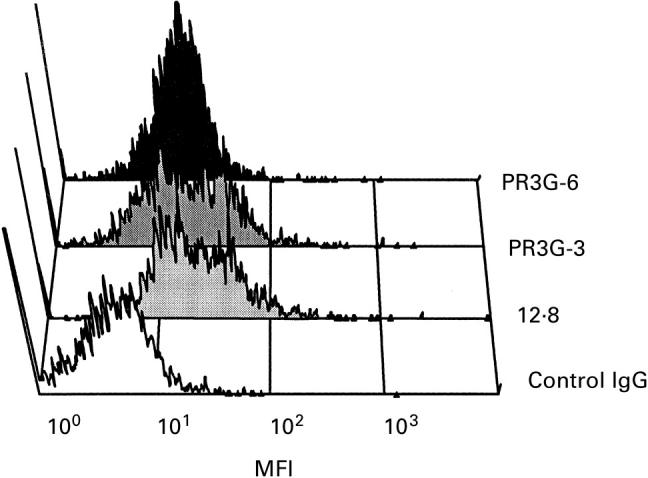
FACS analysis of anti-PR3 MoAbs on primed neutrophils. Neutrophils were isolated and primed with tumour necrosis factor-alpha (TNF-α) for 15 min. MoAbs to PR3 were added and detected with goat anti-mouse FITC. All anti-PR3 MoAbs bound to PR3 expressed by neutrophils; a selection is shown here. Neutrophils incubated with MoAbs 12.8, PR3G-3 and PR3G-6 are shown. As a negative control neutrophils were incubated first with an irrelevant IgG1 MoAb (blank).
PR3 detection by ELISA and BIAcore
All MoAbs directed against PR3 recognized PR3 in a direct as well as in a capture ELISA system (Table 2). Results are shown as antibody concentration in μg/ml at which 50% of maximal binding occurred. MoAbs WGM1 and WGM3, being of the IgM class, could not be tested in a capture ELISA system. Table 2 shows that for each MoAb the concentration required to give 50% of maximal binding to PR3 in a direct ELISA was similar to or above the concentration needed in a capture ELISA system. Furthermore, the antibody concentration at which 50% of maximal binding occurred differed between the anti-PR3 MoAbs, suggesting differences in affinity.
Table 2.
Recognition of PR3 by MoAbs as measured by ELISA and BIAcore
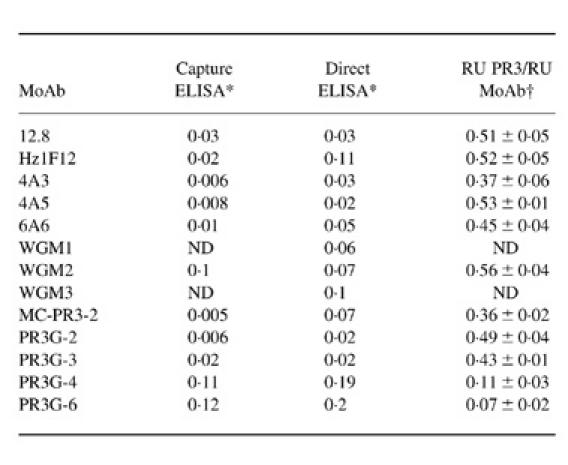
*Concentrations of MoAb (in mg/ml) are given that produce 50% of maximal binding based on an antibody dilution curve (first two columns). Data represent mean of three experiments.
†Represents the amount of PR3 presented by the MoAbs as a ratio of the amount of MoAb present as detected by BIAcore. Data represent mean 6s.d. of 10±15 experiments.
In the last column binding characteristics of the MoAbs in the BIAcore system are presented (Table 2). Based on the molarity of PR3 and IgG, a ratio of the amount of PR3 in RU presented by the MoAbs to the amount of MoAb present in RU is shown. As PR3 (30 kD) is five times smaller than IgG (150 kD), 500 RU antibody and 100 RU PR3 represent the same number of molecules. MoAbs can bind, in ideal binding situations, two PR3 molecules. So, 500 RU of antibody can bind 200 RU of PR3, which gives a PR3/MoAb ratio of 0.4. A ratio of approximately 0.4 represents maximal occupation of a MoAb with PR3. A ratio lower than 0.4 represents submaximal occupation.
Mutual binding interference of MoAbs
With the use of the BIAcore an antigen capture system was set up to test whether anti-PR3 MoAbs compete in binding to PR3. Coupling of RAM-IgG1 or -IgG2a was stable and the individual chip could be used repeatedly. All anti-PR3 MoAbs were bound by RAM-IgG1 and -IgG2a with no detectable difference in affinity, and could recognize PMSF-inactivated PR3. In experiments where active PR3 was used the baseline decreased rapidly, which was most certainly due to proteolytic cleavage of the antibodies coupled to the sensor chip.
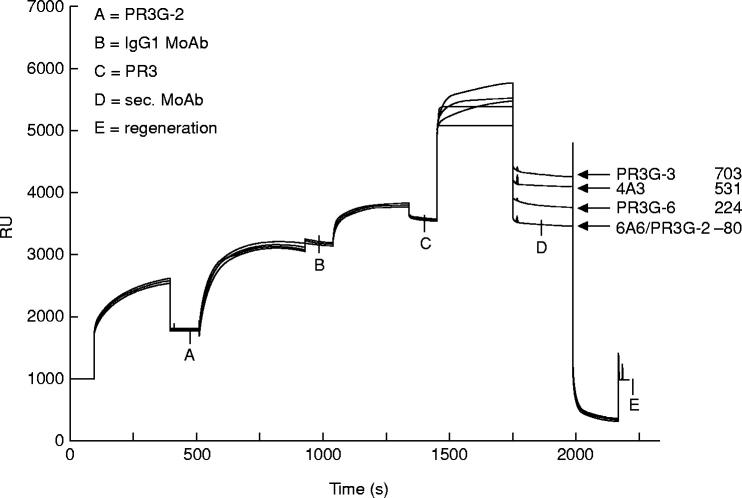
To examine whether MoAbs to PR3 compete in their binding to PR3 all MoAbs were tested pairwise in the BIAcore system. A representative experiment with PR3G-2 as first MoAb is shown in Fig. 3. The figure shows binding of the MoAbs PR3G-3, 4A3, PR3G-6, 6A6 and PR3G-2 to PR3 presented by PR3G-2. The RU depicted on the ordinate give the relative amount of bound protein at each stage. Eight hundred RU of PR3G-2 was bound to RAM-IgG1. The unoccupied RAM-IgG1 sites were occupied by a control IgG1 MoAb (1400 RU) and 400 RU of PR3 was bound by PR3G-2 (with a PR3/MoAb ratio of 0.5). MoAbs PR3G-3, 4A3 and PR3G-6 were able to bind to PR3 presented by PR3G-2, but 6A6 and PR3G-2 itself were not. PR3G-2, when used as second MoAb, did not bind to PR3 presented by the same antibody. This indicates that no unoccupied RAM-IgG1 sites were available. When PR3 was left out no binding was seen. The RAM-IgG1 and RAM-IgG2a proved subclass-specific: MoAbs of the IgG2a or IgM (sub)class were not bound by RAM-IgG1, and no IgG1 or IgM MoAbs were bound by RAM-IgG2a (data not shown).
Fig. 3.
Overlay plot of sensorgrams showing the binding of the MoAbs PR3G-3, 4A3, PR3G-6, 6A6 and PR3G-2 on PR3 presented by PR3G-2. A, 800 Resonance Units (RU) binding of PR3G-2 on a rabbit anti-mouse IgG1 (RAM-IgG1) containing sensor chip. Unoccupied RAM-IgG1 sites were occupied by a control IgG1 MoAb (1400 RU) (B). C, 400 RU of captured PR3. D, binding of PR3G-3, 4A3, PR3G-6, 6A6 and PR3G-2 to PR3. RU values shown represent the increase in RU from point C to D. After regeneration RU came back to base level (E).
The capture system was used to test all anti-PR3 MoAbs pairwise, both as first and as second MoAb. If the second anti-PR3 MoAb was able to bind to PR3 presented by the first anti-PR3 MoAb, these MoAbs were considered to recognize different regions on PR3. If not, the MoAbs were considered to recognize overlapping regions on PR3 [38]. When the first, i.e. the presenting anti-PR3 MoAb, was also used as second, i.e. the detecting anti-PR3 MoAb, no additional binding of the second MoAb was seen. When experiments were repeated up to three times results were consistent.
The pattern of competition between the 13 different MoAbs tested is depicted in Fig. 4. MoAbs 12.8, PR3G-2, 6A6 and Hz1F12 (group 1 MoAbs) mutually inhibited their binding when used either as first or second MoAb. The MoAbs PR3G-4 and PR3G-6 always displayed the same binding characteristics, different from that of the other MoAbs. Therefore, PR3G-4 and PR3G-6 were considered group 2 anti-PR3 MoAbs. MoAbs PR3G-3, 4A5 and WGM2 mutually inhibited the binding of one another, and thus constituted a third group of MoAbs (group 3). Finally, MoAbs MC-PR3-2, 4A3 and WGM3 showed mutual inhibition and were grouped as group 4. The binding characteristics of WGM1 could not be tested accurately, so WGM1 could not be grouped in our analysis.
Fig. 4.
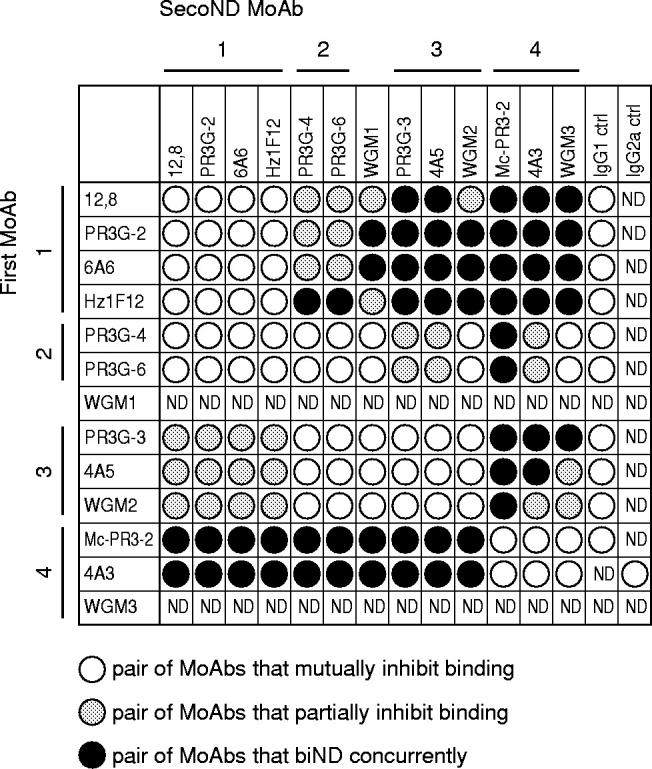
Pattern of competition matrix showing the binding ability of pairs of MoAbs to PR3. Inhibition of binding of the second MoAb (binding of < 10% of maximal binding) is assumed to represent similar or closely related epitopes (○). Binding of the second MoAb of < 50% and > 10% of maximal binding was defined as partial inhibition in binding, assuming overlapping epitopes (hatched). Concurrent binding of a pair of MoAbs to PR3 (a decrease in binding of > 50% of maximal binding) is assumed to represent different epitope specificities (•).
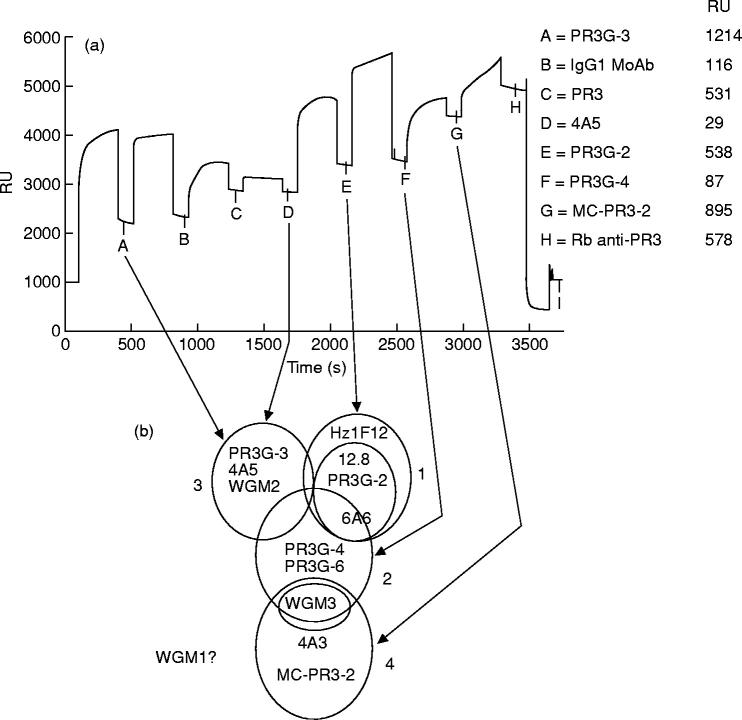
Next, binding to PR3 by MoAbs from all four groups together was investigated. In Fig. 5a PR3G-3 was bound to RAM-IgG1. The unoccupied RAM-IgG1 sites were occupied by a control IgG1 MoAb and PR3 was allowed to bind to PR3G-3. As expected from the previously described grouping, MoAb 4A5 could not bind to PR3 presented by PR3G-3. The MoAbs PR3G-2, PR3G-4, and MC-PR3-2 were all able to bind to PR3 presented by PR3G-3, independently of each other. The ability of large amounts of polyclonal rabbit anti-PR3 antibody to bind at the end of the experiment suggests that still more epitopes on PR3 were available. Figure 5b shows a two-dimensional representation of epitope areas on PR3 derived from the former experiments. This two-dimensional representation is in accord with the results in Fig. 5a.
Fig. 5.
Sequential multideterminant binding of MoAbs to PR3. (a) Addition of five anti-PR3 MoAbs from different epitope groups to PR3 captured by PR3G-3. Point A indicates binding of PR3G-3 on a rabbit anti-mouse IgG1 (RAM-IgG1) containing sensor chip. C, captured PR3. D–G, binding of different MoAbs to PR3. H, a large amount of binding by a polyclonal anti-PR3 antibody. Resonance Unit (RU) values shown represent increase in RU as measured from the previous point. (b) Two-dimensional map of PR3 representing epitope areas based on the matrix of Fig. 4. Overlapping circles indicate groups of MoAbs that cannot bind concurrently. Partly overlapping circles indicate MoAb groups that can bind concurrently.
We further compared binding patterns of MoAbs within one group and determined the amount of overlap in binding to PR3 between the different groups of MoAbs. Within MoAb group 1 the binding pattern of Hz1F12, when used as first MoAb, was slightly different from that of the other MoAbs of this group (Fig. 4). This suggests that MoAb Hz1F12 may recognize a larger epitope area that overlaps the epitopes recognized by 12.8, PR3G-2 and 6A6 because Hz1F12 inhibited their binding and group 1 MoAbs inhibited binding of Hz1F12 (Figs 4 and 5b). When MoAbs of group 1 were used as first MoAb, binding to PR3 by group 2 MoAbs and WGM1 was partially inhibited, whereas binding of group 3 and 4 MoAbs was not inhibited. In contrast, binding of group 1 MoAbs was completely or partially inhibited when group 2 or group 3 MoAbs were used as first MoAb, respectively. Thus, group 1 MoAbs recognized an area on PR3 partly overlapping that of group 2 and 3 MoAbs, because the latter groups partially inhibited the binding of group 1 MoAbs. This is shown as partly overlapping circles in Fig. 5b. Group 2 MoAbs, when used as first antibody, inhibited binding of WGM1, WGM2 (group 3) and partially inhibited binding of the other MoAbs of group 3. In contrast, when group 3 MoAbs were used as first antibody binding of group 2 was inhibited completely. Thus, group 2 MoAbs recognized an epitope area overlapping the area recognized by group 1 and 3 MoAbs (Fig. 5b). Group 4 MoAbs bound concurrently to PR3 presented by group 3 MoAbs. PR3 presented by group 4 MoAbs was bound by all other MoAbs. Furthermore, group 2 MoAbs, when used as first MoAb, inhibited, partially inhibited, and did not inhibit binding of WGM3, 4A3 and MC-PR3-2 (group 4), respectively. Thus, group 4 MoAbs recognized an area on PR3 only overlapping that of group 2 MoAbs (Fig. 5b).
DISCUSSION
This study, using biosensor technology, shows that eight well-established and four newly produced anti-PR3 MoAbs recognize four separate epitope areas on PR3, including one area detected with newly raised MoAbs only.
Four new MoAbs to PR3 of the IgG1 subclass were generated. The results from IIF, immunoblotting, FACS analysis and antigen-specific ELISA verified the antigenic specificity of PR3G-2, -3, -4 and -6, as well as that of nine well-established MoAbs to PR3.
In the direct and capture ELISA the anti-PR3 MoAbs differed considerably in antibody concentration, resulting in 50% maximal binding, which might indicate differences in affinity. Low concentrations probably indicate high affinity for PR3, and high concentrations a low affinity. This suggests that MoAbs PR3G-4, PR3G-6 and WGM2 have a lower affinity for PR3 compared with the other MoAbs of the IgG class. Furthermore, the PR3/MoAb ratio, as detected in BIAcore, of PR3G-4 and PR3G-6 was far below 0.4 (maximal occupation of a MoAb with PR3), suggesting binding of less than two PR3 molecules per antibody molecule.
Biosensor technology was chosen to determine whether MoAbs to PR3 compete in their binding to PR3. The advantages of a biosensor system include its ability to study molecular interactions in real time, with soluble proteins in their native state, unpurified and non-labelled. A capture system was chosen because it allows presentation of PR3 in its native form. PR3 coupling onto a solid phase, as in a direct ELISA, may denature the tertiary structure of PR3, which may lead to partial loss of epitopes. It has indeed been shown that a PR3-specific capture ELISA is more sensitive than a direct ELISA for the detection of anti-PR3-positive sera [30,31]. Furthermore, a capture system was chosen because direct binding of PR3 to the sensor chip surface proved difficult. PR3 could be coupled on the sensor chip but was poorly recognized by MoAbs to PR3, whereas the amount of PR3 coupled was low and the baseline dropped rapidly, so the chip could only be used up to 10 times. Results obtained in this way were not reproducible. The capture system used in this study gave reproducible results with a very low background.
In this capture system it was not possible to calculate the affinity constants of the different anti-PR3 MoAbs, since the final results depended both on the on and off rates of binding of the MoAbs to the RAM, as well as the on and off rates of binding of PR3 to the MoAbs. The affinity constants of MoAb and PR3, which are difficult to determine in this system, will thus not be reliable.
MoAbs pairwise inhibiting the binding of each other were clustered into groups (Fig. 5b), based on the assumption that they recognize similar epitope areas on the PR3 molecule. Four different groups were identified. MoAbs with other epitope specificities apparently were not produced. Possibly, for the mouse humoral immune system only four epitope areas are recognized on PR3, as PR3 is a small extensively folded protein [39]. Within group 3 MoAb WGM2 displayed a difference in binding pattern compared with the other MoAbs of group 3, especially when PR3G-4 or PR3G-6 were used as first antibody. This difference in binding pattern was probably due to the low affinity of all three MoAbs, as shown in the ELISA testing of these antibodies. When a MoAb with low affinity for PR3 is used as first MoAb it will easily release bound PR3 when a second antibody with higher affinity for PR3 is incubated. The total amount of protein bound on the sensor chip may diminish and will thus cause lowering of the signal. Eventually, this may give false-negative results, resulting in falsely grouping of MoAbs based on their epitope specificity. This is illustrated in Fig. 4. Group 2 MoAbs, when used as first MoAb, inhibited the binding of group 1 MoAbs. However, when group 1 MoAbs where used as first MoAb binding of group 2 MoAbs was only partially inhibited. If one considers only the first results and not the latter, MoAbs of group 1 and 2 would be clustered together. The discrepancy in competition pattern within MoAb groups 3 and 4, when group 2 MoAbs and WGM2 were used as first MoAb, can also be explained by the low affinity of these MoAbs. In a final experiment, clustering of the MoAbs in different groups as based on their epitope specificity was confirmed (Fig. 5). All MoAbs from the four groups could bind to PR3 simultaneously and thus recognize non- or only partly overlapping epitopes on PR3. The ability of a polyclonal rabbit anti-PR3 antibody to bind after MoAbs from all four groups had bound to PR3 suggests that for the rabbit immune system still more epitopes on PR3 are available. So, the epitope restriction seen for anti-PR3 MoAbs appears to be due not only to steric hindrance.
Results obtained from these cross-inhibition experiments confirm previously published data on epitope recognition by some MoAbs to PR3. Sommarin et al. [40] showed, by competition in a BIAcore system and in ELISA, that MoAbs 4A3, 4A5 and 6A6 recognize different epitopes on PR3 and that MoAb 12.8 recognizes the same epitope as 6A6. Tested by competition in a direct cytoELISA MoAb MC-PR3-2 did not compete with MoAbs 4A5 and 6A6 in binding to PR3, and MoAb 4A3 seemed to bind a larger epitope that partially overlapped with the epitope recognized by MC-PR3-2 [28]. In the present study we integrally analysed the competition between 13 anti-PR3 MoAbs in binding to PR3. Furthermore, we used a capture system setup in the BIAcore where PR3 is presented in its native state by anti-PR3 MoAbs, in contrast to a direct cytoELISA where PR3 may be denatured by coating to a solid phase.
In preliminary studies some of the established MoAbs have been used in inhibition studies with PR3-ANCA sera. The epitope area recognized by MoAb 4A5 proved to be the most common epitope area being recognized by PR3-ANCA as tested by competition ELISA [40]. This MoAb, a member of MoAb group 3, could completely or partially inhibit the binding of 61% of PR3-ANCA sera. MoAb 4A3 (group 4) partially inhibited eight out of 22 PR3-ANCA sera and MoAb 6A6 (group 1) inhibited 11% of the same sera [40]. Hz1F12 (group 1) partially or completely inhibited the binding of 46% of PR3-ANCA sera from patients with WG [41]. MoAb WGM2 was unable to block binding of five anti-PR3-positive sera as tested by IIF and confirmed by ELISA [42]. So, some anti-PR3 MoAbs recognize comparable epitopes areas as PR3-ANCA patient sera, and thus can serve as tools for epitope mapping and detection of epitope shifts in different stages of the disease.
Thus far it has been difficult to specifically determine epitopes recognized by MoAbs. MoAbs to PR3 and anti-PR3-positive sera recognize conformational epitopes [23]. MoAbs to PR3 do not recognize linear peptides of the whole PR3 sequence. Even PR3 reduced with 2-mercaptoethanol was not detected by all anti-PR3 MoAbs (data not shown).
Some of the anti-PR3 MoAbs are widely used in diagnostic tests, based on the capture ELISA system [28–30,43]. The above mentioned results indicate that PR3-ANCA react with different epitopes on PR3 and that some of the epitopes may be masked by MoAbs. Even in a capture ELISA where a mix of three MoAbs was used for capturing PR3, still less than 100% of the PR3-ANCA sera were positive [30,31]. A capture ELISA with an oligoclonal antibody or a mix of several MoAbs to PR3 as capturing antibody still present all epitopes available on PR3 and thus might give more accurate results.
In this study the capture system set up in the BIAcore gave reproducible results and can be used for studying epitope restriction in PR3-ANCA patient sera. Corresponding experiments have already been performed for MPO-ANCA sera [27]. Recently, using biosensor technology it was shown that sera from patients with anti-MPO-associated vasculitis recognize a restricted number of epitopes on MPO [44]. In one patient an epitope shift was found between sequential relapses [45].
In conclusion, our results demonstrate that eight well-established anti-PR3 MoAbs, produced by different research groups, and four newly produced anti-PR3 MoAbs recognize four separate epitope areas on PR3, including one area detected with newly raised MoAbs only. Two of the four new MoAbs recognize an epitope area on PR3 different from the established MoAbs, thus enabling the detection of a broader range of anti-PR3 antibody specificities. The restricted number of epitope areas recognized by mouse MoAbs on PR3, being a small extensively folded protein, suggests that the human anti-PR3 response in WG is also restricted.
Acknowledgments
We thank Dr E. Csernok and Professor W. Gross (Rheumaklinik, Bad Bremsted, Germany) for the MoAbs WGM1–3, Dr J. Wieslander (Lund, Sweden) for MoAbs 4A3, 4A5 and 6A6, Dr C. M. Lockwood (Cambridge, UK) for MoAb Hz1F12, and Dr U. Specks (Rochester, MN) for the MoAb MC-PR3-2 and his support. Furthermore, the authors would like to thank M. G. Huitema for her help with the PR3 purification. We are especially grateful to Dr M. van Kooij (Department of Medical Microbiology, University Hospital Groningen) who taught the fine work of handling the BIAcore. This work was supported by Research Grant (C1481) from the Dutch Kidney Foundation.
REFERENCES
- 1.van der Woude FJ, Rasmussen N, Lobatto S, et al. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet. 1985;1:425–9. doi: 10.1016/s0140-6736(85)91147-x. [DOI] [PubMed] [Google Scholar]
- 2.Cohen Tervaert JW, van der Woude FJ, Fauci AS, et al. Association between active Wegener's granulomatosis and anticytoplasmic antibodies. Arch Intern Med. 1989;149:2461–5. doi: 10.1001/archinte.149.11.2461. [DOI] [PubMed] [Google Scholar]
- 3.Niles JL, McCluskey RT, Ahmad MF, Arnaout MA. Wegener's granulomatosis autoantigen is a novel neutrophil serine proteinase. Blood. 1989;74:1888–93. [PubMed] [Google Scholar]
- 4.Goldschmeding R, van der Schoot CE, ten Bokkel Huinink D, Hack CE, van den Ende ME, von Kallenberg CG, dem Borne AE. Wegener's granulomatosis autoantibodies identify a novel diisopropylfluorophosphate-binding protein in the lysosomes of normal human neutrophils. J Clin Invest. 1989;84:1577–87. doi: 10.1172/JCI114335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ludemann J, Utecht B, Gross WL. Anti-neutrophil cytoplasm antibodies in Wegener's granulomatosis recognize an elastinolytic enzyme. J Exp Med. 1990;171:357–62. doi: 10.1084/jem.171.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baggiolini M, Bretz U, Dewald B, Feigenson ME. The polymorphonuclear leukocyte. Agents Actions. 1978;8:3–10. doi: 10.1007/BF01972395. [DOI] [PubMed] [Google Scholar]
- 7.Csernok E, Ludemann J, Gross WL, Bainton DF. Ultrastructural localization of proteinase 3, the target antigen of anti-cytoplasmic antibodies circulating in Wegener's granulomatosis. Am J Pathol. 1990;137:1113–20. [PMC free article] [PubMed] [Google Scholar]
- 8.Rao NV, Wehner NG, Marshall BC, Gray WR, Gray BH, Hoidal JR. Characterization of proteinase-3 (PR-3), a neutrophil serine proteinase. Structural and functional properties. J Biol Chem. 1991;266:9540–8. [PubMed] [Google Scholar]
- 9.Kao RC, Wehner NG, Skubitz KM, Gray BH, Hoidal JR. Proteinase 3. A distinct human polymorphonuclear leukocyte proteinase that produces emphysema in hamsters. J Clin Invest. 1988;82:1963–73. doi: 10.1172/JCI113816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leid RW, Ballieux BE, van der Heijden I, Kleyburg van der Keur C, Hagen EC, van Es LA, van der Woude FJ, Daha MR. Cleavage and inactivation of human C1 inhibitor by the human leukocyte proteinase, proteinase 3. Eur J Immunol. 1993;23:2939–44. doi: 10.1002/eji.1830231132. [DOI] [PubMed] [Google Scholar]
- 11.Cohen Tervaert JW, Huitema MG, Hene RJ, Sluiter WJ, The TH, van der Hem GK, Kallenberg CG. Prevention of relapses in Wegener's granulomatosis by treatment based on antineutrophil cytoplasmic antibody titre. Lancet. 1990;336:709–11. doi: 10.1016/0140-6736(90)92205-v. [DOI] [PubMed] [Google Scholar]
- 12.Kerr GS, Fleisher TA, Hallahan CW, Leavitt RY, Fauci AS, Hoffman GS. Limited prognostic value of changes in antineutrophil cytoplasmic antibody titers in patients with Wegener's granulomatosis. Adv Exp Med Biol. 1993;336:411–4. doi: 10.1007/978-1-4757-9182-2_71. [DOI] [PubMed] [Google Scholar]
- 13.Kallenberg CG, Brouwer E, Weening JJ, Tervaert JW. Anti-neutrophil cytoplasmic antibodies: current diagnostic and pathophysiological potential. Kidney Int. 1994;46:1–15. doi: 10.1038/ki.1994.239. [DOI] [PubMed] [Google Scholar]
- 14.Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA. 1990;87:4115–9. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charles LA, Caldas ML, Falk RJ, Terrell RS, Jennette JC. Antibodies against granule proteins activate neutrophils in vitro. J Leukoc Biol. 1991;50:539–46. doi: 10.1002/jlb.50.6.539. [DOI] [PubMed] [Google Scholar]
- 16.van de Wiel BA, Dolman KM, van der Meer Gerritsen CH, von Hack CE, dem Borne AE, Goldschmeding R. Interference of Wegener's granulomatosis autoantibodies with neutrophil Proteinase 3 activity. Clin Exp Immunol. 1992;90:409–14. doi: 10.1111/j.1365-2249.1992.tb05860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daouk GH, Palsson R, Arnaout MA. Inhibition of proteinase 3 by ANCA and its correlation with disease activity in Wegener's granulomatosis. Kidney Int. 1995;47:1528–36. doi: 10.1038/ki.1995.216. [DOI] [PubMed] [Google Scholar]
- 18.Dolman KM, Stegeman CA, van de Wiel BA, von Hack CE, dem Borne AE, Kallenberg CG, Goldschmeding R. Relevance of classic anti-neutrophil cytoplasmic autoantibody (C-ANCA)-mediated inhibition of proteinase 3-alpha 1-antitrypsin complexation to disease activity in Wegener's granulomatosis. Clin Exp Immunol. 1993;93:405–10. doi: 10.1111/j.1365-2249.1993.tb08192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolman KM, Jager A, von Sonnenberg A, dem Borne AE, Goldschmeding R. Proteolysis of classic anti-neutrophil cytoplasmic autoantibodies (C-ANCA) by neutrophil proteinase 3. Clin Exp Immunol. 1995;101:8–12. doi: 10.1111/j.1365-2249.1995.tb02269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulder AH, Stegeman CA, Kallenberg CG. Activation of granulocytes by anti-neutrophil cytoplasmic antibodies (ANCA) in Wegener's granulomatosis: a predominant role for the IgG3 subclass of ANCA. Clin Exp Immunol. 1995;101:227–32. doi: 10.1111/j.1365-2249.1995.tb08343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulder AH, Heeringa P, Brouwer E, Limburg PC, Kallenberg CG. Activation of granulocytes by anti-neutrophil cytoplasmic antibodies (ANCA): a Fc gamma RII-dependent process. Clin Exp Immunol. 1994;98:270–8. doi: 10.1111/j.1365-2249.1994.tb06137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman GS, Specks U. Antineutrophil cytoplasmic antibodies. Arthritis Rheum. 1998;41:1521–37. doi: 10.1002/1529-0131(199809)41:9<1521::AID-ART2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 23.Bini P, Gabay JE, Teitel A, Melchior M, Zhou JL, Elkon KB. Antineutrophil cytoplasmic autoantibodies in Wegener's granulomatosis recognize conformational epitope(s) on proteinase 3. J Immunol. 1992;149:1409–15. [PubMed] [Google Scholar]
- 24.Williams RC, Jr, Staud R, Malone CC, Payabyab J, Byres L, Underwood D. Epitopes on proteinase-3 recognized by antibodies from patients with Wegener's granulomatosis. J Immunol. 1994;152:4722–37. [PubMed] [Google Scholar]
- 25.Chang L, Binos S, Savige J. Epitope mapping of anti-proteinase 3 and anti-myeloperoxidase antibodies. Clin Exp Immunol. 1995;102:112–9. doi: 10.1111/j.1365-2249.1995.tb06644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffith ME, Coulthart A, Pemberton S, Pusey CD. c-ANCA recognise a restricted number of linear epitopes of proteinase 3 which involve the catalytic site. Sarcoidosis Vasc Diff Lung Dis. 1996;13:256. (Abstr.) [Google Scholar]
- 27.Audrain MA, Baranger TA, Moguilevski N, Martin SJ, Devys A, Lockwood CM, Muller JY, Esnault VL. Anti-native and recombinant myeloperoxidase monoclonals and human autoantibodies. Clin Exp Immunol. 1997;107:127–34. doi: 10.1046/j.1365-2249.1997.d01-895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun J, Fass DN, Hudson JA, Viss MA, Wieslander J, Homburger HA, Specks U. Capture-ELISA based on recombinant PR3 is sensitive for PR3-ANCA testing and allows detection of PR3 and PR3-ANCA/PR3 immunecomplexes. J Immunol Methods. 1998;211:111–23. doi: 10.1016/s0022-1759(97)00203-2. [DOI] [PubMed] [Google Scholar]
- 29.Hagen EC, Andrassy K, Csernok E, et al. Development and standardization of solid phase assays for the detection of anti-neutrophil cytoplasmic antibodies (ANCA). A report on the second phase of an international cooperative study on the standardization of ANCA assays. J Immunol Methods. 1996;196:1–15. doi: 10.1016/0022-1759(96)00111-1. [DOI] [PubMed] [Google Scholar]
- 30.Baslund B, Segelmark M, Wiik A, Szpirt W, Petersen J, Wieslander J. Screening for anti-neutrophil cytoplasmic antibodies (ANCA): is indirect immunofluorescence the method of choice? Clin Exp Immunol. 1995;99:486–92. doi: 10.1111/j.1365-2249.1995.tb05577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westman KW, Selga D, Bygren P, Segelmark M, Baslund B, Wiik A, Wieslander J. Clinical evaluation of a capture ELISA for detection of proteinase-3 antineutrophil cytoplasmic antibody. Kidney Int. 1998;53:1230–6. doi: 10.1046/j.1523-1755.1998.00873.x. [DOI] [PubMed] [Google Scholar]
- 32.Borregaard N, Heiple JM, Simons ER, Clark RA. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983;97:52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiik A. Delineation of a standard procedure for indirect immunofluorescence detection of ANCA. APMIS Suppl. 1989;6:12–13. [PubMed] [Google Scholar]
- 34.Kallenberg CG, Cohen Tervaert JW, Huitema MG, van der Giessen M. Towards standard sera for the determination of anti-neutrophil cytoplasmic (ANCA) and anti-myeloperoxidase (aMPO) antibodies. APMIS Suppl. 1989;6:14–15. [PubMed] [Google Scholar]
- 35.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 36.Malmqvist M. Biospecific interaction analysis using biosensor technology. Nature. 1993;361:186–7. doi: 10.1038/361186a0. [DOI] [PubMed] [Google Scholar]
- 37.Johnsson B, Lofas S, Lindquist G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Anal Biochem. 1991;198:268–77. doi: 10.1016/0003-2697(91)90424-r. [DOI] [PubMed] [Google Scholar]
- 38.Fagerstam LG, Frostell A, Karlsson R, Kullman M, Larsson A, Malmqvist M, Butt H. Detection of antigen–antibody interactions by surface plasmon resonance. Application to epitope mapping. J Mol Recognit. 1990;3:208–14. doi: 10.1002/jmr.300030507. [DOI] [PubMed] [Google Scholar]
- 39.Fujinaga M, Chernaia MM, Halenbeck R, Koths K, James MN. The crystal structure of PR3, a neutrophil serine proteinase antigen of Wegener's granulomatosis antibodies. J Mol Biol. 1996;261:267–78. doi: 10.1006/jmbi.1996.0458. [DOI] [PubMed] [Google Scholar]
- 40.Sommarin Y, Rasmussen N, Wieslander J. Characterization of monoclonal antibodies to proteinase-3 and application in the study of epitopes for classical anti-neutrophil cytoplasm antibodies. Exp Nephrol. 1995;3:249–56. [PubMed] [Google Scholar]
- 41.Huang Z, Zhao MH, Lockwood CM. Epitope mapping on the proteinase 3 molecule using monoclonal antibodies and sera from patients with Wegener's granulomatosis. Clin Exp Immunol. 1995;101:50. (Abstr.) [Google Scholar]
- 42.Braun MG, Csernok E, Gross WL, Muller Hermelink HK. Proteinase 3, the target antigen of anticytoplasmic antibodies circulating in Wegener's granulomatosis. Immunolocalization in normal and pathologic tissues. Am J Pathol. 1991;139:831–8. [PMC free article] [PubMed] [Google Scholar]
- 43.Hagen EC, Daha MR, Hermans J, et al. Diagnostic value of standardized assays for anti-neutrophil cytoplasmic antibodies in idiopathic systemic vasculitis. EC/BCR Project for ANCA Assay Standardization. Kidney Int. 1998;53:743–53. doi: 10.1046/j.1523-1755.1998.00807.x. [DOI] [PubMed] [Google Scholar]
- 44.Short AK, Lockwood CM. Studies of epitope restriction on myeloperoxidase (MPO), an important antigen in systemic vasculitis. Clin Exp Immunol. 1997;110:270–6. doi: 10.1111/j.1365-2249.1997.tb08327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chapman PT, Short AK, Lockwood CM. The use of Biosensor technology in epitope mapping studies on human myeloperoxidase. Clin Exp Immunol. 1998;112:44. (Abstr.) [Google Scholar]
- 46.Zhao MH, Lockwood CM. A comprehensive method to purify three major ANCA antigens: proteinase 3, myeloperoxidase and bactericidal/permeability-increasing protein from human neutrophil granule acid extract. J Immunol Methods. 1996;197:121–30. doi: 10.1016/0022-1759(96)00123-8. [DOI] [PubMed] [Google Scholar]
- 47.Ludemann J, Utecht B, Gross WL. Detection and quantitation of anti-neutrophil cytoplasm antibodies in Wegener's granulomatosis by ELISA using affinity-purified antigen. J Immunol Methods. 1988;114:167–74. doi: 10.1016/0022-1759(88)90169-x. [DOI] [PubMed] [Google Scholar]
- 48.Braun MG, Csernok E, Rogener Schwarz W, Ludwig WD, Muller Hermelink HK, Gross WL, Feller AC. Monoclonal antibody WGM1 directed against proteinase 3: an immunohistochemical marker for naphthol ASD chloroacetate. Hematol Oncol. 1996;14:83–90. doi: 10.1002/(SICI)1099-1069(199606)14:2<83::AID-HON570>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]