Abstract
We have analysed the effects of cocaine, administered to mice during the in vivo differentiation of effector T cells stimulated by antigen (influenza virus) recognition, on the frequency of IL-2-, IL-4- and interferon-gamma (IFN-γ)-expressing CD4+ and CD8+ T cells. Each animal was injected intraperitoneally with 10 mg/kg of cocaine 6, 24, 48 and 72 h after immunization with A/PR8 influenza virus (PR8). This enabled the determination of the pharmacological effects of cocaine on T cells during the initial step of the immune response, which is characterized by the production of large amounts of immunoregulatory cytokines. The distribution of IL-2-, IL-4- and IFN-γ-producing CD4+ and CD8+ T cells was assayed on unseparated PR8-immune spleen cells, obtained from mice treated with cocaine or vehicle, and restimulated in vitro with UV-inactivated PR8 virus. The frequency of T cells singly or co-expressing the above three cytokines was determined at single-cell level by simultaneous flow cytometric analysis of intracellular cytokines and surface antigen expression. In parallel, the levels of IL-2, IL-4 and IFN-γ in the culture supernatants were quantified by ELISA. The results showed that cocaine, administered during the in vivo virus-induced differentiation of T cells, caused an increase of both the frequencies of CD8+ T cells singly and co-expressing IL-2 and IFN-γ and the levels of these cytokines in virus-restimulated spleen cell culture supernatants, compared with those of untreated controls. In contrast, no effect was found on IL-4-positive CD8+ T cells and on IL-2-, IFN-γ- and IL-4-positive CD4+ T cells. Our findings suggest that the immunomodulatory effects of cocaine may be due to the up-regulation of the production of IL-2 and IFN-γ by CD8+ T cells with a type 0 cytokine profile.
Keywords: cocaine, T cell, cytokine, in vivo, influenza virus
INTRODUCTION
Evidence of an interaction between cocaine and the immune response has been accumulating for the past decade. Both human and animal studies (reviewed in [1]) have shown that cocaine can influence both cell-mediated and humoral immunity by affecting the effector functions of different immunocompetent cells, including T cells, natural killer (NK) cells, macrophages and neutrophils.
In particular, cocaine was found to modulate both in vitro and in vivo production of different immunoregulatory cytokines by T cells. In in vitro studies cocaine was found to inhibit the concanavalin A (Con A)-stimulated mouse spleen cell production of IL-2, IL-4, IL-5, IL-10 and interferon-gamma (IFN-γ) in a concentration-dependent manner [2,3], and to down-regulate the production of IFN-γ and IL-8 by IL-2-stimulated human peripheral blood lymphocytes [4]. Thus, in vitro effects of cocaine on cytokine production by T cells were found to be generally suppressive [1]. However, by using different methods for stimulation of T cells and determination of cytokine production, we found some discrepancies. In fact, in the previous studies, the cytokine production was induced by polyclonal activation and was measured by cytokine-specific ELISA in cell culture supernatants. Differently, we assayed the in vitro effects of cocaine on antigen-specific induced cytokine production in ex vivo ovalbumin (OVA)-immune murine splenocytes. The cytokine production was evaluated both by ELISA quantification of the cytokines in culture supernatants and by enumeration of the single cytokine-producing CD4+ T cells, performed by combined flow cytometric analysis of intracytoplasmic cytokine production and surface antigen expression [5]. The results showed that exposure to cocaine reduced the levels in culture supernatants of IL-2 and IFN-γ, without affecting IL-4 and IL-5 levels, and increased the percentage of CD4+ T cells positive for IL-2 (CD4+/IL-2+ cells), whereas the percentage of CD4+/IL-4+ cells was unchanged. These results suggested that cocaine exerts distinct effects on the production of the different cytokines by CD4+ T cells following antigen-specific stimulation and that, at least in vitro, cocaine may act by interfering with the secretion rather than the synthesis of cytokines.
Less information has been gathered on the in vivo effects of cocaine on cytokine production by immune cells. In one study [6], acute (1 mg/kg body weight) or subchronic (1 mg/kg per day for 7 consecutive days) i.p. administration of cocaine to male BALB/c mice was found to significantly reduce IL-2, IFN-γ and IL-4 production by mitogen-stimulated spleen cells. Different results were obtained when the pattern of cytokine production from Con A-stimulated spleen cells was studied in female C57Bl/6 mice injected intraperitoneally with increasing dosages of cocaine (from 10 to 40 mg/kg per day) for 6 weeks [3]. Cocaine administration enhanced the production of IL-2 and IL-5, inhibited the production of IL-4 and IL-10, but did not affect IFN-γ. A recent study [7] reported that acute cocaine administration (30 mg/kg) to mice 1 day before sensitization with sheep erythrocytes induced an increase in IL-4 and IL-10 production by anti-CD3 stimulated spleen cells, while IL-2 and IFN-γ were unaffected. These contradictory results could be due to the different experimental conditions used (i.e. cocaine dose levels, duration and frequency of treatment, strain and sex of the mouse used) which could affect differently the various cytokine-producing T cell subsets. It is now known that different subsets of both CD4+ and CD8+ cells, defined by their cytokine production profile, are involved in the regulation of different types of immunity [8–10]. Type 1 cells are characterized by the secretion of IL-2, IFN-γ and lymphotoxins; type 2 cells are characterized by the production of IL-4, IL-5, IL-10 and IL-13, while a third population of cells, type 0 cells, which produce both type 1 and type 2 cytokines, probably represents the majority of circulating T cells [11]. It is believed that the production of distinct combinations of cytokines by the different subsets of CD4+ and CD8+ T cells is associated with distinct immune functions and that the perturbation of the cytokine production and balance may lead to disease [12]. Currently no data are available on the in vivo effects of cocaine on the cytokine profiles of the different T cell subpopulations. In fact, in the above mentioned studies the pattern of cytokine production was exclusively determined by measuring cytokine concentration in culture supernatants of unseparated spleen cells and therefore no direct determination of the effects of cocaine on cytokine production by the different T cell subsets was possible.
The present study was designed to evaluate the in vivo effects of cocaine on cytokine profile of both antigen-specific stimulated CD4+ and CD8+ T cells. For this purpose, we used a model system [14] in which unseparated spleen cells obtained from mice, injected intraperitoneally with PR8 influenza virus, were restimulated in vitro with UV-inactivated PR8 virus. The distribution of IL-2-, IL-4- and IFN-γ-producing CD4+ and CD8+ T cells was assayed at single-cell level by simultaneous flow cytometric analysis of intracellular cytokines and surface antigen expression. In parallel, the levels of IL-2, IL-4 and IFN-γ in the culture supernatants were quantified by ELISA.
The results show that cocaine administration in mice immunized with influenza virus induced an increase in the number of individual splenic CD8+ T cells singly expressing or co-expressing IL-2 or IFN-γ, while the frequency of IL-4-expressing CD8+ T cells remained unchanged. In contrast, no effect was found in the frequencies of CD4+ T cells expressing any one of the three cytokines.
MATERIALS AND METHODS
Animals
Male BALB/c mice (5–7 weeks) purchased from Charles River Italia (Co., Italy) and attested to be negative for antibodies to murine hepatitis virus, Sendai virus and mycoplasma by ELISA, were used.
Virus and immunization
A/PR8 (H1N1) influenza virus (PR8) was grown in the allantoic cavities of 10-day-old embryonated chicken eggs. UV-inactivated PR8 was prepared by exposing virus to UV light (40 W, 254 nm, 8 cm distance) for 5 min. Mice to be immunized were intraperitoneally injected with 500 haemagglutinating units (HAU)/animal of PR8.
Cocaine treatment
Cocaine hydrochloride (SALARS, Como, Italy) was dissolved, just before use, in 0.9% saline solution and diluted so that each mouse received 0.2 ml of the drug solution. Control mice received 0.2 ml of saline. Each animal was injected intraperitoneally with cocaine (10 mg/kg body weight) or saline 6, 24, 48 and 72 h after immunization with PR8. The dosage of cocaine was chosen on the basis of previous experimental works [4,13] which demonstrated that cocaine administered to mice at the dose of 10 mg/kg for different periods of time, up to 7 weeks, induced significant effects on different immunological parameters without inducing general toxic effects.
Cell cultures
Spleens were removed 5 days after immunization. Splenocytes were plated into 24-well plates (Becton Dickinson, Lincoln Park, NJ) at 1 × 107/ml (2 ml/well) in culture medium (RPMI 1640 plus 10% fetal calf serum (FCS), 1% l-glutamine and 1% penicillin–streptomycin). Cells were cultured alone or with 250 HAU/ml of UV-inactivated PR8 for 24 h at 37°C in humidified 5% CO2 in air.
Detection of cytokine-producing cells by intracellular immunofluorescence staining
The frequency of cytokine-producing CD4+ and CD8+ T cells was determined as described [14]. In brief, cultured cells were fixed in 4% paraformaldehyde for 20 min, resuspended in PBS containing 0.5% bovine serum albumin (BSA) and 0.1% NaN3 and aliquoted for staining at 1 × 106 cells. Then Cy-chrome conjugated rat anti-mouse CD4 MoAb (clone RM4-5) or rat anti-mouse CD8 MoAb (clone 53-6.7) was added (30 min at 4°C). Cells were resuspended in PBS containing 0.5% BSA, 0.1% NaN3 and 0.3% saponin (Sigma, St Louis, MO) for staining with rat anti-mouse cytokine MoAbs (0.25–1 mg MoAb/106 cells). The following MoAbs, all purchased from PharMingen (San Diego, CA), were used: FITC anti-IL-2 (clone JES6-5H4), PE anti-IL4 (clone 11B11), PE anti-IFN-γ (clone XGM1.2) and FITC anti-IFN-γ (clone XGM1.2). As negative controls, aliquots of cell suspensions were incubated with an irrelevant isotype-matched MoAb conjugated to the same fluorochrome as the sample. The cytofluorometric analysis was carried out using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA). Ten thousand cells were computed in list mode and analysed using the FACScan Research Software (Becton Dickinson). List mode files were analysed for cytokine production by first defining an analysis gate on anti-CD4+ or anti-CD8+ T cells. Statistical markers were set using the irrelevant isotype-matched controls as reference. Typically, 1% positive cells were allowed beyond the statistical markers in these controls. Earlier results [14,15] showed presence of no significant cytokine immunofluorescence in fixed but not saponin-permeabilized cells.
Quantification of cytokines in culture supernatants
Concentrations of IL-2, IL-4 and IFN-γ in the culture supernatants were measured by two-side sandwich ELISA as described earlier [5], using commercial reagents under conditions recommended by the manufacturer (PharMingen).
Statistical analysis
Statistical significance was determined using Student's t-test.
RESULTS
Effect of cocaine on the expression of IL-2, IL-4 and IFN-γ by individual CD4+ and CD8+ T cells
Mice immunized with PR8 were given cocaine (10 mg/kg body weight) by i.p. injection or saline 6, 24, 48 and 72 h after immunization. Spleen cells from individual cocaine-treated or untreated animals were cultured for 24 h in the presence of UV-inactivated PR8. As a control, cultures of naive spleen cells stimulated with PR8 and immune cells lacking the inactivated virus were established. The effect of the treatment was examined by assessing the frequencies of CD4+ and CD8+ T cells expressing IL-2, IL-4 or IFN-γ as determined by two-colour flow cytometric procedure, fixed and saponin-permeabilized cells fluorescent stained for the CD4 or CD8 surface molecule, and for one of the three intracytoplasmic cytokines. Despite the lack of an amplification step, such as the presence of brefeldin A or monensin during in vitro restimulation, we were able to detect, by using appropriate isotype controls, significant percentages of cytokine-positive cells in all PR8-stimulated samples. The cut-off point between positively and negatively stained cells was set at 1% of isotype controls.
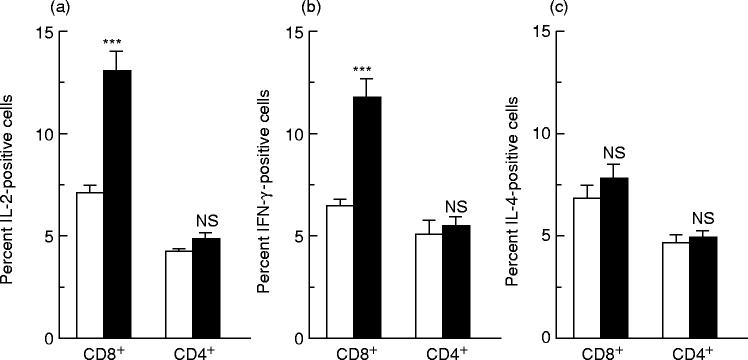
As demonstrated earlier [14], both CD4+ and CD8+ PR8-immune T cells produced IL-2, IL-4 and IFN-γ upon restimulation in vitro with virus (Fig. 1). The analysis of the frequency of the cells producing the single cytokine showed that the percentage of IL-2-, IL-4- and IFN-γ-producing cells, within the two subsets of cells, was essentially the same. Cocaine treatment induced a statistically significant increase versus untreated controls of the number of individual CD8+ T cells expressing IL-2 (Fig. 1a) or IFN-γ (Fig. 1b), while the frequency of IL-4-expressing CD8+ T cells remained unchanged (Fig. 1c). In contrast, no statistically significant differences in the frequencies of splenic CD4+ T cells expressing any one of the three cytokines were found between cocaine-treated and untreated mice (Fig. 1). No significant numbers of cytokine-producing cells were observed in control cultures of naive spleen cells stimulated with PR8 and of immune cells lacking the inactivated virus (data not shown).
Fig. 1.
Effects of cocaine administration to influenza virus-immunized mice on the frequency of IL-2-, IL-4- or IFN-γ-expressing CD8+ and CD4+ T cells, as determined by two-colour flow cytometry analysis. The percentage of cytokine-producing cells was evaluated on in vitro PR8-restimulated spleen cells derived from PR8-immunized animals treated (▪) or not (□) with cocaine. All the samples were analysed for their CD4 or CD8 surface antigen expression versus intracellular IL-2 (a), IFN-γ (b) and IL-4 (c) expression. Samples were analysed by first gating on the CD4+ or CD8+ population. The threshold for detection of positive cytokine-producing cells was set at 1% above the negative control consisting of cells treated with an isotype-specific antibody. Results represent the mean + s.e.m. for each group. The spleen cells from 19 and 20 individual mice were used for the control and the cocaine-treated group, respectively. Statistical analysis (Student's t-test) was performed comparing cocaine-treated animals with untreated animals. ***P < 0.001. NS, Not significant.
Effects of cocaine on cytokine co-expression pattern of individual CD8+ T cells
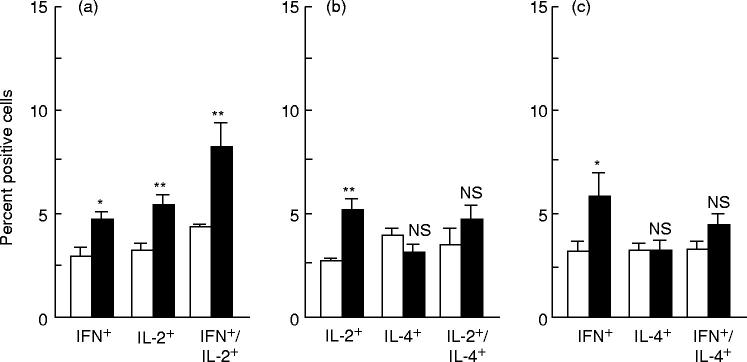
The findings indicate that cocaine treatment affected the antigen-specific induced cytokine production exclusively in the CD8+ T cell subset. To analyse further the in vivo effects of cocaine on this cell population, we determined the co-expression of cytokines in splenic CD8+ T cells obtained from individual PR8-immune mice treated as above. The frequencies of CD8+ T cells singly or co-expressing IL-2, IL-4 and IFN-γ were determined by analysing, using three-colour flow cytometry, fixed and saponin-permeabilized cells fluorescent stained for the CD8 surface molecule and for one of the following combinations of two intracytoplasmic cytokines: IL-2/IL-4, IL-2/IFN-γ and IL-4/IFN-γ. In agreement with our previous results [14], PR8 restimulation of immune spleen cells resulted in the activation of distinct CD8+ T cell subsets expressing either IL-2, IL-4 and IFN-γ singly or co-expressing IL-4/IL-2, IL-4/IFN-γ and IL-2/IFN-γ (Fig. 2) Cocaine treatment induced a statistically significant increase, versus untreated controls, of CD8+ T cell subsets singly expressing IL-2 and IFN-γ, without affecting cells singly expressing IL-4. Also we found that cocaine induced a significant increase of CD8+ T cells co-expressing IL-2 and IFN-γ (Fig. 2a), whereas the percentages of CD8+ T cells double-positive for IL-2/IL-4 and IFN-γ/IL-4 (Fig. 2b,c, respectively) remained unchanged.
Fig. 2.
Effects of cocaine administration to influenza virus-immunized mice on the frequency of CD8+ T cells expressing one or more of the cytokines IL-4, IL-2 and IFN-γ, as determined by three-colour flow cytometry analysis. The percentage of cytokine-producing cells was evaluated on in vitro PR8-restimulated spleen cells derived from PR8-immunized animals treated (▪) or not (□) with cocaine. All the samples were analysed for single and combinatorial (IFN-γ/IL-2 (a), IL-2/IL-4 (b) and IFN-γ/IL-4 (c)) cytokine expression. Samples were analysed by first gating on the CD8+ population. The threshold for detection of positive cytokine-producing cells was set at 1% above the negative control consisting of cells treated with an isotype-specific antibody. Results represent the mean + s.e.m. for each group. The spleen cells from six and eight individual mice were used for the control and the cocaine-treated group, respectively. Statistical analysis (Student's t-test) was performed comparing cocaine-treated animals with untreated animals. *P < 0.05; **P < 0.01. NS, Not significant.
Effect of cocaine on cytokine secretion by spleen cells
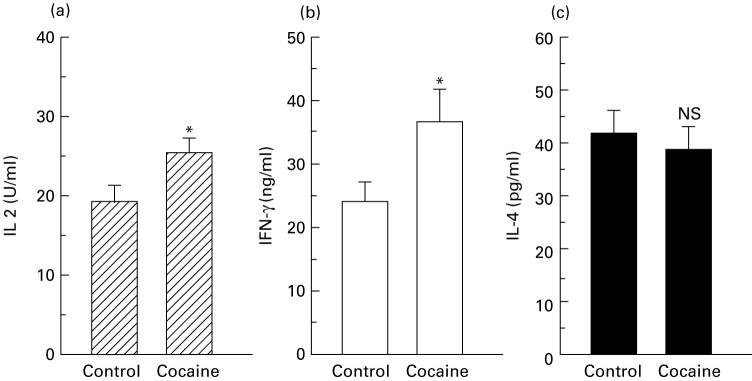
To evaluate the effects of in vivo cocaine administration on cytokine secretion, the levels of IL-2, IL-4 and IFN-γ were measured by ELISA in the supernatants of the same spleen cell samples tested for intracellular cytokines. The results (Fig. 3) showed a statistically significant increase in the titres of IL-2 and IFN-γ in the supernatants of PR8-restimulated spleen cells obtained from cocaine-treated animals, when compared with untreated animals. In contrast, the titre of IL-4 remained unchanged, thus confirming the results obtained by determination of cytokine phenotypes of individual cells.
Fig. 3.
Effect of cocaine administration to influenza virus-immunized mice on in vitro production of cytokines by spleen cells. The levels of IL-2 (a), IFN-γ (b), and IL-4 (c) were determined by ELISA in culture supernatants of PR8-restimulated spleen cells derived from cocaine-treated or untreated PR8-immunized animals. Results represent the mean + s.e.m. for each group. The spleen cells from 12 individual mice were used for each group. Statistical analysis (Student's t-test) was performed comparing cocaine-treated animals with control animals. *P < 0.05. NS, Not significant.
Effect of cocaine on CD4+ and CD8+ spleen T cell frequency
The immunophenotypic analysis of splenic T cells did not show significant changes in the proportion of either CD4+ or CD8+ T cells between cocaine-treated and untreated influenza virus-immune mice (Fig. 4).
Fig. 4.
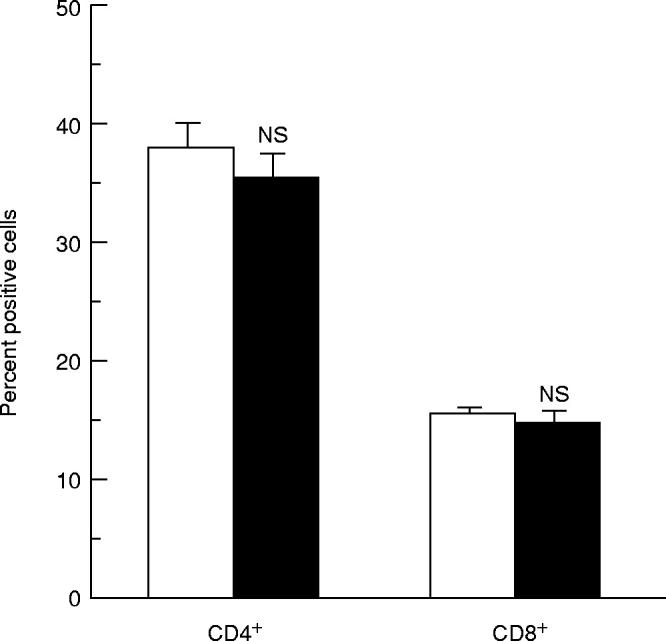
Immunophenotypic analysis of splenic T cells derived from PR8-immunized animals treated (▪) or not (□) with cocaine. The immunophenotypes were determined by FACS analysis and expressed as percentages of total lymphocytes. All the samples were analysed for both CD4+ and CD8+ expression. Results represent the mean values + s.e.m. for each group. The spleen cells from 12 individual mice were used for each group. Statistical analysis (Student's t-test) was performed comparing cocaine-treated animals with untreated animals. NS, Not significant.
DISCUSSION
In the present study we analysed the effects of repeated administrations of cocaine in mice at 6, 24, 48 and 72 h after immunization with PR8 influenza virus on the frequency of IL-2-, IL-4- and IFN-γ-expressing CD4+ and CD8+ T cells. We found that under these conditions of exposure, cocaine induced an increase, versus untreated controls, of the frequencies of virus-restimulated immune splenic CD8+ T cells singly or co-expressing IL-2 and IFN-γ, while the frequency of CD8+ T cells either singly expressing IL-4 or co-expressing this cytokine in combination with IL-2 or IFN-γ remained unchanged. In contrast, no effects were found on the frequencies of CD4+ T cells expressing any one of the three cytokines. We also observed a significant increase, in comparison with untreated animals, of the titres of IL-2 and IFN-γ, but not of IL-4, as determined by ELISA in the supernatants of PR8-restimulated spleen cells obtained from cocaine-treated animals. Thus, our data indicate that cocaine administration specifically up-regulates type 1 cytokine production by the CD8+ T cell subset.
Our results differ from previous reports, some of which have been contradictory. In fact, it has earlier been reported that acute (1 mg/kg) and subchronic (1 mg/kg per day for 7 consecutive days) cocaine administration to male BALB/c mice reduced IL-2, IFN-γ and IL-4 production by mitogen-stimulated spleen cells [6], while chronic administration (40 mg/kg per day for 6 weeks) to female C57Bl/6 mice enhanced the production by Con A-stimulated splenocytes of IL-2 and IL-5, and inhibited the production of IL-4 and IL-10, whereas IFN-γ was not affected [3]. Finally, acute cocaine administration (30 mg/kg) to female B6C3F1 mice induced an increase of IL-4 and IL-10 production in anti-CD3-stimulated spleen cells while IL-2 and IFN-γ production was unaffected [7]. Whether the different effects are due to the experimental conditions used or reflect the complexity of the pharmacological action of cocaine remains to be determined. However, our experimental model has at least three features which support the ‘physiological’ relevance of the results obtained. First, to generate cytokine-producing T cells we used a naturally occurring virus-specific stimulus rather than polyclonal stimuli like Con A or anti-CD3 which are known to induce different patterns of cytokine production [16,17]. Second, regarding the schedule of cocaine administration, each animal was injected with the drug 6, 24, 48 and 72 h after immunization with influenza virus. This allowed the determination of the in vivo effects of cocaine on T cells during the initial step of the immune response, i.e. at the time of the antigen (virus)-induced differentiation of naive T cells into ‘effector’ T cells, which are characterized by the capacity to produce very large amounts of immunoregulatory cytokines upon antigen restimulation [18,19]. In addition, we chose a cocaine dose (10 mg/kg) that did not significantly alter the percentage of both CD4+ and CD8+ T cells, even when administered for 30 consecutive days [13]. Third, the effects of cocaine on the cytokine profiles of the single CD4+ and CD8+ T cells were determined in bulk cultures of virus-immune spleen cells restimulated with PR8. This provided conditions for possible cross-regulatory activities by the different T cell subsets through the cytokines produced by these cells.
The mechanisms by which cocaine may selectively act on CD8+ T cells, at least in the present experimental system, remain unclear. However, we have earlier reported [14] that primary immunization with influenza virus induces heterogeneous patterns of cytokine response in both CD4+ and CD8+ T cells, but with substantial differences between these two populations. In fact, immune CD4+ T cells were seen to express almost exclusively a single cytokine per cell, whereas immune CD8+ T cells were found to express either a single cytokine or co-express combinations (i.e. IL-4/IL-2, IL-4/IFN-γ and IL-2/IFN-γ). As the frequencies of CD8+ T cells co-expressing different cytokines were not statistically different, we suggested these cells might belong to a single subset of type 0 cells co-expressing all three cytokines; therefore, influenza virus-immune CD8+ T cells can express, unlike the CD4+ T cells, a type 0 phenotype at the single-cell level. This may explain the different effects of cocaine on the two T cell subsets. In fact, type 0 cells represent a subset of cells that exhibit an unrestricted cytokine profile and that, unlike cells already committed to either type 1 or type 2 phenotype [9], can be induced to acquire a specific cytokine profile under the influence of different immunological, hormonal and environmental factors [9,20,21]. The finding that cocaine increased the percentage of cytokine-positive CD8+ T cells while the total percentage of CD8+ T cells in the spleen remained unchanged, supports the hypothesis that cocaine may act by specifically altering the pattern of cytokine production in a subset of precursor CD8+ T cells, rather than by altering their absolute number or distribution. Furthermore, we can reasonably exclude that the differences in the effect of cocaine on the two T cell subsets could depend on differences in experimental conditions. In fact, the cytokine profiles of both the T cell populations were evaluated in spleen cells obtained from the same animal, under similar conditions of activation, at the same time after restimulation with influenza virus, in the presence of the same complexity of immunocompetent cells and at comparable assay sensitivity.
Whether the up-regulation of IL-2 and IFN-γ expression by CD8+ T cells may be related to the immunopathogenic mechanism responsible, either partially or completely, for cocaine-induced immune dysfunctions remains to be determined. However, recent studies [20–22] have shown that CD8+ T cells can be divided into two distinct subsets that secrete type 1 or type 2 patterns of cytokines with different physiological roles. In particular, type 1 CD8+ T cells, which produce IL-2 and IFN-γ, can inhibit type 2 (antibody) responses [20]. Thus, the inhibitory effects of cocaine, e.g. on B lymphocyte response to lipopolysaccharide (LPS) and antibody response to sheep erythrocytes [23–26], could be caused by the increase of IL-2- and IFN-γ-expressing CD8+ cells.
Our findings demonstrating that CD8+ T cells are the target (or one of the targets) of immune effects of cocaine are in agreement with earlier data suggesting that cocaine can induce an increased susceptibility to infections [1]. Indeed, CD8+ T cells play a central role either in anti-viral immunity [27,28] or in many bacterial and protozoan infections [29]. Recent studies have also clearly established that these cells, in addition to their cytotoxic activity against virally infected cells, can exert, through differential cytokine production, a regulatory role on differentiation and activation of other immune cells involved in the anti-viral response [10,22,28,30]. Thus, the fact that cocaine induces a dysregulation of CD8+ T cell cytokine production suggests that by this mechanism the drug can affect the immune response to infections, and may also explain the data in humans suggesting an increased risk of infection, including HIV infection, among drug users [31–33].
In conclusion, we demonstrated that cocaine administered during the in vivo differentiation of effector T cells stimulated by antigen (influenza virus) increased both the frequency of CD8+ T cells singly or co-expressing IL-2 and IFN-γ and the titres of these cytokines in virus-restimulated spleen cell culture supernatants. In contrast, no effect was found either on IL-4-positive CD8+ T cells or on IL-2-, IFN-γ- and IL-4-positive CD4+ T cells. Our findings suggest that the immunomodulatory effect of cocaine may be due to the up-regulation of the production of IL-2 and IFN-γ in CD8+ T cells of type 0 cytokine profile.
Acknowledgments
This work was supported by the Italian National Research Council (CNR 97.02455, PS 04, 97.04808 ST74 and Target Project on Biotechnology 97.01093, PF49) and by grant from MURST 60%.
REFERENCES
- 1.Pellegrino T, Bayer MB. In vivo effects of cocaine on immune cell function. J Neuroimmunol. 1998;83:139–47. doi: 10.1016/s0165-5728(97)00230-0. [DOI] [PubMed] [Google Scholar]
- 2.Watzl B, Chen G, Scuderi P, Pirozhkov S, Watson RR. Cocaine-induced suppression of interferon-gamma secretion in leukocytes from young and old C57BL/6 mice. Int J Immunopharmacol. 1992;14:1125–31. doi: 10.1016/0192-0561(92)90158-h. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Huang DS, Watson RR. In vitro and in vivo cocaine modulation on production of cytokine in C57BL/6 mice. Life Sci. 1994;54:401–11. doi: 10.1016/0024-3205(94)00698-9. [DOI] [PubMed] [Google Scholar]
- 4.Mao JT, Huang M, Wang J, Sharma S, Tashkin DP, Dubinett SM. Cocaine down-regulates IL2-induced peripheral blood lymphocytes IL8 and IFN-γ production. Cell Immunol. 1996;172:217–23. doi: 10.1006/cimm.1996.0235. [DOI] [PubMed] [Google Scholar]
- 5.Falchetti R, Di Francesco P, Lanzilli G, Gaziano R, Casalinuovo IA, Ravagnan G, Garaci E. In vitro effects of cocaine on cytokine secretion induced in murine splenic CD4+ T cells by antigen-specific stimulation. Cell Immunol. 1995;164:57–64. doi: 10.1006/cimm.1995.1142. [DOI] [PubMed] [Google Scholar]
- 6.Di Francesco P, Marini S, Pica F, Cartesio F, Tubaro E, Garaci E. In vivo cocaine administration influences lymphokine production and humoral immune response. Immunol Res. 1992;11:74–79. doi: 10.1007/BF02918610. [DOI] [PubMed] [Google Scholar]
- 7.Stanulis ED, Jordan SD, Rosecrans JA, Holsapple MP. Disruption of Th1/Th2 cytokine balance by cocaine is mediated by corticosterone. Immunopharmacology. 1997;37:25–33. doi: 10.1016/s0162-3109(96)00167-1. [DOI] [PubMed] [Google Scholar]
- 8.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 9.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 10.Carter LL, Dutton RW. Type 1 and type 2: a fundamental dichotomy for all T cell subsets. Curr Opin Immunol. 1996;8:336–42. doi: 10.1016/s0952-7915(96)80122-1. [DOI] [PubMed] [Google Scholar]
- 11.McHugh S, Deighton J, Rifkin I, Ewan P. Kinetics and functional implications of Th1 and Th2 cytokine production following activation of peripheral blood mononuclear cells in primary culture. Eur J Immunol. 1996;26:1260–5. doi: 10.1002/eji.1830260612. [DOI] [PubMed] [Google Scholar]
- 12.Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–57. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 13.Falchetti R, Di Francesco P, Lanzilli G, Gaziano R, Casalinuovo IA, Palamara AT, Ravagnan G, Garaci E. Determination of cytokine coexpression in individual splenic CD4+ and CD8+ T cells from influenza virus-immune mice. Immunology. 1998;95:346–51. doi: 10.1046/j.1365-2567.1998.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Francesco P, Falchetti R, Gaziano R, Lanzilli G, Belogi L, Ravagnan G, Garaci E. Differential effects of short-term or prolonged cocaine exposure on peripheral blood cells in mice. Life Sci. 1994;54:2015–20. doi: 10.1016/0024-3205(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 15.Assenmacher M, Schmitz J, Radbruck A. Flow cytometric determination of cytokines in activated murine T helper lymphocytes: expression of interleukin-10 in interferon-γ and in interleukin-4-expressing cells. Eur J Immunol. 1994;24:1097–101. doi: 10.1002/eji.1830240513. [DOI] [PubMed] [Google Scholar]
- 16.ElGhazali GEB, Paulie S, Andersson G, et al. Number of interleukin-4- and interferon-gamma-secreting human T cells reactive with tetanus toxoid and the mycobacterial antigen PPD or phytohemagglutinin: distinct response profiles depending on the type of antigen used for activation. Eur J Immunol. 1993;11:2740–5. doi: 10.1002/eji.1830231103. [DOI] [PubMed] [Google Scholar]
- 17.Sander B, Cardell S, Moller G, Moller E. Differential regulation of lymphokine production in mitogen-stimulated murine spleen cells. Eur J Immunol. 1991;8:1887–92. doi: 10.1002/eji.1830210816. [DOI] [PubMed] [Google Scholar]
- 18.Swain SL. Who does the polarizing? Curr Biol. 1995;5:849–51. doi: 10.1016/s0960-9822(95)00170-9. [DOI] [PubMed] [Google Scholar]
- 19.Swain SL, Croft M, Dubey C, Haynes L, Rogers P, Zhang X, Bradley LM. From naive to memory T cells. Immunol Rev. 1996;150:143–67. doi: 10.1111/j.1600-065x.1996.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 20.Sad S, Marcotte R, Mosmann T. Cytokine induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271–9. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 21.O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–83. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 22.Croft M, Carter L, Swain SL, Dutton RV. Generation of polarized antigen specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994;180:1715–28. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watzel B, Watson RR. Immunomodulation by cocaine—a neuroendocrine mediated response. Life Sci. 1990;46:1319–29. doi: 10.1016/0024-3205(90)90331-k. [DOI] [PubMed] [Google Scholar]
- 24.Biron CA. Cytokines in the generation of immune responses to, and resolution of, virus infection. Curr Opin Immunol. 1994;6:530–8. doi: 10.1016/0952-7915(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 25.Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+ T cells during acute virus infection. Immunity. 1998;8:167–75. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott P, Kaufmann SHE. The role of T-cell subsets and cytokines in the regulation of infection. Immunol Today. 1991;10:346–8. doi: 10.1016/0167-5699(91)90063-Y. [DOI] [PubMed] [Google Scholar]
- 27.Srikiatkhachorn A, Braciale TJ. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J Exp Med. 1997;186:421–32. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaisson RE, Bacchetti P, Osmond P, Brodie B, Sande MA, Moss AR. Cocaine use and HIV infection in intravenous drug users in San Francisco. JAMA. 1989;261:561–5. [PubMed] [Google Scholar]
- 29.Anthony JC, Vlahov D, Nelson KE, Cohn S, Astemborki J, Solomon L. New evidence on intravenous cocaine use and the risk of infection with human immunodeficiency virus type 1. Am J Epidemiol. 1991;134:1175–89. doi: 10.1093/oxfordjournals.aje.a116021. [DOI] [PubMed] [Google Scholar]
- 30.Baldwin GC, Roth MD, Tashkin DP. Acute and chronic effects of cocaine on the immune system and the possible link to AIDS. J Neuroimmunol. 1998;83:133–8. doi: 10.1016/s0165-5728(97)00229-4. [DOI] [PubMed] [Google Scholar]