Abstract
To investigate the spontaneous turning off mechanism of endogenous uveitis, EAAU was induced in Lewis rats. Immunohistochemical and terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) stains revealed that CD4+ T cells were predominant in the uveal tissue of EAAU and that the apoptosis of these cells had occurred and progressed throughout the inflammatory period in EAAU eyes. The immunohistochemistry and in situ hybridization for Fas ligand (FasL) expression showed that the expression of Fas ligand was increased in the EAAU eyes compared with control eyes. These results suggest that the apoptosis of CD4+ T cells may play a key role in the spontaneous turning off mechanism of intra-ocular inflammation and that the induction of apoptosis may be mediated by the Fas–FasL system in EAAU.
Keywords: experimental autoimmune anterior uveitis, apoptosis, FasL, CD4+ T cell
INTRODUCTION
Uveitis is a major cause of visual impairment, affecting approximately 10% of visually handicapped patients. The most frequent form of human uveitis is the acute anterior uveitis of unknown aetiology, accounting for approximately 75% of all uveitis cases. The aetiology of endogenous uveitis has been thought to be of autoimmune origin [1]. Most cases of acute uveitis regress spontaneously, but some cases develop into chronic relapsing uveitis to cause permanent visual loss. Therefore, if the regression could be maintained the visual loss from endogenous uveitis would be prevented. However, the mechanism of the spontaneous regression of this endogenous inflammation is not yet understood.
The termination of the intra-ocular inflammation occurs after the disappearance of the infiltrating inflammatory cells. These cells in the eye can disappear in situ or in other sites. Recently, an apoptotic pathway has been shown to play a key role in the regression of experimental autoimmune disease of the central nervous system [2,3]. The eye is an immune-privileged organ like the central nervous system, and the expression of Fas, which is one of the most well-known mediators of apoptosis, is reported to be increased in patients of posterior uveitis [4]. To investigate the spontaneous turning off mechanism of endogenous uveitis, the present study was designed to determine the types of infiltrating cells, the apoptotic incidence of these cells, and whether the expression of Fas ligand (FasL) increased in EAAU using Lewis rats.
MATERIALS AND METHODS
Animals
For the experiment male Lewis rats (Charles River Japan, Yokohama, Japan), 6–8 weeks old and weighing 125–160 g, were used. A total of 50 animals was used for the experiments. The rats were anaesthetized intraperitoneally with xylazine (10 mg/kg) and ketamine hydrochloride (20 mg/kg). All experiments were conducted in accordance with the National Institutes of Health Guiding Principles in the Care and Use of Animals and the guidelines established by the Declaration of Helsinki.
Antigen and induction of EAAU
Melanin-associated antigen (MAA) was prepared as previously described by Bora et al. [5] with a modification. The preparation was as follows. The iris and ciliary body were carefully excised from fresh pigmented bovine eyes. The tissue was homogenized gently and filtered through a wire mesh to remove cellular debris and connective tissues. The homogenate was centrifuged at 1.2 × 105 g at 4°C for 15 min and washed once with PBS pH 7.4. The resulting pellet was resuspended in 2% SDS and incubated at 70°C for 10 min. After centrifugation the pellet was washed three times with water. The insoluble antigen was vacuum dried and stored at −20°C.
Forty-six rats were immunized with 100 μg of MAA, and four animals were used as a control. The antigen was suspended in PBS, emulsified (1:1) in Freund's complete adjuvant (FCA) and injected into the left hind footpad in a volume of 100 μl. The emulsion also contained Bordetella pertussis toxin (Sigma, St Louis, MO; 1 μg/animal).
Clinical examination
The rats were observed daily with slit-lamp biomicroscopy for clinical signs of ocular inflammation. Prior to slit-lamp examination, the rats were anaesthetized using inhalant ether. Disease severity was clinically assessed employing a scale ranging from 0 to 4 [6]. The ranges were: 0 = normal; 1 = slight iris vessel dilation and some anterior chamber cells; 2 = iris hyperaemia, some limitation in pupil dilation, anterior chamber cells and slight flare; 3 = miotic, irregular, hyperaemic and sometimes slightly damaged iris, considerable flare and cells, especially accumulating near the iris; 4 = seriously damaged and hyperaemic iris, miotic pupil often filled with protein, and cloudy gel-like aqueous humor.
Histology
Twenty-four rats were enucleated at 7, 10, 12, 14, 16, 18, 19, 21, 23, 25, 28 and 30 days after immunization, and two control rats were killed for normal histology and immunohistochemistry.
Eyes were placed in OCT compound (Tissue Tek; Miles, Elkhart, IN), snap-frozen and sectioned with a cryostat as 8 μm thick sections. The tissue was air-dried overnight and fixed in 4% formaldehyde for 10 min. The sections were stained with conventional haematoxylin and eosin. Disease was graded in a masked fashion using the scores described previously [7]. The scores used were: 0 = normal; 1 = dilated iris vessel and thickened iris stroma, exudate in the anterior chamber with protein and/or a few scatted inflammatory cells, or both; 2 = moderate infiltration of inflammatory cells in the stroma of the iris, ciliary body or the anterior chamber; 3 = heavy infiltration of inflammatory cells within the iris stroma and ciliary body or the anterior chamber; 4 = heavy exudation of cells with dense protein aggregation in the anterior chamber and inflammatory cell deposits on the corneal endothelium.
Immunohistochemistry
For immunohistochemical analysis, the sections were fixed with cold acetone for 10 min. After washing in 0.01 m PBS pH 7.4, which was used for all washes, the slides were then incubated with a mouse anti-rat CD3, CD4, CD8 monoclonal IgG (PharMingen, San Diego, CA) or a goat anti-rat FasL polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at a dilution of 1:70 for 1 h. All incubations were conducted at room temperature. The slides were next washed for 5 min three times with PBS. Following incubation with a secondary biotinylated rabbit anti-mouse IgG (CD3, CD4, CD8; Dako, Carpenteria, CA) or anti-goat immunoglobulin (FasL; Dako) at a dilution of 1:200 for 30 min, the slides were washed again. Sections were then incubated for 30 min with alkaline phosphatase-conjugated streptavidin (Dako) at a dilution of 1:100. Sections were then incubated for 5–10 min with an alkaline phosphatase substrate solution (fast red; Sigma).
CD4/TUNEL double-staining
Cell death was detected in situ in a cryosection by enzymatic labelling of DNA strand breaks using terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL; Oncor, Gaithersburg, MD) according to the manufacturer's instructions. CD4/TUNEL dual staining was performed to allow the apoptotic eye-infiltrating CD4+ T cells to be identified and enumerated. CD4 staining was performed first on cryosections, as described. Following fixation in 1% paraformaldehyde, post-fixation was done in ethanol:acetic acid (2:1) for 5 min at −20°C. Sections were washed, and endogenous peroxidase activity was blocked in 3% hydrogen peroxide in PBS for 30 min. Next, the slides were washed with PBS. This and all subsequent washes were in PBS (0.05 m, pH 7.4). After washing, the labelling reaction was performed using a solution containing terminal deoxynucleotidyl transferase, its buffer, and digoxygenin (DIG)-dUTP. During this step, the slides were coverslipped and incubated at 37°C for 60 min in a humidity chamber. Terminal deoxynucleotidyl transferase was omitted from negative control slides, which were included in each run. To localize the cells containing labelled DNA strands, sections were washed and incubated with an anti-DIG conjugated with horseradish peroxidase at room temperature in a humidity chamber for 30 min. Following washing, TUNEL-positive colour development was obtained by incubating the section with 0.05% diaminobenzidine substrate solution for 10 min. When viewed under light microscopy, the CD4 single-positive cells stained red, while CD4/TUNEL double-positive cells appeared with black nuclear staining with red cytoplasmic/cell surface staining.
Preparation of cells from iris-ciliary body and anterior chamber
Twelve immunized rats were used for flow cytometric analysis and enucleated at 14, 17, 19, 21, 23 and 25 days after the injection of the antigen. The eyes were removed from the rats and placed in RPMI medium containing 10% fetal bovine serum (FBS) and 1 mg/ml collagenase. The anterior portion of an eye was microdissected to obtain the iris-ciliary body, and the remaining portion of the eye was removed. After 45 min incubation at 37°C, a single-cell suspension was prepared by pipetting followed by filtering through a nylon filter. Mononuclear cells were isolated using Ficoll–Paque Plus (Pharmacia Biotech, Uppsala, Sweden). Cells were washed twice by centrifugation and resuspended in RPMI containing 10% FBS.
Flow cytometric analysis
The cells were stained with FITC-conjugated MoAb specific for CD3, CD4 and CD8. Cells (106 cells/ml) were incubated with the antibody for 30 min in the dark at 4°C in PBS containing 1% bovine serum albumin (BSA). Cells were washed in the same buffer.
Apoptotic cells were quantified using a combination of propidium iodide (PI) and FITC-labelled annexin V (both from PharMingen). Cells for annexin V and PI staining were washed twice with cold PBS and resuspended in binding buffer at a concentration of 106 cells/ml. One hundred microlitres of suspension (105 cells) were transferred to a tube and 2 μl of FITC-conjugated annexin V and 5 μl PI were added. The cells were incubated for 15 min in the dark at room temperature, and then binding buffer was added. These samples were used to evaluate the numbers of early apoptotic cells (annexin-labelled) and late apoptotic cells labelled with annexin and PI. Becton Dickinson FACS analyser (San Jose, CA) was used for flow cytometry.
Generation of a FasL-specific RNA probe (riboprobe)
A DIG-labelled RNA hybridization probe was synthesized by in vitro transcription using DIG-11-UTP and T7 RNA polymerase (Boehringer Mannheim, Mannheim, Germany). Template for the in vitro transcription reaction was generated by the polymerase chain reaction (PCR) amplification of a fragment of rat FasL cDNA using a proofreading thermostable polymerase (UlTma DNA polymerase; Perkin-Elmer, Norwalk, CT) and an anti-sense primer, to which a T7 promoter sequence was added.
RNA was isolated from 100 mg of testis of Lewis rat by homogenization in guanidine thiocyanate (Sigma), followed by phenol extraction and ethanol precipitation. cDNA was synthesized using the AMV reverse transcriptase (Promega, Madison, WI) and random hexanucleotide primers (Boehringer Mannheim).
PCR was performed on the cDNA using the following sense and anti-sense primers, respectively: TGCCTCCACTAAGCCCTCTA and ATTCCAGAGGGATGGACCTT. Primer pairs were chosen to span introns in the FasL genomic sequence, thus, ensuring a mRNA-specific amplification [8]. Primers were selected that showed no significant homology to any other genes in the EMBL DNA sequence database.
Thermal cycling (40 cycles) was as follows: denaturation at 96°C for 15 s, annealing at 55°C for 30 s, and extension at 72°C for 3 min. Primers were used at a final concentration of 0.1 μm each, dNTPs at 50 μm, and MgCl2 at 1.5 mm. One unit of UlTma DNA polymerase was used per 50-μl reaction. PCR product specificity was confirmed by restriction mapping.
The nucleotide sequence of the FasL probe showed no significant homology to any other sequence in the EMBL DNA sequence database. Northern blot was performed for the eye tissue of 14 day EAAU, testes and kidney to confirm that this new riboprobe would bind at 1.7 kb. An unlabelled riboprobe was also synthesized for competitive control hybridization.
Localization of FasL mRNA expression by in situ hybridization
Twelve animals were used for the localization of FasL mRNA. Ten animals were enucleated at 7, 13, 19, 25 and 30 days after immunization and two non-immunized animals were used as a control. In situ hybridization was performed on cryo-embedded eyeball sections (8 μm thick) of Lewis rat that were mounted on aminopropylethoxysilane (APES)-treated slides. Frozen sections were thawed, rehydrated for 10 min in PBS, placed in 0.2 N HCl for 20 min, washed in water, digested with 2 μg/ml of proteinase K (Boehringer Mannheim), and placed in 2 mm CaCl2 and 10 mm Tris–HCl pH 7.4 at 37°C for 10 min. Sections were post-fixed in 3% paraformaldehyde for 5 min, rinsed twice in 2× SSC; acetylated for 10 min in 0.1 m triethanolamine–HCl pH 8.0 containing 0.25% acetic anhydride, rinsed in 2× SSC, incubated in 0.1 m Tris–HCl and 0.1 m glycine pH 7.0 for 30 min, washed twice in 2× SSC, dehydrated via sequential washes in 70%, 80%, 90% and 100% ethanol, and air-dried. Sections were prehybridized for 2 h with a solution containing 50% deionized formamide, 4× SSC, 50 mm sodium phosphate buffer pH 6.5, 0.1% SDS, 1% glycine, l× Denhardt's solution, 200 μg/ml of yeast transfer RNA, and 500 μg/ml of heat-denatured herring sperm DNA (Sigma) at 56°C in a humid environment. Hybridization was performed at 56°C overnight with a solution containing 50% deionized formamide, 4× SSC, 20 mm sodium phosphate buffer pH 6.5, 0.1% SDS, 1× Denhardt's solution, 10% dextran sulphate, 500 μg/ml of heat-denatured herring sperm DNA, and 20 μg/ml of heat-denatured, DIG-labelled probe. After hybridization, tissues were washed with increasing stringency to 0.1× SSC at 37°C. Hybridized probe was detected immunologically using alkaline phosphatase-conjugated sheep anti-DIG antibody (Boehringer Mannheim) and visualized with nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphates (NBT/BCIP; purple/black precipitating product). Control slides involved a competitive inhibition of hybridization by adding a 10-fold excess of unlabelled riboprobe to the DIG-labelled riboprobe before hybridization. This resulted in a marked reduction of signal intensity, thus confirming the specificity of hybridization.
RESULTS
Time course of EAAU
Anterior uveitis was not detected with slit-lamp biomicroscopy prior to day 11 in Lewis rats immunized with MAA. The peak inflammation was noted between days 18 and 21, followed by a resolution lasting up to 28 days. Histologically, between 12 and 14 days after immunization, mononuclear cells started to infiltrate the ciliary body, iris and anterior chamber. At the peak of EAAU, the ciliary body and iris became swollen and heavily infiltrated with inflammatory cells. Most invading inflammatory cells were mononuclear cells. Polymorphonuclear cells were rarely found. Dense protein aggregates and cellular infiltration were present in the anterior chamber during the peak stage of the disease (Fig. 1A). Most eyes which had been observed through the peak stage had a clinical and histological score > 3. Based on these observations, EAAU was subdivided into four stages: preinflammation (days 7–10); onset (days 11–14); active (days 15–21); resolution (days 23–28) according to the sum of the clinical and histological scores.
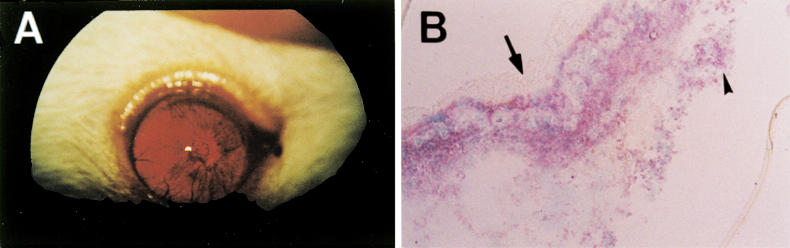
Fig. 1.
The EAAU eye at day 19. (A) Photography, the iris is miotic, irregular and hyperaemic, and the pupil is secluded with cloudy gel-like aqueous humor. (B) Immunohistochemistry for CD4. CD4+ T cells stained red (fast red). The iris vessels are dilated and the iris is infiltrated heavily with CD4+ cells. The anterior surface of the iris is covered with protein aggregation (arrow) and CD4+ T cells in cluster are found in the posterior chamber (arrowhead). Counter stain with methyl green. (Original mag., × 100.)
CD4+ T cells are predominant lymphocytes in the EAAU eye
CD4+ T cells were predominant in the uveal tissue throughout all stages of EAAU (Fig. 1B). Thus, mononuclear cells were separated from the eye and analysed using flow cytometry to phenotype the invading cells. Flow cytometric analysis showed that up to 70% of the cells in the EAAU eye were CD4+, while no more than 10% of CD8− cells was present through the stages of EAAU (Table 1).
Table 1.
Analysis of the types of invading T lymphocytes in the EAAU eye by flow cytometry
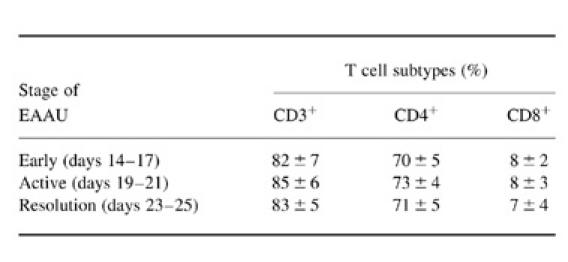
The numbers represent the percentage of the positive cells from the iris and ciliary body of EAAU. Results are expressed as the mean of three different experiments.
Apoptosis occurs in infiltrating lymphocytes in EAAU eyes
Apoptotic cells were found in the EAAU eye with TUNEL stain; these TUNEL+ cells were also positive for CD4; thus, it was concluded that almost all apoptosis in the EAAU eye occurred in infiltrating lymphocytes. The number of apoptotic cells increased in the peak stage of EAAU (days 18–21) (Fig. 2A,B). This increase may account for the fact that the number of infiltrating lymphocytes was relatively high in the peak stage. In the resolution stage the proportion of apoptotic CD4+ T cells was markedly increased while the number of CD4+ T cells was decreased (Fig. 2C,D). Flow cytometric analysis showed that the proportion of apoptotic lymphocytes increased as EAAU progressed. Initially, the proportion of PI+ cells was < 10% in the early active stage of EAAU (days 14–17) and increased to about 30% in the resolution stage (days 23–25) (Fig. 3).
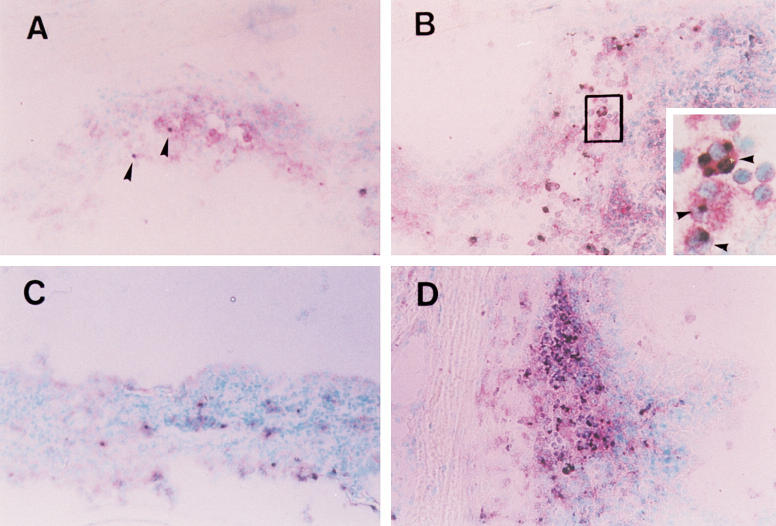
Fig. 2.
Apoptotic depletion of CD4+ T cells in EAAU. CD4+ T cells are stained red in the cytoplasm and cell surface. Terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL)+ cells are stained black. CD4/TUNEL double-positive cells appear with black nuclear staining with red cytoplasmic/cell surface staining. Counterstain was done with methyl green. (A) Only a small proportion of CD4+ T cells is shown TUNEL+ (arrowheads) in the iris of onset stage of EAAU (day 14). (B) The junctional area of ciliary process and iris is infiltrated with CD4+ T cells (red) and many CD4/TUNEL double-positive cells are found in that area of the active stage of EAAU (day 19). The inset shows a magnified rectangular area, which shows double-positive cells (arrowheads). (C,D) In resolution stage (day 23), although CD4+ T cells are decreased compared with those in the EAAU iris in the active stage, the proportion of double-positive cells increases markedly. (Original mag., × 200.)
Fig. 3.
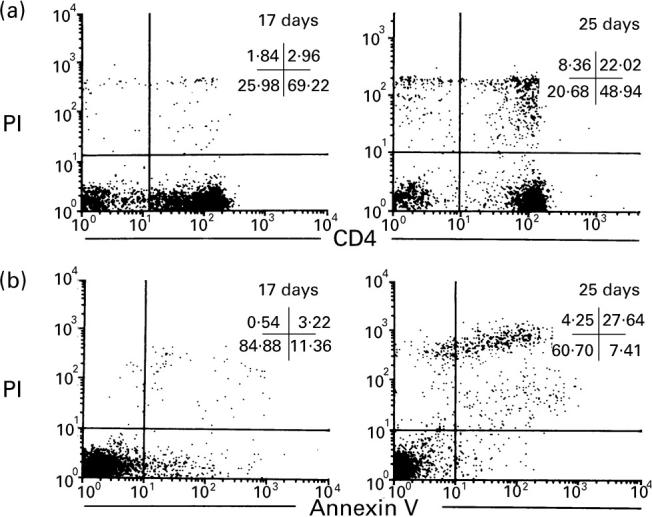
Two-colour flow cytometry of lymphocytes from EAAU eyes. (a) Double-labelling of CD4 and propidium iodide (PI). The percentage of PI+ cells is about 5% among CD4+ cells in the EAAU eye at the early stage (17 days after immunization). The percentage is increased to about 30% in the EAAU eye at resolution stage (25 days after immunization). (b) Double-labelling of annexin V and PI. The early stage apoptotic cells (annexin V+ and PI−) are found in the early stage EAAU (17 days after immunization) and the later stage apoptotic cells (annexin V and PI double-positive) are markedly increased in the later stage of EAAU (25 days after immunization).
Expression of FasL increases in the EAAU eye
FasL was expressed in the eye of the Lewis rat before immunization with MAA. Corneal epithelium and endothelium showed a strong FasL positivity, and aqueous fluid of the anterior chamber also showed a diffused positivity. Retina, iris and aqueous fluid of the anterior chamber were stained diffusely. In the EAAU eye, FasL expression increased in the anterior chamber and iris (Fig. 4A,B). The expression of FasL of iris was higher in the eye at 30 days after immunization compared with the control eye, even though the inflammation of the eye subsided at that time (Fig. 4C). Most invading inflammatory cells in the anterior chamber were stained positively.
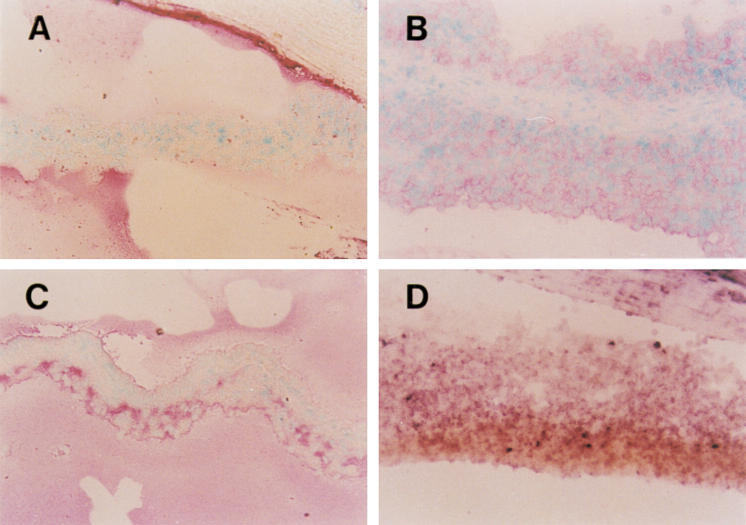
Fig. 4.
Immunohistochemistry (A,B,C) and in situ hybridization (D) for Fas ligand (FasL) in the EAAU iris. (A) Control. The expression of FasL in the iris is weak and soluble FasL is stained red (fast red) in the anterior chamber. Corneal endothelium is stained strongly for FasL. (B) Nineteen days after immunization. EAAU strongly induced FasL expression in the iris. The infiltrating lymphocytes including chamber cells express FasL in the active stage of EAAU. (C) Thirty days after immunization. The expression of FasL in the iris remains increased after the invading cells have completely disappeared. (D) The iris is thickened by the infiltrating lymphocytes 19 days after immunization. Both the iris and the infiltrating lymphocytes express mRNA of FasL at this stage. Counterstain with methyl green. (Original mag., × 200.)
In situ hybridization of FasL mRNA confirmed the results of immunohistochemistry. The expression of FasL mRNA, of which riboprobe was binding to a band of 1.7 kb, increased in the eye tissue, including cornea, iris and ciliary body at the time of EAAU (Fig. 4D). The infiltrating cells including anterior chamber cells also expressed FasL mRNA. The retina, which is not the target of EAAU, also expressed FasL mRNA more than in the control eye.
DISCUSSION
Experimental autoimmune uveitis, which has been induced by soluble retinal proteins such as S-antigen, interphotoreceptor retinoid binding protein, rhodopsin and phosducin [9–12], destroyed retina, infiltrated pineal gland and was primarily confined to the posterior segment of eyes. In contrast, human acute anterior uveitis occurs in the anterior segment of eyes with the posterior segment usually being spared.
Broekhuyse and associates developed an experimental uveitis model in Lewis rats using bovine protein derived from the retinal pigment epithelium [6]. This uveitis has been called experimental autoimmune anterior uveitis, since the inflammation is primarily confined to the anterior segment of the eye. Recently MAA, which is derived from the iris and ciliary body, has been used for the induction of EAAU because it can be more easily extracted [5]. The inflammation of EAAU subsides very quickly, leaving no pathologic changes in the eye compared with experimental uveoretinitis and autoimmune models in other organs [10]. EAAU is similar in these characteristics to human acute anterior uveitis. So, we used EAAU as an experimental model of human endogenous anterior uveitis in this study to investigate the mechanism of its spontaneous regression.
The mechanism of this fast spontaneous termination of EAAU is not yet understood. Little is known about the fate of activated T cells after infiltrating the eye. The activated lymphocytes could be decreased and reach homeostasis via passive deletion or active suppression mechanism by lymphoid organ or target organ, the eye. Lymphocytes that are deprived of survival stimuli, such as costimulators and cytokines, lose expression of anti-apoptotic proteins, mainly of the Bcl family, and die ‘by neglect’ [13]. The activation of lymphocytes in the eye may trigger feedback mechanisms that limit their proliferation and differentiation [14–16].
The eye is an immune-privileged organ like the nervous system and has many immune deviation mechanisms [17] including the presence of immunosuppressive cytokines [18] and neuropeptides [19], strategic localization of phagocytic cells [20], and the ability to induce immune deviation following antigen presentation [21]. Therefore, the eye is supposed to be the active site where the infiltrating inflammatory cells die in EAAU.
We observed that CD4+ T cells were predominant over both the CD8+ T cells and macrophages in the uvea during each stage of EAAU (Fig. 1 and Table 1), which has been reported by Kim et al. [7]. The results of this study showed that apoptosis of infiltrating CD4+ T cells occurred in the EAAU eye (Figs 2 and 3) and the proportion of apoptotic cells increased in the resolution stage of EAAU. Apoptosis (or programmed cell death) is known to play a key role in lymphocyte homeostasis [22,23]. It is also the mechanism for the orderly elimination of unwanted cells during normal development, homeostasis, response to infection, and wound healing in multicellular organisms [24]. Apoptosis, in contrast to necrosis, occurs with minimal collateral damage to surrounding tissues and would provide an ideal non-inflammatory mechanism to terminate the attack of autoimmune T cells in vulnerable tissues like the eye, assuring a minimum of detrimental bystander damage to the local parenchyma. Apoptosis of autoreactive T cells has been shown in autoimmune diseases of the nervous system and other sites with a specialized immune defence mechanism [2,25–28] and recognized to contribute to the preservation of these vulnerable tissues from excessive inflammation. However, it has not yet been demonstrated in the eye of autoimmune uveitis. Our results suggest that in situ apoptosis in the infiltrating lymphocytes of EAAU might play an important role in protecting the eye where the excessive inflammation is fatal to the visual function. However, the increasing proportion of apoptotic CD4+ T cells during the course of experimental uveitis could result from the increased turnover of the cells or just from the fact that they stay around longer and are not destroyed. Therefore, the active mechanism of inducing apoptosis in the EAAU eye should be elucidated in order to confirm that in situ apoptosis plays a major role in the regression of intra-ocular inflammation.
Our results show that the expression of FasL is markedly increased in the active stages of EAAU (Fig. 4), although FasL is expressed in the non-immunized eye (data not shown). The results of in situ hybridization suggest that FasL in the EAAU eye is derived from both the eye tissue and the infiltrating lymphocytes (Fig. 4D). Fas and FasL are two of the best characterized molecules involved in apoptosis induction [23]. The Fas receptor (CD95) and its ligand (FasL, CD95L) are transmembrane proteins of the tumour necrosis factor (TNF) family of receptors and ligands. Engagement of Fas by FasL triggers a cascade of subcellular events that result in apoptosis.
Fas-mediated apoptosis has a functional role in immune homeostasis [29] and may involve the termination of autoimmune disease [2,25–28]. Recently, it has been also demonstrated in vitro and in situ in tumour-infiltrating lymphocytes in melanomas and hepatocellular carcinomas [30,31], and the expression of FasL in the various tumours may contribute to tumour survival and malignancy. Griffith et al. demonstrated that FasL produced by ocular cells could stimulate apoptosis of inflammatory cells expressing Fas [32]. Autoreactive CD4+ T cells have been known to express Fas and FasL for autoregulation [33,34]. The Fas/FasL system in the cornea has been shown to modulate apoptosis of the keratocytes occurring following corneal epithelial injury [35]. Increased expression of Fas and FasL was found in the ocular tissues with uveitis and infiltrating CD4+ cells in the anterior chamber of the eye with autoimmune uveitis [4,36]. These facts imply that the interaction of Fas–FasL may be involved in the spontaneous regression of autoimmune uveitis. Here, the increased expression of FasL in the EAAU eye may be involved in the development of apoptosis of the infiltrating CD4+ T cells, and might play a role in the autoregulation of the self-reactive cells in EAAU. However, in situ apoptosis could develop through many different pathways other than Fas–FasL interaction, and other mechanisms may co-operate to induce in situ apoptosis in the EAAU eye. So further study should be done to confirm that the Fas-mediated apoptosis of infiltrating lymphocytes plays a major role in the spontaneous turning off of EAAU.
In conclusion, apoptosis of infiltrating CD4+ T cells occurred in the EAAU eye, and a significant number of TUNEL+ CD4 cells also expressed FasL. Acute anterior uveitis in humans is expected to regress through similar mechanisms to EAAU, and chronic relapsing uveitis might occur due to a defect in the terminating mechanism of inflammation in the eye. A therapeutic approach to induce selective apoptosis in the autoreactive cells that cause the disease would be helpful in the treatment of chronic uveitis.
REFERENCES
- 1.Nussenblatt RB, Whitcup SM, Palestine AG. 2. St Louis: Mosby Year Book Inc.; 1996. Uveitis: fundamentals and clinical practice. [Google Scholar]
- 2.Schmied M, Breitschopf H, Gold R, Zischler H, Rothe G, Wekerle H, Lassmann H. Apoptosis of T lymphocytes in experimental autoimmune encephalomyelitis. Am J Pathol. 1993;143:445–52. [PMC free article] [PubMed] [Google Scholar]
- 3.Zettle UK, Gold R, Toyka KV, Hartung BP. In situ demonstration of T cell activation and elimination in the peripheral nervous system during experimental autoimmune neuritis in the Lewis rat. Acta Neuropathol. 1996;91:360–7. doi: 10.1007/s004010050437. [DOI] [PubMed] [Google Scholar]
- 4.Chan CC, Dawn M, Matteson MS, Li Q, Whitcup SM, Nussenblatt RB. Apoptosis in patients with posterior uveitis. Arch Ophthalmol. 1997;115:1559–67. doi: 10.1001/archopht.1997.01100160729010. [DOI] [PubMed] [Google Scholar]
- 5.Bora NS, Kim MC, Kabeer NH, et al. Experimental autoimmune anterior uveitis. Induction with melanin-associated antigen from the iris and ciliary body. Invest Ophthalmol Vis Sci. 1995;36:1056–66. [PubMed] [Google Scholar]
- 6.Broekhuyse RM, Kuhlmann ED, Winkens HJ, van Vugt AM. Experimental autoimmune anterior uveitis (EAAU), a new form of experimental uveitis. I. Induction by a detergent-insoluble, intrinsic protein fraction of the retinal pigment epithelium. Exp Eye Res. 1991;52:465–74. doi: 10.1016/0014-4835(91)90044-f. [DOI] [PubMed] [Google Scholar]
- 7.Kim MC, Kabeer NH, Tandhasetti MT, Kaplan HJ, Bora NS. Immunohistochemical studies on melanin associated antigen (MAA) induced experimental autoimmune anterior uveitis (EAAU) Curr Eye Res. 1995;14:703–10. doi: 10.3109/02713689508998498. [DOI] [PubMed] [Google Scholar]
- 8.Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–78. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 9.Gery I, Wiggert B, Redmond TM, Kuwabara T, Crawford MA, Vistica BP, Chader GJ. Uveoretinitis and pinealitis induced by immunization with interphotoreceptor retinoid-binding protein. Invest Ophthalmol Vis Sci. 1986;27:1296–300. [PubMed] [Google Scholar]
- 10.Wacker WB, Donoso LA, Kalsow CM. Experimental allergic uveitis. Isolation, characterization, and localization of a soluble uveitopathogenic antigen from bovine retina. J Immunol. 1977;119:1949–58. [PubMed] [Google Scholar]
- 11.Schalken JJ, Winkens HJ, van Vugt AHM, Bovee-Geurts PH, deGrip WJ, Broekhuyse RM. Rhodopsin-induced experimental autoimmune uveoretinitis: dose-dependent clinicopathological features. Exp Eye Res. 1988;47:135–45. doi: 10.1016/0014-4835(88)90030-9. [DOI] [PubMed] [Google Scholar]
- 12.Dua HS, Lee RH, Lolley RN, Barrett JA, Abrams M, Forrester JV, Donoso LA. Induction of experimental autoimmune uveitis by the retinal photoreceptor cell protein, phosducin. Curr Eye Res. 1992;11:107–11. doi: 10.3109/02713689208999519. [DOI] [PubMed] [Google Scholar]
- 13.Van Parijs L, Abbas AK. Role of Fas-mediated cell death in the regulation of immune responses. Curr Opin Immunol. 1996;8:355–61. doi: 10.1016/s0952-7915(96)80125-7. [DOI] [PubMed] [Google Scholar]
- 14.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–50. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 15.Cleveland JL, Ihle JN. Contenders in FasL/TNF death signaling. Cell. 1995;81:479–82. doi: 10.1016/0092-8674(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 16.Van Parijs L, Peterson DA, Abbas AK. The Fas/Fas ligand pathway and Bcl-2 regulate T cell responses to model self and foreign antigens. Immunity. 1998;8:265–74. doi: 10.1016/s1074-7613(00)80478-1. [DOI] [PubMed] [Google Scholar]
- 17.Streilein JW. Unraveling immune privilege. Science. 1995;270:1158–9. doi: 10.1126/science.270.5239.1158. [DOI] [PubMed] [Google Scholar]
- 18.Hooper P, Bora NS, Kaplan HJ, Ferguson TA. Inhibition of lymphocyte proliferation by resident ocular cells. Curr Eye Res. 1991;10:363–72. doi: 10.3109/02713689108996342. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson TA, Fletcher SM, Hemdon JM, Griffith TS. Neuropeptides modulate immune deviation induced via the anterior chamber of the eye. J Immunol. 1995;155:1746–56. [PubMed] [Google Scholar]
- 20.Kinesely T, Anderson T, Sherwood ME, Flotte T, Albert D, Granstein RD. Morphologic and ultrastructural examination of I-A+ cells in the murine iris. Invest Ophthal Vis Sci. 1991;32:2423–31. [PubMed] [Google Scholar]
- 21.Streilein JW, Ksander BR, Taylor AW. Immune deviation in relation to ocular immune privilege. J Immunol. 1997;158:3557–60. [PubMed] [Google Scholar]
- 22.Ju S-T, Panka DJ, Cui H, Ettinger R, El-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–8. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 23.Suda T, Nagata S. Purification and characterization of the Fas-ligand that induces apoptosis. J Exp Med. 1994;179:873–9. doi: 10.1084/jem.179.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellamy CO, Malcomson RD, Harrison EJ, Wyllic AH. Cell death in health and disease: the biology and regulation of apoptosis. Sem Cancer Biol. 1995;6:3–16. doi: 10.1006/scbi.1995.0002. [DOI] [PubMed] [Google Scholar]
- 25.Pender M. Apoptosis of alpha beta T lymphocytes in the nervous system in experimental autoimmune encephalomyelitis: its possible implications. J Autoimmun. 1992;5:401–10. doi: 10.1016/0896-8411(92)90001-7. [DOI] [PubMed] [Google Scholar]
- 26.Dowling P, Shang GF, Raval S, Menonna J, Cook S, Husar W. Involvement of the CD95 (APO-I/Fas) receptor/ligand system in multiple sclerosis brain. J Exp Med. 1996;184:1513–8. doi: 10.1084/jem.184.4.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennet MW, O'Connell J, O'Sullivan GC, Brady C, Roche D, Collines JK, Shanahan F. The Fas counterattack in vivo: apoptotic depletion of tumor-infiltrating lymphocytes associated with Fas ligand expression by human esophageal carcinoma. J Immunol. 1998;160:5669–75. [PubMed] [Google Scholar]
- 28.Gold R, Hartung HP, Lassmann H. T-cell apoptosis in autoimmune disease: termination of inflammation in the nervous system and other sites with specialized immune-defense mechanisms. Trends Neurosci. 1997;20:399–404. doi: 10.1016/S0166-2236(97)01079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–65. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 30.Hahne M, Rimoldi D, Schroeter M, et al. Melanoma cell expression of Fas(Apo-l/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–6. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 31.Strand S, Hofmann WJ, Hug H, et al. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand expressing tumor cells—a mechanism of immune evasion? Nat Med. 1996;2:1361–6. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- 32.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–92. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 33.Brunner T, Mogil RJ, LaFace D, et al. Cell-autonomous Fas (CD95)/FasL interaction mediates activation-induced apoptosis in T cell hybridomas. Nature. 1995;373:441–4. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 34.Dhein J, Walczac H, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1. Nature. 1995;373:438–41. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 35.Moban RR, Liang Q, Kim WJ, Helena MC, Baerveldt F, Wilson SE. Apoptosis in the cornea: further characterization of Fas/Fas ligand system. Exp Eye Res. 1997;65:575–89. doi: 10.1006/exer.1997.0371. [DOI] [PubMed] [Google Scholar]
- 36.Ohta K, Yoshimura N. Expression of Fas antigen on helper T lymphocytes in Vogt–Koyanagi–Harada disease. Gruefes Arch Clin Exp Ophthalmol. 1998;236:434–9. doi: 10.1007/s004170050102. [DOI] [PubMed] [Google Scholar]