Abstract
In the present study we report that the appearance of oligo-monoclonal immunoglobulins (oligoM-Igs) in the sera of transplanted individuals is concurrent with the detection of coincident active CMV infection and EBV replication. Eighty-four renal allograft patients were monitored with respect to CMV isolation, to CMV conventional serology and humoral response against the EBV trans-activator ZEBRA (an immediate-early antigen also called BZLF1). Titration of anti-ZEBRA antibodies (IgG and IgM) and amount of EBV DNA in serum were evaluated. Using the combination of four techniques (agarose gel electrophoresis, analytical isoelectric focusing, high resolution immunoelectrophoresis, immunofixation electrophoresis), oligoM-Igs were found in 25% of patients after allografting and significantly associated with rejection episodes (P < 0.001). Twenty out of 23 (86%) concurrent CMV/EBV infections were associated with serum oligoM-Igs (P < 0.001). One can thus reasonably assume that a sustained EBV replication following iatrogenic immunosuppression can promote the immunoglobulin heavy chain expression in EBV-infected B lymphocytes. The proliferation of immunoglobulin-secreting clones might occur after active CMV infection, through a transient over-immunosuppression or via immune subversion.
Keywords: monoclonal immunoglobulins, Epstein–Barr virus, ZEBRA, cytomegalovirus, transplantation
INTRODUCTION
EBV is a gammaherpesvirus of humans which has a potent B cell growth-transforming activity [1] and is associated with diverse malignancies including B and T cell lymphomas, adenocarcinomas, nasopharyngeal carcinoma and others [2]. Whereas immunocompetent individuals can limit proliferation of EBV-infected cells, those with congenital or acquired immunodeficiency are highly susceptible to EBV-associated lymphoproliferation [3]. The frequency of EBV+ cells in the blood of normal EBV+ donors is low and has been estimated to be in order of < 0.5–2/106 circulating B lymphocytes or < 1/107 blood mononuclear cells [4]. After organ transplantation a 100- to 1000-fold increase of circulating EBV+ cells was demonstrated [5,6]. Viral reactivation was found in 25–30% of patients following transplantation because of the heavy immunosuppressive regimens to which they must adhere to prevent graft rejection [7,8]. Post-transplant lymphoproliferative disease (PTLD, for which symptomatology may vary from infectious mononucleosis-like illness to solid localized tumours) results from EBV-induced proliferation of B cells in the immunosuppressed transplant population. Therapy with OKT3, CMV seromismatch and seronegativity for EBV before transplantation [9] are recognized to be major risk factors of lymphoproliferative disorder. Human CMV continues to be an important post-transplant pathogen in allograft recipients and CMV infection increases the risk of other opportunistic infections, probably by the intrinsic immunosuppression [10,11] and subversion [12] induced by this virus. The occurrence of serum monoclonal immunoglobulins in kidney transplant recipients is well known and one can reasonably suppose that this might be the first step of a patent immunoproliferative disorder [13–15]. Less is known about the influence of viral infections, especially by the above-described pathogens, on the development of oligo-monoclonal immunoglobulins (oligoM-Igs, also called monoclonal gammopathies). The presence of oligoM-Igs in serum reflects the expansion of a B cell clone, which after proliferation produces its individual monoclonal immunoglobulin. CMV and/or EBV have been suspected to be responsible for oligoM-Ig development. Our study relates that detection of both active CMV infection and EBV reactivation in transplanted individuals was concurrent with the development of an oligoclonal pattern of immunoglobulins, rather than a ‘typical’ monoclonal pattern as can be seen in myeloma patients.
In the present study we monitored 84 patients after they received a renal allograft with respect to their humoral response against the EBV trans-activator BZLF1 (an immediate-early antigen also called ZEBRA) [16,17], which is the early sign of EBV replication [18]. Such replication involves the ZEBRA protein, which plays a crucial role in the switch from EBV latency to EBV replicative cycle. EBV replication may also be associated with the release of virions, and there is a growing interest in the detection of the EBV load in the serum [19,20]. In the present work, both the titration of anti-ZEBRA antibodies (IgG and IgM) and the level of EBV DNA in serum were evaluated. Furthermore, we analysed associations of EBV reactivation and CMV infection and attempted to correlate them to the appearance of serum oligoM-Ig.
PATIENTS AND METHODS
Sera (n = 288) from 84 patients (including 26 patients followed up for > 100 days after renal transplantation) were screened prospectively for oligoM-Ig, detection of CMV active infection, serological markers of EBV infection (including anti-ZEBRA antibodies—see below), EBV DNA. All patients were transplanted between January 1993 and December 1993 at the Transplantation Unit, E. Herriot Hospital, Lyon.
As immunosuppressive treatment: (i) 78 patients received a quadruple induction therapy (with anti-thymocyte globulins (ATG) in 74); (ii) anti-CD3 MoAb (OKT3) was administered in six others (two received both ATG and OKT3 for intolerance to ATG) for 10 days, associated with cyclosporin (dosage adapted to reach 150 μg/ml level), azathioprine (2–3 mg/kg per day) and steroids (1 mg/kg per day). Six patients did not receive ATG, but cyclosporin associated with mycophenolate mofetil (2 or 3 g/day) and steroids. Long-term therapy consisted of triple therapy with cyclosporin, azathioprine or mycophenolate mofetil and steroids, and was slightly tapered with time. Rejection therapy consisted of high-pulse steroids or OKT3.
Four patients received prophylactic anti-CMV therapy with i.v. ganciclovir, then oral acyclovir for 3 months.
Standard laboratory diagnosis
Serologic diagnosis of EBV infection was performed by standard methods: IgG antibodies to VCA and EA were determined by indirect immunofluorescence (IF) on antigen-producing P3HR-1 cells and TPA/butyrate-induced Raji cells, respectively. Antibodies against EBNA were determined by anti-complement IF (ACIF) on Raji cells. Reactivation of EBV infection was defined by the existence of elevated antibody titres to VCA IgG (> 1:320), EA (> 1:80), and the pre-existence of anti-EBNA IgG antibodies (> 1:10).
Anti-ZEBRA antibody detection by enzyme-linked immunoassay
Two antigen preparations were used for testing human sera in an ELISA test [21]: (i) Recombinant protein: rZEBRA recombinant protein (GST-ZEBRA from pGEXZ25 plasmid kindly supplied by A. Sergeant, Lyon, France) was used for IgG detection; (ii) one synthetic peptide, designated ZEBRAp130 (carboxyterminal region of ZEBRA protein) was used for IgM detection. Results were read by optical absorbance at 450 nm (A450). Patients with anti-ZEBRA IgG antibodies were separated into three groups according to the signal obtained (A450): a high-titre group with absorbance > 1, an intermediate-titre group giving signals between 0.5 and 1 A450 unit, and a low-titre group giving signals between cutoff value (0.3 A450) and 0.5 A450 unit. Healthy blood donors (n = 398) were used as controls. Under the above-described conditions, anti-ZEBRA IgG antibodies were detected in 14.8% (59/398) of them, among whom 16 individuals (4%) showed high antibody titres. Finally, anti-ZEBRAp130 IgM could be detected in 40% of patients with EBV reactivation (5% in a population of healthy blood donors).
Detection of EBV DNA in serum samples
A quick alkaline lysis technique for extraction of DNA from serum sample was used [20]. Serum (20 μl) was mixed with 2 μl of 1 m NaOH and incubated at 37°C in a water bath for 60 min, then neutralized with 2 μl of 1 m HCl. Flotation dialysis was then performed using 0.05-μm filters (Millipore, Bedford, MA) to eliminate salts. Ten microlitres of the preparation were used in a total volume of 100 μl in the polymerase chain reaction (PCR). PCR protocols and analysis of the PCR products have been previously described in detail [20]. Finally, we defined as patients with EBV replication those patients bearing anti-ZEBRA IgG and/or anti-ZEBRA IgM and/or EBV DNA detected in serum.
Detection of active CMV infection
CMV cultures were performed as described elsewhere [22]. Briefly, virus isolation from peripheral blood leucocytes and urine on fibroblast monolayers was performed by two methods: the conventional tube culture method and isolation by centrifugation (1000 g for 10 min) in 24-well flat-bottomed culture plates. Foci were detected with MoAb E13 [23] directed against CMV immediate–early antigens by IF. Active CMV infection was defined when CMV was isolated from a body fluid (blood, urine) or by seroconversion or the presence of CMV-specific IgM. Active infection in an initially seronegative patient was considered a primary infection. When it occurred in a seropositive patient it was designated reactivation. CMV infection was considered symptomatic when active infection occurred in association with clinical symptoms. CMV syndrome was defined as a CMV infection associated with fever of unknown origin of > 38.5°C for more than 5 days, leukopenia of < 2000/mm3, or thrombocytopenia of < 100 000/mm3.
Qualitative analysis of serum oligoM-Igs
Qualitative analysis of serum immunoglobulin in grafted patients was performed routinely using four different techniques with distinct abilities in detecting oligo and monoclonal immunoglobulins.
First, sera were submitted to agarose gel electrophoresis using Helena's Rapid Electrophoresis Analyser. Second, serum IgGs were studied using analytical isoelectric focusing (IEF) [24–26]. Briefly, IEF was performed on a home-made ultrathin (500 μm) T4C3 polyacrylamide gel permanently bound to a 1 mm thick glass plate by using Polyfix 1000 (Serva FeinBiochemica, Heidelberg, Germany). Gels contained 2 m urea, 3% ampholine pH 3.5–9.5 (Pharmacia Biotech, Uppsala, Sweden) and 2 mm Lys-Arg-Asp-Glu (Sigma, St Quentin-Fallavier, France). The anode solution was 1 m H3PO4, and the cathode solution was 1 m NaOH. The gel was run on a Multiphor electrophoresis system (Pharmacia Biotech). Neat sera were applied on the anodal side of the gel after a prefocusing step of 30 min at 10 W and 4°C. To assign apparent pIs to focused bands a protein mixture (Protein test mixture 9; Serva Feinbiochemica) was run in parallel to the sera. While urea is known to give apparently higher pI values for the carrier ampholytes, we did not use any correction factors for the reported pI values. IEF was completed after a 150-min run at 15 W. The proteins were fixed in trichloracetic acid (20%) for 30 min, stained in a coomassie solution (Phast gel Blue R; Pharmacia Biotech) and destained in a solution of ethanol:acetic acid:distilled water 30:8:62. Third, sera were submitted to a highly resolutive immunoelectrophoresis run on home-made plates prepared as follows. Ultrathin layers of 1.5% agarose gels bound to a Gelbond film (FMC, Rockland, ME) were obtained after pouring in a preheated mould a boiling suspension of agarose SeaKem HEEO (FMC) in a barbital buffer pH 8.6 (Kallestadt, Chaska, MN). The main improvements consisted of using an ultrathin layer gel (500 μm) with precut troughs, agarose with high electroendosmosis, high gradient voltage (25 V/cm) and minute amounts (25 μl) of commercially available polyvalent antiserum anti-immunoglobulins (γ-, α-, μ-, κ- and λ-chain-specific) (Silenus, Hawthorn, Australia). In a final step, sera were submitted to standard immunofixation electrophoresis (IFE) using the Paragon Immunofixation electrophoresis kit (Beckman Instruments, Fullerton, CA) according to the manufacturer's instructions.
Interpretation of the results
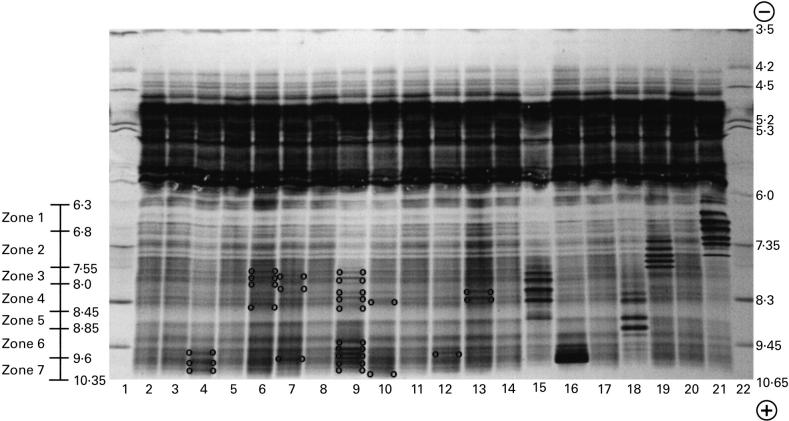
After visual inspection of the electrophoregram, samples were classified according to the presence of unique or multiple discrete bands appearing in the α2 to the γ zone. Improvements in the standard procedure of immunoelectrophoresis permitted a reliable and easy qualitative screening of mini monoclonal IgMs due to an increased separation and sharpness of the precipitin arcs. In most cases mini monoclonal IgMs could not be detected by zonal electrophoresis (concerning IgG interpretation, see legend to Fig. 1). In order to detect newly developing IgG bands, patient samples were run in parallel with normal samples. A serum was classified as normal in IEF when no differences could be demonstrated between normal sera and the patient's serum. Abnormal patterns were extremely heterogeneous, ranging from one extra band localized in any part of the post-albumin region to a great number of ‘extra bands’ with very close isoelectric points or with widely varying isoelectric points.
Fig. 1.
Isoelectric focusing spectrotypes of normal and oligo/monoclonal IgGs containing sera. Thanks to the high resolving power of isoelectric focusing, IgGs appeared in normal sera as more than 30 bands (most often 33) spreading over a 4-cm length from the albumin fraction to the anode and thus from apparent pH 6.3 to pH 10.35. In normal sera, IgG bands were distributed in seven zones from the anode to the cathode: zone 1, five bands ranging from apparent pH 6.3 to 6.8 in the post-albumin part of the gel; zone 2, seven distinct bands of various intensities ranging from apparent pH 6.8 to 7.55; zone 3, eight bands ranging from apparent pH 7.55 to 8.0; zone 4, three bands ranging from apparent pH 8.0 to 8.45. However, this zone was separated from zone 3 by a blurred narrow region where no bands could be seen in normal sera. Zone 5, ranging from apparent pH 8.45 to 8.85: no bands in normal sera; zone 6, three bands defining two zones ranging from apparent pH 8.85 to 9.6; zone 7, four or five bands ranging from apparent pH 9.6 to 10.35. Lane 1, apparent pH gradient; lane 22, protein test mixture ensuring assignment of apparent pIs of focused bands; lanes 2, 3, 5, 8, 11, 14, 17 and 20, normal sera with a normal distribution of the 33 bands of IgG; lane 4, serum with a normal distribution of IgG bands from zone 1 to zone 6 and with three extra bands in zone 7; lane 6, serum exhibiting extra bands in zone 3 and a normal distribution of the IgG bands in the other zone; lane 7, serum with one extra band in zones 3, 4 and 7; lane 9, serum with multiple extra bands distributed all over the spectrum but zones 1 and 2 (patient 17); lane 10, one extra band in zones 4 and 7 (patient 19); lane 12, serum with two extra bands in zone 4 and one extra band in zone 7 (patient 20); lane 13, serum with two heavy extra bands in zone 4 and an increase in the concentration of the normal IgG bands of zones 2 and 3 (see patient 11, Table 1); lanes 15, 16, 18, 19 and 21, as an example these sera exhibit the characteristic patterns of myelomatous monoclonal IgGs focusing into several bands of various apparent pIs and regularly spaced. This microheterogeneity is attributed to post-translational glycosylation and/or amidation/deamidation. One must be aware that newly synthesized IgGs occurring in kidney transplant recipients never exhibited such a microheterogeneity. In such patients, one extra band appearing on isoelecric focusing corresponds to one monoclonal IgG which according to its concentration will (or will not) be displayed on zone elctrophoresis and typed by immunofixation electrophoresis (IFE). The coexistence of two or more extra bands in the IgG region of IEF characterizes the so-called oligoclonal pattern which is constituted with multiple mini monoclonal IgGs.
By immunofixation, the unique or multiple discrete bands displayed in zonal electrophoresis could be assigned to one heavy and light chain type of immunoglobulin. Likewise, the light chain of mini monoclonal IgM detected with immunoelectrophoresis could be typed using immunofixation electrophoresis. However, some sera displaying no suspicious bands in zonal electrophoresis, no localized thickening of the IgM arc, but displaying one or several bands of a rather weak intensity on the IgG isoelectric spectrum did not happen to reveal oligo/monoclonal bands by standard immunofixation. However, using more sensitive immunodetection techniques, such as immunoblot [25], would permit the assignment to IgGs of these extra bands.
Statistical analysis
χ2 test was used for statistical comparisons of concurrent EBV/CMV and non-concurrent EBV/CMV infections for the incidence of oligoM-Ig (P < 0.05 was taken as statistically significant).
RESULTS
Sera screening for oligoM-Ig
Using the combination of the techniques described above, oligoM-Igs were found in 21/84 (25%) of patients after allografting (Table 1). Nine patients had one monoclonal immunoglobulin typed by IFE (patients 1, 2, 5, 7, 10, 12) or visible on IEF (patients 18, 20, 21) (see Table 1), whereas two or more different oligoM-Igs were detected in the sera of the others (for an example see patients 11 and 13 in Table 1). Sera shown in Fig. 1, lanes 4, 6, 7, 10, 12 and 13, did not reveal distinct immunoprecipitates of IgG after standard immunofixation, while serum shown in lane 9 revealed two monoclonal IgGs, one of the κ type, localized in the cathodal part of the γ zone, and the other one of the λ type (patient 17, Table 1), localized in the middle part of the γ zone. Most of these oligoM-Igs belonged to the IgG class. Sera from four patients (patients 18–21, see Table 1) exhibited ‘extra bands’ not fixed by IFE (Fig. 1: patient 19/lane 10; patient 20/lane 12). Appearance of oligoM-Igs did not seem to be related to any primary or secondary infection. Finally, no patient developed immunoproliferative and/or lymphoproliferative disease within the following 2 years.
Table 1.
Oligo-monoclonal immunoglobulins, anti-ZEBRA antibodies, EBV DNA and CMV markers from 21 transplanted patients (patients 1–17 exhibited more than two extra bands except patient 7; patients 18–21 exhibited one extra band except patient 19)
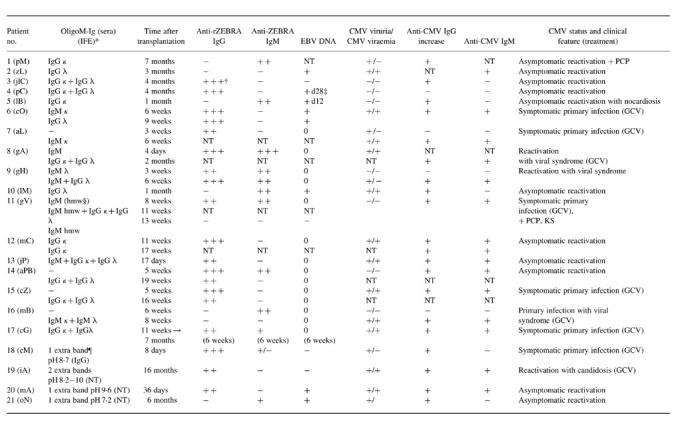
NT, Not tested; PCP, Pneumocystis carinii pneumonitis; KS, Kaposi's sarcoma; GCV, ganciclovir.
*IFE, Immunofixation electrophoresis only considered (patients 1–17).
†+++, High antibody titres (A450 > 1.5).
‡d28, 28 days post-transplantation.
§hmw, High molecular weight.
¶Isoelectric focusing (IEF) only considered (patients 18–21).
Concerning rejection episodes, 20 patients without oligoM-Ig (32%) experienced at least one rejection crisis and received steroid pulses or OKT3. Among patients with oligoM-Ig, 15 (71%) had rejection crises (P < 0.001) and received rejection treatment (nine patients with steroids and six with OKT3).
Detection of activation of EBV replication
All the tested patients experienced an EBV reactivation. When considering only the anti-ZEBRA IgG, rates of positivity were quite similar in the whole cohort and in the subgroup of oligoM-Ig patients (70% and 71%, respectively). Interestingly, EBV viral load was detected by PCR in 25% and 27% of patients, respectively. Using these two markers, there were no differences between the two populations of patients considered. High anti-EA IgG titres correlated strongly with high anti-ZEBRA IgG absorbances (A450 > 1.5). When such increases were detected, anti-ZEBRA IgG and anti-ZEBRA IgM were detectable 44 days and 60 days, respectively, before an increase in anti-EA IgG titres.
In most cases (55.8%) an anti-ZEBRA IgG was detected just after transplantation (beginning of follow up). This phenomenon was also observed for anti-ZEBRA IgM (30.4% positivity rate at the beginning of follow up). The average delay for EBV DNA detection was 27.8 days post-transplantation.
When considering EBV alone, no significant correlation was found between EBV replication and oligoM-Ig appearance (P > 0.05). When results were dichotomized into high replicative activity (high titres of anti-ZEBRA antibodies with A450 > 1.5 and/or anti-ZEBRA IgM) and low/intermediate replicative activity (A450 < 1.5 and/or without IgM), high level of EBV replication correlated with oligoM-Ig appearance (P < 0.05).
Active CMV infection
During the 100 days post-transplantation, active CMV infection occurred in 42 patients (including 34 with CMV isolation from blood). Fifteen patients in the group without oligoM-Ig received ganciclovir therapy, and nine patients with oligoM-Ig. Coincidentally, EBV replication and CMV infection were found in 23 patients. In terms of clinical symptoms, no difference was observed between singly and doubly infected individuals.
Correlation between EBV reactivation/CMV active infection and oligoM-Igs
After graft, the 21 patients with oligoM-Igs found included 20 patients with double CMV/EBV infection and one patient (patient pC) with a disseminated EBV infection with high AZA titres and positive PCR in serum (Table 1) (Fig. 2a,b, patients gH and cO). Serum oligoM-Ig was never detected in patients with an active CMV infection alone. Of 63 patients with complete documentation over 6 months, 20/23 (86%) concurrent CMV/EBV infections were associated with serum oligoM-Ig (P < 0.001). In 11 patients with oligoM-Igs, signs of EBV replication measured by increased titres of AZA (or EBV DNA in serum) were detected concurrently. In the 10 other cases, increase of AZA antibodies was observed before CMV isolation or increase of anti-CMV antibodies.
Fig. 2.
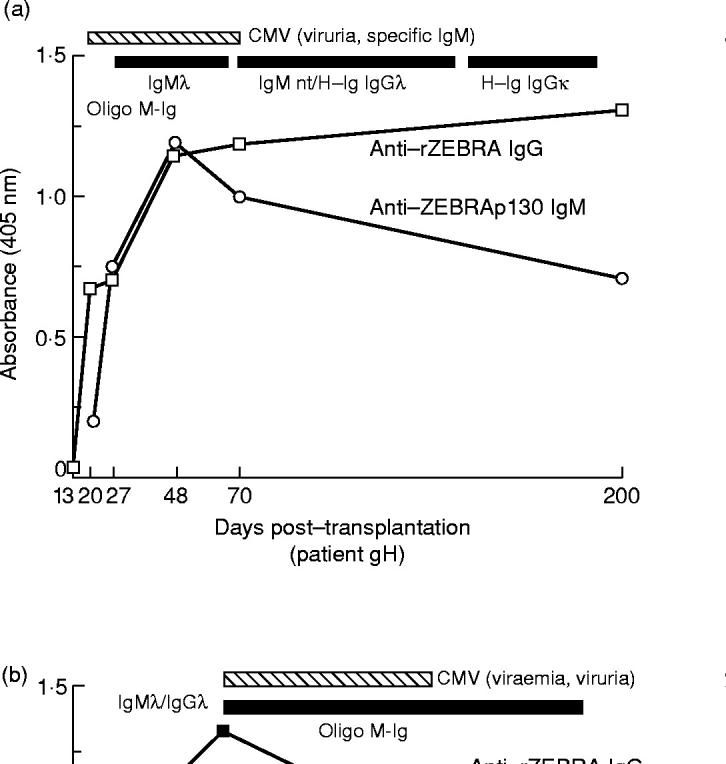
(a,b) Concurrent EBV replication and active CMV infection, oligoM-Ig development in two followed up patients
DISCUSSION
Both EBV and CMV are notable for their ubiquity, latency, and tendency to reactivate in immunosuppressed hosts. Management of patients infected with these two herpesviruses revealed some similarities. First, viral persistence is controlled in the immunocompetent host by an intact cellular immune system. Immunosuppressive therapy administered following transplantation may lead to an uncontrolled viral replication and, consequently, to symptomatic or asymptomatic active infection. Second, a patient with no prior immunity to CMV or EBV before transplantation who receives an organ harbouring a latent or persistent virus (primary infection) is at higher risk of uncontrolled replication than a patient who benefits from immunity to CMV or EBV pretransplantation. Third, these viruses can infect cells involved in the immune response (monocytes, B lymphocytes) and can lead to immune subversion and dysregulate the host immune balance [27]. Fourth, laboratory tests for disseminated infection (viral DNA in blood and viraemia) allow a careful monitoring of transplanted patients. Quantification of PCR-amplified viral DNA has been successfully used to predict the development of PTLD [28,29]. Whereas there is a well-known association between EBV infection and the incidence of PTLD in immunosuppressed individuals, less is known about the clinical significance of the EBV replication itself. Such replication is mediated by two trans-activators, ZEBRA and R (also called BRLF1). ZEBRA is a key immediate–early transactivator of early EBV lytic expression genes, and is also essential for activation of the viral DNA origin of replication used for lytic infection (oriLyt) [30,31]. Thus, ZEBRA plays an essential role in switching on the lytic replication of EBV in B cells latently infected with EBV. Transient expression of ZEBRA in latently infected B cells induces lytic replication of the virus.
We previously used an immunoassay (EIA) to detect IgG and IgM to the IEA ZEBRA [18] in transplanted patients: both recombinant protein and ZEBRA-derived peptide were targeted in the ELISA test and results correlated well with standard immunofluorescence assays as well as with primary versus reactivated infection. The major improvement of the test was due to the nature of ZEBRA—immediate–early protein—and consisted of an early detection of specific antibodies, long before conventional tests, both in secondary and primary infection. Moreover, this was the first demonstration of EBV reactivation in transplanted patients using a ZEBRA polypeptide as an antigen. Indeed, the characteristics of antibody responses to ZEBRA are unique among reactions to EBV proteins [32]. So far, as found by others, no clinical symptoms could clearly be associated with the appearance of serological markers of EBV replication.
About half of the transplant patients had an active CMV infection. We found a striking coincidence of active CMV infection and active EBV replication. This interesting observation was underlined by its relevant association with oligoM-Ig in serum. Of grafted patients, 40–50% exhibited restriction of immunoglobulin heterogeneity or monoclonal peaks of serum immunoglobulin [33,34]. Such serum oligoM-Igs represent monoclones of at least 1 × 109 cells of B lymphocyte lineage which apparently proliferate without adequate suppressive control [33], but the inducing role of viral agents is questionable. EBV-associated B cell proliferation in transplanted patients was found to be polyclonal, multiclonal or oligoclonal, as indicated by the examination of immunoglobulin heterogeneity restriction [35]. It was shown that conventional EBV serology was not sufficient to define a population at risk of developing lymphomas [36]. Concerning oligoM-Igs, several investigators tried to find a correlation between herpesviruses (EBV and/or CMV) and a restricted electrophoretic heterogeneity of serum immunoglobulins by using more conventional serological methods for EBV diagnosis (such as IF), but could draw no clear-cut conclusions about the triggering role of viruses [34,37]. In a previous report [20] we found that EBV DNA was detected in 73% and 68% of patients with AIDS-related non-Hodgkin's lymphoma (ARNHL) and infectious mononucleosis, respectively, and that high EBV DNA levels in the bloodstream (> 20 000 EBV genome equivalents/104) demonstrate the high frequency of EBV replication after renal transplantation, as evidenced by the high anti-ZEBRA positivity rate. When present, anti-ZEBRA antibodies (IgG and IgM) were early markers of EBV reactivation and were detected before anti-EA or anti-VCA IgG antibodies. After transplantation, high EBV replication (high anti-ZEBRA antibody titres, or anti-ZEBRA IgM) and, in some cases, circulating EBV DNA, may signal patients at risk for monoclonal gammopathy. In the future, quantification of EBV DNA in blood (leucocyte or serum) might be a relevant marker of lymphoproliferative disorders in such patients.
The coincidence of EBV reactivation and active CMV infection frequently leads to the expansion of B cell clones and to the detection of serum oligoM-Igs. If these two herpesviruses seemed to be closely associated in most of the patients investigated, this association did not systematically lead to the phenomenon. Other investigators found no correlation between concurrent CMV/EBV infection and clinical symptoms, except rejection episodes [38], proving that in most cases oligoM-Igs were not related to a specific symptomatology. However, it was demonstrated recently that the role of CMV disease was relevant in the development of PTLD in primary EBV infection after liver transplantation [39]. Interestingly, we already observed lower CD4+ T cell counts and decreased proliferative T cell responses to mitogens in patients with oligoM-Igs, compared with other transplanted patients without oligoM-Ig [40].
In our series of patients, although the basal immunosuppressive treatment was the same, more patients with oligoM-Igs experienced acute rejection during the period of follow up. The reinforcement of immunosuppression in such patients obviously did alter T cell responses and the control of the viral burden and could lead to opportunistic diseases. After rejection therapy, two patients presented with Pneumocystis carinii infection; one of them, without oligoM-Ig, died as a consequence, and another one with oligoM-Ig presented with Kaposi's sarcoma (patients pM and gV in Table 1).
The mechanisms by which both activation of EBV replication and CMV infection promote the expansion of B cells warrant further study. ZEBRA antigen has been previously detected in B lymphocytes by immunostaining and this detection was confirmed in a transplanted patient 1 month after grafting [41]. One can imagine that ZEBRA expression in B lymphocytes induces transcription of cellular genes by itself or in synergy with cytokines. CMV is known to induce transcription and secretion of transforming growth factor-beta (TGF-β) [42]. The immunosuppressive effect of CMV may help the pathogenic chain of events leading to the appearance of oligoM-Ig. Other clinical situations are associated with detection of anti-ZEBRA antibodies, the most prominent diseases being nasopharyngeal carcinoma (NPC) [43] and EBV+ Hodgkin's disease [44]. This suggests that tumorogenesis could involve reactivation of EBV. ZEBRA is a DNA-binding transcription factor of the bZIP family and is related to the AP-1 family of transcription factors [45]. It binds specifically as a homodimer to a DNA sequence motif called ZRE. It was recently found that ZEBRA also activates transcription in transiently transfected mammalian cells via a consensus binding site located in the IgH (immunoglobulin heavy chain) intron enhancer [46]. We may thus speculate that sustained ZEBRA expression in B cells following iatrogenic immunosuppression could influence IgH expression in EBV-infected B lymphocytes. The proliferation of immunoglobulin-secreting clones might occur after active CMV infection through a transient over-immunosuppression through induction of cytokines (TGF-β) or via immune subversion.
Acknowledgments
We are indebted to Susan Michelson for critical reading of this manuscript and to Daniel Garin for statistical analysis.
REFERENCES
- 1.Rickinson AB, Kieff E. Epstein-Barr virus. In: Fields BN, Knipe DM, Howley PM, et al., editors. Fields virology. 3. Philadelphia: Lippincott-Raven Publishers; 1996. pp. 2397–446. [Google Scholar]
- 2.Niedobitek G, Young LS. Epstein-Barr virus peristence and virus-associated tumours. Lancet. 1994;343:333–5. doi: 10.1016/s0140-6736(94)91167-3. [DOI] [PubMed] [Google Scholar]
- 3.Hanto D. Classification of Epstein-Barr virus-associated posttransplant lymphoproliferative diseases: implications for understanding their pathogenesis and developing rational treatment strategies. Ann Rev Med. 1995;46:381–94. doi: 10.1146/annurev.med.46.1.381. [DOI] [PubMed] [Google Scholar]
- 4.Rocchi G, de Felici A, Ragona G, et al. Quantitative evaluation of Epstein-Barr virus-infected mononuclear peripheral blood leukocytes in infectious mononucleosis. N Engl J Med. 1977;296:132–4. doi: 10.1056/NEJM197701202960302. [DOI] [PubMed] [Google Scholar]
- 5.Crompton CH, Cheung RK, Donjon C, et al. Epstein-Barr virus surveillance after renal transplantation. Transplantation. 1994;57:1182–9. doi: 10.1097/00007890-199404270-00008. [DOI] [PubMed] [Google Scholar]
- 6.Riddler SA, Breinig MC, McKnight JLC. Increase levels of circulating Epstein-barr virus-infected lymphocytes and decreased EBV nuclear antigen antibody responses are associated with the development of post-transplant lymphoproliferative diseases in solid-organ recipients. Blood. 1994;20:1346–53. [PubMed] [Google Scholar]
- 7.Ho M, Miller G, Atchinson RW, et al. Epstein-Barr virus infections and DNA hybridization studies in posttransplantation lymphoma and lymphoproliferative lesions: the role of primary infection. J Infect Dis. 1985;152:876–86. doi: 10.1093/infdis/152.5.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho M, Jaffe R, Miller G, et al. The frequency of Epstein-Barr virus infection and associated lymphoproliferative syndrome after transplantation and its manifestation in children. Transplantation. 1988;45:719–27. doi: 10.1097/00007890-198804000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker RC, Marshall WF, Strickler JG, et al. Pretransplantation assessment of the risk of lymphoproliferative disorder. Clin Infect Dis. 1995;20:1346–53. doi: 10.1093/clinids/20.5.1346. [DOI] [PubMed] [Google Scholar]
- 10.Rinaldo CR, Carney WP, Richter BS, et al. Mechanisms of immunosuppression in cytomegalovirus mononucleosis. J Infect Dis. 1980;141:488–95. doi: 10.1093/infdis/141.4.488. [DOI] [PubMed] [Google Scholar]
- 11.Nokta MA, Hassan MI, Kimberly L, et al. Human cytomegalovirus-induced immunosuppression. J Clin Invest. 1996;97:2635–41. doi: 10.1172/JCI118713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oldstone MBA. How viruses escape from cytotoxic lymphocyte: molecular parameters and players. Virology. 1997;234:179–85. doi: 10.1006/viro.1997.8674. [DOI] [PubMed] [Google Scholar]
- 13.Touraine JL. Immunoglobulin abnormalities and infectious lymphoproliferative syndrome in cyclosporine-treated transplant patients. Transplant Proc. 1983;15:2798–804. [Google Scholar]
- 14.Radl J, Valentijn RM, Haaijman JJ, et al. Monoclonal gammapathies in patients undergoing immunosuppressive treatment after renal transplantation. Clin Immunol Immunopathol. 1985;37:98–102. doi: 10.1016/0090-1229(85)90140-0. [DOI] [PubMed] [Google Scholar]
- 15.Stanko CK, Jeefery JR, Rush DN. Monoclonal and muticlonal gammapathies after renal transplantation. Transplant Proc. 1989;21:3330–2. [PubMed] [Google Scholar]
- 16.Chevallier-Greco A, Manet E, Chavrier P, et al. Both Epstein-Barr virus (EBV)-encoded transacting factors EB1 and EB2 are required to activate transcription from an EBV early promoter. EMBO J. 1986;5:3243–9. doi: 10.1002/j.1460-2075.1986.tb04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Countryman J, Jenson H, Seibl R, et al. Polymorphic proteins encoded within BZLF1 of defective and standard Epstein-Barr viruses disrupt latency. J Virol. 1987;61:3672–9. doi: 10.1128/jvi.61.12.3672-3679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drouet E, Chapuis-Cellier C, Garnier JL, Touraine JL. Early detection of Epstein-Barr virus infection and meaning in transplant patients. In: Touraine JL, Traeger J, Betuel H, et al., editors. Cancer in transplantation prevention and treatment. Dordrecht: Kluwer Academic Publishers; 1996. pp. 207–13. [Google Scholar]
- 19.Gan YJ, Sullivan JL, Sixbey JW. Detection of cell-free Epstein-Barr virus DNA in serum during acute infectious mononucleosis. J Infect Dis. 1994;170:436–9. doi: 10.1093/infdis/170.2.436. [DOI] [PubMed] [Google Scholar]
- 20.Laroche C, Drouet E, Brousset P, et al. Measurement by polymerase chain reaction of the Epstein-Barr virus load in infectious mononucleosis and AIDS-related non-Hodgkin's lymphomas. J Med Virol. 1995;46:66–74. doi: 10.1002/jmv.1890460115. [DOI] [PubMed] [Google Scholar]
- 21.Brousset P, Drouet E, Schlaifer D, et al. Epstein-Barr virus (EBV) replicative gene expression in tumor cells of AIDS-related non Hodgkin's lymphoma in relation to CD4 cell number and antibody titers to EBV. AIDS. 1994;8:583–90. doi: 10.1097/00002030-199405000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Drouet E, Colimon R, Michelson S, et al. Monitoring levels of human cytomegalovirus DNA in blood after liver transplantation. J Clin Microbiol. 1995;33:389–94. doi: 10.1128/jcm.33.2.389-394.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazeron MC, Colimon R, Roseto A, et al. Detection of cytomegalovirus using monoclonal antibodies. Dev Biol Standard. 1984;57:287–91. [PubMed] [Google Scholar]
- 24.Confavreux C, Chapuis-Cellier C, Arnaud P, et al. Oligoclonal «fingerprint» of cerebrospinal fluid IgG in multiple sclerosis patients is not modified following intrathecal administration of natural β-interferon. J Neurol Neurosurg Psychiatry. 1986;49:1308–12. doi: 10.1136/jnnp.49.11.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haas H, Lange A, Schlaak M. Homogeneous immunoglobulins in the sera of lung carcinoma patients receiving cytotoxic chemotherapy—detection with the use of isoelectric focusing and immunoblotting. Clin Exp Immunol. 1987;70:630–9. [PMC free article] [PubMed] [Google Scholar]
- 26.Deteix P, Chapuis-Cellier C, Ghais Z, et al. Systematic survey of immunoglobulin abnormalities: frequency, and evolution in organ transplant recipients. Transplant Proc. 1985;17:2651–4. [Google Scholar]
- 27.Michelson S. Mechanisms of immunosuppression by human cytomegalovirus. In: Scholz M, Rabenau MF, Doerr HW, et al., editors. CMV-related immunopathology. Monogr Virol Basel: Karger; 1998. pp. 12–28. [Google Scholar]
- 28.Rooney CA, Loftin SK, Holladay MS, et al. Early identification of Epstein-Barr virus-associated post-transplantation lymphoproliferative diseases. Br J Haematol. 1995;89:98–103. doi: 10.1111/j.1365-2141.1995.tb08904.x. [DOI] [PubMed] [Google Scholar]
- 29.Green M, Reyes J, Jabbour N, et al. Use of quantitative PCR to predict onset of Epstein-Barr viral infection and post-transplant lymphoproliferative disease after intestinal transplantation in children. Transplant Proc. 1996;28:2759–60. [PubMed] [Google Scholar]
- 30.Schepers A, Pich D, Hammerschmidt W. A transcription factor with homology to the AP-1 family links RNA transcription and DNA replication in the lytic cycle of EBV. EMBO J. 1993;12:3921–9. doi: 10.1002/j.1460-2075.1993.tb06070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schepers A, Pich D, Hammerschmidt W. Activation of oriLyt, the lytic origin of DNA replication of Epstein-Barr virus, by BZLF1. Virology. 1996;220:367–76. doi: 10.1006/viro.1996.0325. [DOI] [PubMed] [Google Scholar]
- 32.Tedeschi R, Foong YT, Cheng HM, et al. The disease associations of the antibody response against the Epstein-Barr virus transactivator protein ZEBRA can be separated into different epitopes. J Gen Virol. 1995;76:1393–400. doi: 10.1099/0022-1317-76-6-1393. [DOI] [PubMed] [Google Scholar]
- 33.Peest D, Schaper B, Nashan B, et al. High incidence of homogeneous Igs in patients after liver or heart transplantation. Transplantation. 1988;46:389–93. doi: 10.1097/00007890-198809000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Garnier JL, Berger F, Betuel H, et al. Epstein-Barr virus associated lymphoproliferative diseases (B cell lymphoma) after transplantation. Nephrol Dial Transplant. 1989;4:818–23. [PubMed] [Google Scholar]
- 35.Hanto DW, Birkenbach M, Frizzera G, et al. Confirmation of the heterogeneity of post-transplant Epstein-Barr virus associated B cell proliferation by immunoglobulin gene rearrangement analyses. Transplantation. 1989;47:458–64. doi: 10.1097/00007890-198903000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Preiksaitis JK, Diaz-Mitoma F, Mirzayans F, et al. Quantitative oropharyngeal Epstein-Barr virus shedding in renal and cardiac tranplant patients. Relationship to immunosuppressive therapy, serologic responses and the risk of posttransplant lymphoproliferative disorder. J Infect Dis. 1992;166:986. doi: 10.1093/infdis/166.5.986. [DOI] [PubMed] [Google Scholar]
- 37.Gerritsen EJA, Van Tol MJD, Lankester AC, et al. Immunoglobulin levels and monoclonal gammapathies in children after bone marrow transplantation. Blood. 1993;82:3493–502. [PubMed] [Google Scholar]
- 38.Hornef MW, Bein G, Fricke L, et al. Coincidence of Epstein-Barr virus reactivation, cytomegalovirus infection and rejection episodes in renal transplant recipients. Transplantation. 1996;60:474–80. doi: 10.1097/00007890-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 39.Manez R, Breinig MC, Linden P, et al. Posttransplant lymphoproliferative disease in primary Epstein-Barr virus infection after liver transplantation: the role of cytomegalovirus disease. J Infect Dis. 1997;176:1462–7. doi: 10.1086/514142. [DOI] [PubMed] [Google Scholar]
- 40.Bancel J, Plotnicky H, Rousset F, Touraine JL, Touraine F. Diagnosis and management of overimmunosuppression after transplantation. In: Touraine JL, Traeger J, Betuel H, et al., editors. Transplantation and clinical immunology, evaluation and monitoring in transplantation. Amsterdam: Elsevier; 1992. pp. 541–7. [Google Scholar]
- 41.Hornef MW, Wagner HJ, Fricke L, et al. Immunocytochemical detection of Epstein-Barr virus antigens in peripheral B lymphocytes after renal transplantation. Transplantation. 1995;59:138–40. doi: 10.1097/00007890-199501150-00025. [DOI] [PubMed] [Google Scholar]
- 42.Michelson S, Alcami J, Kim SJ, et al. Human cytomegalovirus infection induces transcription and secretion of transforming growth factor β1 (TGF β1) J Virol. 1994;68:5730–7. doi: 10.1128/jvi.68.9.5730-5737.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathew A, Cheng HM, Sam CK, et al. A high incidence of serum IgG antibodies to the Epstein-Barr virus replication activator protein in nasopharyngeal carcinoma. Cancer Immunol Immunother. 1994;38:68–70. doi: 10.1007/BF01517172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drouet E, Brousset P, Fares F, et al. High Epstein-Barr virus (EBV) serum load and elevated titers of anti-ZEBRA antibodies in patients with EBV-harboring tumor cells of Hodgkin's disease. J Med Virol. 1999;57:383–9. doi: 10.1002/(sici)1096-9071(199904)57:4<383::aid-jmv10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 45.Farrell P, Rowe D, Kouzarides JT. Epstein-Barr virus BZLF1 transactivator specifically binds to a consensus AP-1 site and is related to c-fos. EMBO J. 1989;8:127–32. doi: 10.1002/j.1460-2075.1989.tb03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gstaiger M, Hovens C, Georgiev O, et al. BZLF1 (ZEBRA, Zta) protein of Epstein-Barr virus selected in a yeast one-hybrid system by binding to a consensus in the IgG intronic enhancer: a role in immunoglobulin expression. Biol Chem. 1996;377:669–73. [PubMed] [Google Scholar]