Abstract
Certain patients with silicosis have been reported to exhibit immunological abnormalities such as the appearance of antinuclear antibodies and the occurrence of autoimmune diseases. Fas ligand (FasL) is a type II membrane protein which induces apoptosis by binding to its membrane receptor, Fas. FasL is converted to a soluble form by a metalloproteinase-like enzyme. We have already found serum soluble Fas (sFas) levels in silicosis patients as well as in patients with systemic lupus erythematosus (SLE) to be significantly higher than those in healthy volunteers. To examine further the role of the Fas/FasL system in silica-induced immunological abnormalities, we investigated serum soluble FasL (sFasL) levels in silicosis patients with no clinical symptoms of autoimmune diseases, using ELISA for sFasL. Although the serum sFasL levels in patients with SLE were significantly higher than those in healthy volunteers and showed a slight positive correlation with serum sFas levels, those in silicosis patients exhibited no significant difference from those in healthy volunteers, and there was no correlation with serum sFas levels. However, sFasL levels were elevated in silicosis patients with slight dyspnoea or normal PCO2 among various clinical parameters of silicosis. It may be speculated that the immunological disturbances presented by the abnormalities of apoptosis-related molecules in silicosis patients do not occur with a similar degree of respiratory involvement. Further studies are required to clarify which kinds of factors are involved in silicosis patients who exhibit immunological abnormalities.
Keywords: silicosis, soluble Fas ligand, soluble Fas, apoptosis, autoimmunity
INTRODUCTION
Certain patients with silicosis have been reported to exhibit immunological abnormalities such as hypergammaglobulinaemia, the appearance of antinuclear antibodies (ANA) and the occurrence of autoimmune diseases [1–5]. We have been investigating the mechanisms involved in immunological disturbances found in silicosis, with focus on the Fas-mediated apoptotic pathway, because Fas is a cell surface receptor protein which belongs to the tumour necrosis factor (TNF) receptor family [6–9] and because abnormalities in Fas and related molecules have been reported in human idiopathic autoimmune diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) [10–12]. In addition, it has also been suggested that Fas-mediated apoptosis plays a crucial role in the acquisition of autoimmunity, because mutations of the Fas and Fas ligand (FasL) genes have been identified in autoimmune strains of mice, i.e. lpr mice for the former [13] and gld mice for the latter [14]. We have found the serum levels of the soluble Fas (sFas) molecule to be elevated in silicosis patients with no clinical symptoms of autoimmune diseases such as sclerotic skin, Raynaud's phenomenon, facial erythema or arthralgia (Table 1) [15]. Furthermore, the sFas message derived from peripheral blood mononuclear cells (PBMC) was dominantly expressed in these patients [16]. Based on these investigations, dysregulation of the Fas-mediated apoptotic pathway may play an important role in the pathogenesis of the immunological abnormalities found in silicosis. In addition, it has been reported that silicone-containing macrophages prevent activation-induced cell death in murine lymphocytes [17]. These results have led us to consider that it may be difficult for the T lymphocytes in patients with silicosis to proceed into apoptosis mediated by the Fas-related pathway. Moreover, based on our previous results which demonstrated that silica compounds act as superantigens against human T cells [18], these T cells might include self-recognizing clones. On the other hand, Smalley et al. [19] reported that the T cell response to silicon dioxide is monocyte-dependent and not a superantigen.
Table 1.
Serum levels of soluble Fas (sFas) in healthy volunteers (Cont.) and patients with silicosis, progressive systemic sclerosis (PSS) or systemic lupus erythematosus (SLE)
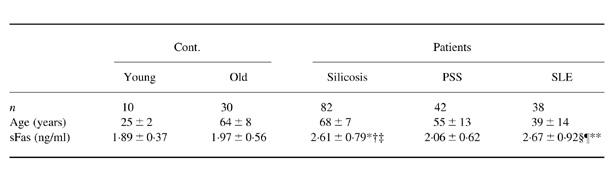
Values are the mean 6s.d.
* P < 0.005 versus Cont. (young); † P < 0.0001 versus Cont. (old); ‡ P < 0.0001 versus PSS; § P < 0.01 versus Cont.(young); ¶ P < 0.001 versus Cont. (old); ** P < 0.005 versus PSS. Details were reported in a previous paper [15].
It has been reported that FasL, the principle ligand for Fas receptor [8], is expressed in activated T cells and natural killer (NK) cells [20,21], and that the soluble form of FasL, which is cleaved by a matrix metalloproteinase-like enzyme from membrane-bound FasL [22,23], is elevated in the serum of patients with large granular lymphocytic leukaemia [20], NK cell lymphoma [20], acute graft-versus-host diseases [24], myocarditis [25], alcoholic liver disease [26], and Sjögren's syndrome [10]. To determine further the role of the Fas/FasL system in silica-induced immunological abnormalities, we investigated the serum levels of sFasL in silicosis patients with no clinical symptoms of autoimmune diseases, and analysed the relationship between sFasL levels and clinical parameters or sFas levels.
PATIENTS AND METHODS
Patients and sera
Serum samples were obtained from 82 patients with silicosis (74 men and eight women, average age 68 ± 7 years) with no clinical symptoms of autoimmune diseases such as sclerotic skin, Raynaud's phenomenon, facial erythema or arthralgia, 42 patients with progressive systemic sclerosis (PSS) (all women, average age 55 ± 13 years), 38 patients with SLE (nine men and 29 women, average age 39 ± 14 years), and 10 younger (all women, average age 25 ± 2 years) and 30 older (14 men and 16 women, average age 64 ± 8 years) healthy volunteers. Specimens were taken only from cases from whom informed consent had been obtained.
sFasL analysis
Serum levels of sFasL were determined by a sandwich ELISA using two anti-FasL MoAbs, 4H9 and 4A5 (sFas Ligand ELISA Kit; MBL, Nagoya, Japan). Briefly, 100 μl of diluted human serum were incubated in a 96-well microplate coated with 4H9 for 1 h. After washing, peroxidase-conjugated 4A5 antibody was added to the wells and the microplate was incubated for 1 h. Then the wells were washed and treated with a peroxidase substrate mixture (TMB and H2O2) for 30 min. The optical density (OD) at 450 nm was read using a microplate reader (Model 450; BioRad, Hercules, CA). A solution of sFasL for calibration was used to standardize the ELISA.
sFas analysis
Serum levels of sFas were analysed using a sFas(s) ELISA kit (MBL) as described previously [15].
Statistical analysis
Data are expressed as the mean ± s.d. Statistical significance was calculated using the Mann–Whitney U-test, and P < 0.05 was considered significant. Pearson's correlation coefficient was also used to examine correlations between the levels of sFas and sFasL, and the deviation was examined by Fisher's Z exchange.
RESULTS
Serum levels of sFasL
In the healthy volunteers no significant differences were observed in serum sFasL levels (mean ± s.d.) between the younger (0.18 ± 0.06 ng/ml) and older (0.16 ± 0.07 ng/ml) generations (Fig. 1) or between men and women, which were similar to those observed in the serum sFas levels (Table 1). These findings imply that the age and gender of patients are not important factors in either sFasL analysis or sFas analysis. As shown in Fig. 1, there were no significant differences in serum sFasL levels between the silicosis patients (0.16 ± 0.07 ng/ml) and healthy volunteers (0.16 ± 0.07 ng/ml), although the serum sFas levels in silicosis patients increased significantly (2.61 ± 0.79 ng/ml, P < 0.0005) compared with those in healthy volunteers (1.97 ± 0.56 ng/ml) (Table 1). No increase in sFasL was observed in PSS patients either (0.18 ± 0.06 ng/ml). However, serum sFasL levels in SLE patients (0.23 ± 0.08 ng/ml) were significantly higher than those in healthy volunteers (P < 0.0005, Fig. 1), as were the sFas levels (Table 1). Furthermore, sFasL levels in SLE patients were significantly higher than those in silicosis (P < 0.0001) and PSS (P < 0.005) patients.
Fig. 1.
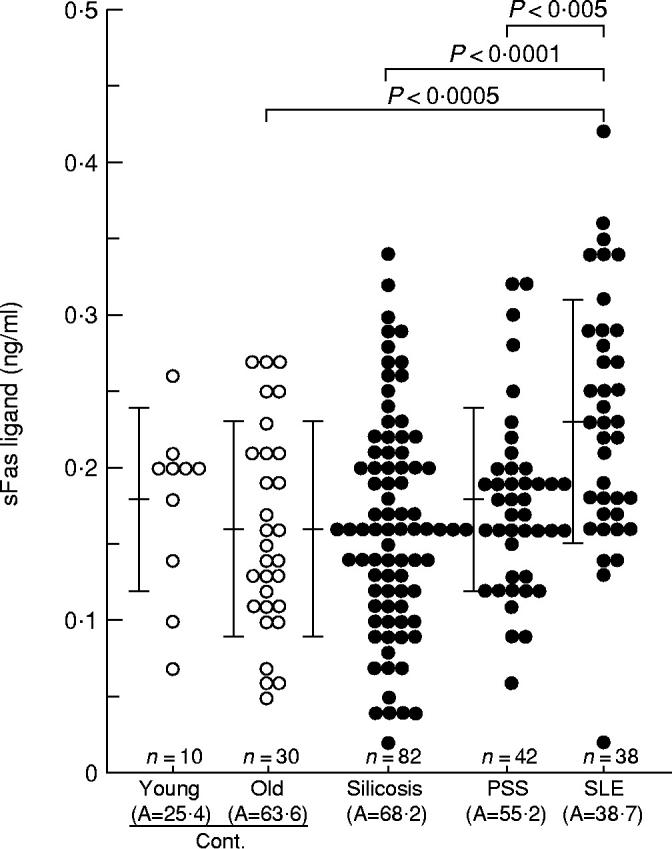
Serum levels of soluble Fas ligand (sFasL) in healthy volunteers (Cont.) and patients with silicosis, progressive systemic sclerosis (PSS) or systemic lupus erythematosus (SLE). Serum sFasL levels were analysed using a sFasL ELISA kit. A, Average age. Bars indicate the mean ± s.d. in each group.
Relationship between sFasL levels and clinical parameters in patients with silicosis
The relationship between serum levels of sFasL and the clinical parameters in silicosis patients was determined. As shown in Table 2, there was no relationship between levels of sFasL and the radiological classification (PR0–4), PO2 values (≥ 80, < 80 torr), the duration of exposure to silica dust (< 30, ≥ 30 years) or the titres of ANA (< 1:40, 1:40 or 1:80 and ≥ 1:160). However, when sFasL levels were analysed according to the stages of pulmonary dysfunction, sFasL levels in patients with slight dyspnoea were significantly higher than those in patients with severe dyspnoea (P < 0.01). In addition, patients with normal PCO2 values (PCO2 < 40 torr) showed significantly higher sFasL levels (P < 0.05) than patients in the advanced stages of pulmonary dysfunction (PCO2≥ 40 torr).
Table 2.
Serum sFasL levels of patients with silicosis according to clinical parameters
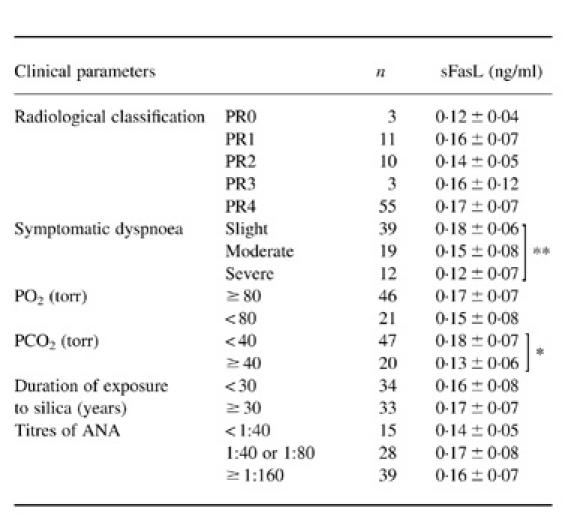
Values are the mean ±s.d.
* P < 0.05; ** P < 0.01.
PR, Profusion rate.
Correlation between sFas and sFasL levels
There was no correlation between serum levels of sFas and sFasL in patients with silicosis (Fig. 2a) or PSS (data not shown). On the other hand, SLE patients showed a slight positive correlation between sFas and sFasL levels (r = 0.313), even though the P value was 0.0522 (Fig. 2b).
Fig. 2.
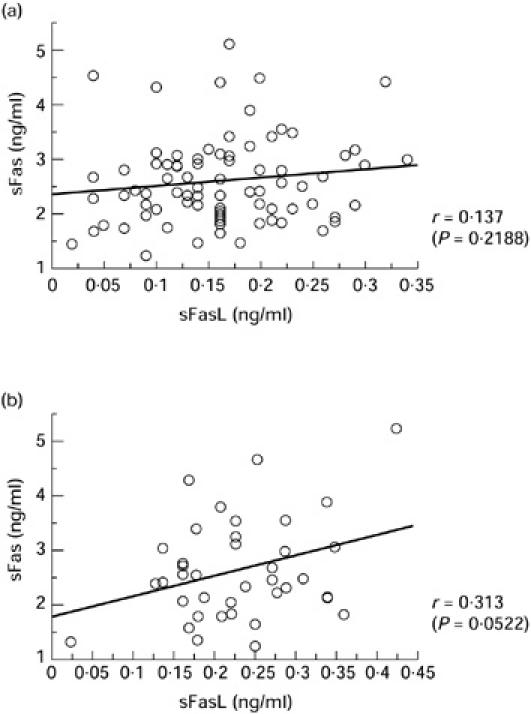
Correlation between the serum levels of sFas and soluble Fas ligand (sFasL) in patients with silicosis (a) and systemic lupus erythematosus (SLE) (b). r, Pearson's correlation coefficient.
DISCUSSION
Although the pathogenesis of autoimmune diseases has not been fully defined, the inability to eliminate self-reactive T or B cells may play a role in the development of immunological disorders. Mutations of genes, which lead to defects in programmed cell death, have been identified in autoimmune strains of mice, e.g. lpr mice with spontaneous mutation in the Fas-encoding locus [13] and gld mice with mutation in the Fas ligand locus [14].
In this study, findings showed the serum levels of sFasL in patients with silicosis to be not significantly elevated compared with those of healthy volunteers, whereas those of SLE were higher than healthy volunteers. In addition, although the correlation between the serum levels of sFas and sFasL in silicosis patients was not significant, there was a slight positive correlation (r = 0.313) in SLE patients, even though the P value was 0.0522. This result in SLE patients coincides with findings reported by Nozawa et al., in which SLE patients with high levels of sFasL tended to have high levels of sFas [10]. We have previously reported that serum sFas levels in silicosis patients with no autoimmune disease manifestations and patients with SLE were elevated significantly compared with those of healthy volunteers [15]. Based on these findings, it seems that the elevated sFasL levels may contribute to the clinical manifestations of autoimmune diseases, such as SLE. Further investigations to determine the role of sFasL in autoimmune diseases as well as in silicosis must be done. Therefore, we are planning to follow up the patients studied here, harvesting serum and measuring serum levels of apoptosis-related molecules such as sFas and sFasL.
Serum sFasL levels were elevated in silicosis patients with slight dyspnoea or normal PCO2 among various clinical parameters for silicosis. In addition, our previous study of serum sFas levels also showed serum sFas levels to be elevated in silicosis patients with slight dyspnoea or normal PO2 [15]. Taken together, these findings indicate that abnormal manifestations in Fas-related molecules; i.e. sFas and sFasL, principally involved patients with slight respiratory disorders. It may be speculated that the immunological disturbances presented by the abnormalities of apoptosis-related molecules in silicosis patients do not occur with a similar degree of respiratory involvement. In fact, the correlation between the duration of exposure to silica dust and the serum levels of sFas or sFasL was not significant (data not shown). The severity of the lung disorders and the development of autoimmune diseases might be dependent on different factors, such as HLA class I and class II alleles or environmental factors. Furthermore, there is an increased prevalence of both ANA and rheumatoid factor in silicosis even in patients without evidence of autoimmune diseases [27]. However, the relationship of changes in the Fas/FasL to autoantibody production per se is also unclear.
In the present study, no elevation of serum sFasL levels was observed in silicosis patients, but it was exhibited in patients with SLE. It remains to be clarified whether silicosis patients with abnormal apoptosis-related molecules, such as Fas and FasL, have a tendency to develop autoimmune diseases later, or whether some other distinct factor(s) is necessary to initiate the progression of autoimmune diseases. Furthermore, the effect of silica exposure on the Fas/FasL system in workers without silicosis must also be determined.
Acknowledgments
The authors thank Miss H. Sakaguchi for her excellent technical assistance and Professor David H. Waterbury for his editorial contribution to the manuscript. This work was supported by a Kawasaki Medical School Project Grant (10–603) and a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Science and Culture (1998).
REFERENCES
- 1.Haustein UF, Ziegler V, Herrmann K, Mehlhorn J, Schmidt C. Silica-induced scleroderma. J Am Acad Dermatol. 1990;22:444–8. doi: 10.1016/0190-9622(90)70062-m. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez-Roman J, Wichmann I, Salaberri J, Varela JM, Nunez-Roldan A. Multiple clinical and biological autoimmune manifestations in 50 workers after occupational exposure to silica. Ann Rheum Dis. 1993;52:534–8. doi: 10.1136/ard.52.7.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rustin MH, Bull HA, Ziegler V, Mehlhorn J, Haustein UF, Maddison PJ, James J, Dowd PM. Silica-associated systemic sclerosis is clinically, serologically and immunologically indistinguishable from idiopathic systemic sclerosis. Br J Dermatol. 1990;123:725–34. doi: 10.1111/j.1365-2133.1990.tb04189.x. [DOI] [PubMed] [Google Scholar]
- 4.Uber CL, McReynolds RA. Immunotoxicology of silica. Crit Rev Toxicol. 1982;10:303–19. doi: 10.3109/10408448209003370. [DOI] [PubMed] [Google Scholar]
- 5.Haslam PL. Parkes WR. Occupational lung disorders. 3. London: Butterworth Heinemann; 1994. Basic immunology and immunopathology; pp. 50–99. [Google Scholar]
- 6.Itoh N, Yonehara S, Ishii A, et al. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–43. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 7.Itoh N, Nagata S. A novel protein domain required for apoptosis. Mutational analysis of human Fas antigen. J Biol Chem. 1993;268:10932–7. [PubMed] [Google Scholar]
- 8.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–56. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 9.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–65. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 10.Nozawa K, Kayagaki N, Tokano Y, Yagita H, Okumura K, Hasimoto H. Soluble Fas (APO-1, CD95) and soluble Fas ligand in rheumatic diseases. Arthritis Rheum. 1997;40:1126–9. doi: 10.1002/art.1780400617. [DOI] [PubMed] [Google Scholar]
- 11.Hasunuma T, Kayagaki N, Asahara H, et al. Accumulation of soluble Fas in inflamed joints of patients with rheumatoid arthritis. Arthritis Rheum. 1997;40:80–86. doi: 10.1002/art.1780400112. [DOI] [PubMed] [Google Scholar]
- 12.Tsokos GC, Kovacs B, Liossis SN. Lymphocytes, cytokines, inflammation, and immune trafficking. Curr Opin Rheumatol. 1997;9:380–6. doi: 10.1097/00002281-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–7. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–76. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 15.Tomokuni A, Aikoh T, Matsuki T, et al. Elevated soluble Fas/APO-1 (CD95) levels in silicosis patients without clinical symptoms of autoimmune diseases or malignant tumours. Clin Exp Immunol. 1997;110:303–9. doi: 10.1111/j.1365-2249.1997.tb08332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otsuki T, Sakaguchi H, Tomokuni A, et al. Soluble Fas mRNA is dominantly expressed in cases with silicosis. Immunology. 1998;94:258–62. doi: 10.1046/j.1365-2567.1998.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald AH. Silicone-containing macrophages prevent activation-induced cell death in murine lymphocytes. Exp Biol. 1999 Abstr. 847.2. [Google Scholar]
- 18.Ueki A, Yamaguchi M, Ueki H, Watanabe Y, Ohsawa G, Kinugawa K, Kawakami Y, Hyodoh F. Polyclonal human T-cell activation by silicate in vitro. Immunology. 1994;82:332–5. [PMC free article] [PubMed] [Google Scholar]
- 19.Smalley DL, Shanklin DR, Hall MF. Monocyte-dependent stimulation of human T cells by silicon dioxide. Pathobiology. 1998;66:302–5. doi: 10.1159/000028037. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka M, Suda T, Haze K, et al. Fas ligand in human serum. Nat Med. 1996;2:317–22. doi: 10.1038/nm0396-317. [DOI] [PubMed] [Google Scholar]
- 21.Arase H, Arase N, Saito T. Fas-mediated cytotoxicity by freshly isolated natural killer cells. J Exp Med. 1995;181:1235–8. doi: 10.1084/jem.181.3.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kayagaki N, Kawasaki A, Ebata T, et al. Metalloproteinase-mediated release of human Fas ligand. J Exp Med. 1995;182:1777–83. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka M, Suda T, Takahashi T, Nagata S. Expression of the functional soluble form of human Fas ligand in activated lymphocytes. EMBO J. 1995;14:1129–35. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanda Y, Tanaka Y, Shirakawa K, et al. Increased soluble Fas-ligand in sera of bone marrow transplant recipients with acute graft-versus-host disease. Bone Marrow Transplant. 1998;22:751–4. doi: 10.1038/sj.bmt.1701427. [DOI] [PubMed] [Google Scholar]
- 25.Toyozaki T, Hiroe M, Tanaka M, Nagata S, Ohwada H, Marumo F. Levels of soluble Fas ligand in myocarditis. Am J Cardiol. 1998;82:246–8. doi: 10.1016/s0002-9149(98)00300-2. [DOI] [PubMed] [Google Scholar]
- 26.Taieb J, Mathurin P, Poynard T, Gougerot-Pocidalo MA, Chollet-Martin S. Raised plasma soluble Fas and Fas-ligand in alcoholic liver disease. Lancet. 1998;351:1930–1. doi: 10.1016/S0140-6736(05)78614-1. [DOI] [PubMed] [Google Scholar]
- 27.Doll NJ, Stankus RP, Hughes J, et al. Immune complexes and autoantibodies in silicosis. J Allergy Clin Immunol. 1981;68:281–5. doi: 10.1016/0091-6749(81)90152-4. [DOI] [PubMed] [Google Scholar]


