Abstract
IL-4 plays a key role in driving the differentiation of CD4+ Th precursors into Th2 cells, both in mice and in humans. The source of IL-4 during primary immune responses is, however, still debated. When IL-4 consumption in in vitro T cell cultures was blocked with a MoAb to the IL-4 receptor α-chain (IL-4Rα), it became evident that freshly isolated naive (CD45RO−) CD4+ T cells from adults or cord blood produce IL-4 upon activation with anti-CD3 and CD80. IL-4 production by naive T cells is strictly IL-2-dependent. Endogenous IL-4 activity in naive CD4+ T cell cultures modulates the production of interferon-gamma (IFN-γ) on the one hand and IL-5 and IL-13 on the other hand in opposite directions, and it is partly responsible for the low IFN-γ production by cord blood T cells. Comparison of the ratio of IL-4/IFN-γ in supernatants of T cell cultures reveals a skewing towards IL-4 production by cord blood T cells, while naive T cells from (non-atopic) adults predominantly produce IFN-γ. We conclude that CD4+ naive T cells can produce IL-4 without the need for Th2 differentiation, and therefore that they can be the initial source of IL-4 required at the time of priming for T cell differentiation into Th2 cells.
Keywords: IL-4, CD45RA+ T cells, CD80, IL-4R, neonatal
INTRODUCTION
IL-4 is a 15-kD protein that has been shown to regulate a wide spectrum of functions in B cells, T cells, monocytes/macrophages and other haematopoietic and non-haematopoietic cells [1]. The high-affinity IL-4R consists of a 140-kD α receptor subunit, associated to the common γc chain, which in T cells is needed for IL-4 signal transduction [2,3]. IL-4 plays a key role in driving the differentiation of CD4+ Thp into Th2 cells [4,5] and in inducing B cells to switch to IgE production. IL-4 is therefore thought to be an essential component of atopic diseases [1,5,6].
Among T cell clones, IL-4 is produced by Th0 and Th2 cells, but not Th1 cells, and this has now been demonstrated both in mice [4] and in humans [5]. Since differentiation of Thp into Th2 cells requires IL-4 [7], the question arises which cells produce the initial IL-4 at the time of T cell priming [8]. In mice NK1.1+ T cells might be the initial IL-4 producers [9,10]. However, mice depleted of NK1.1 cells have normal Th2 responses [11]. γδ+ T cells and Mel-14low memory T cells have also been proposed as the primary source of IL-4 [12,13]. Besides T cells, mast cells and basophils can produce IL-4 and mRNA for IL-4 has also been found in eosinophils [14–16]. However, the IL-4 released by these cells in response to several stimuli is unlikely to be significant in the early encounter between T cell and antigen-presenting cell. Moreover, reconstitution of IL-4-deficient mice with purified wild-type CD4+ cells reveals that IL-4 production does not require non-T cells [17]. Among human CD4+ T cells, IL-4 protein synthesis was restricted to the CD4+CD45RA− (memory) subpopulation [18,19]. The initial source of IL-4 production is therefore still debated [8,20], although recent data indicate that CD4+ naive cells themselves may be able to provide the early IL-4 required for Th2 cell differentiation [21,22]. Data in mice indicate that naive CD4+ T cells release IL-4 at priming and develop into Th2 effectors upon in vitro priming with a low concentration of antigen or altered peptide ligands [23]. Human naive CD4+ T cells also can produce low levels of IL-4 during the first 3 days of anti-CD3/B7.1 activation [24]. Moreover, upon repeated stimulation in vitro with anti-CD3 MoAb captured on CD32/B7.1 cells and IL-2, virtually every human naive CD4+ cell grown in a single-cell culture can develop into a Th2 cell as a result of this low level IL-4 production [24–26].
We have recently shown that IL-4 production in cell cultures is often underestimated as a result of consumption, which can however be inhibited by the use of a MoAb against the IL-4Rα chain [27]. We used this technique here to study whether naive CD45RO− T cells can indeed produce IL-4 and therefore be the initial source of IL-4 production in primary immune reactions.
MATERIALS AND METHODS
Isolation of peripheral and cord blood cells
Peripheral blood mononuclear cells (PBMC) were obtained from healthy adult volunteers of both sexes. Only donors with a negative history of asthma, obstructive airway disease, hay fever, eczema or urticaria, obtained by a standard questionnaire, were selected. Heparinized venous blood was centrifuged on Ficoll–Hypaque density gradients. Interphase cells were washed three times with Dulbecco's PBS (Boehringer Ingelheim Bioproducts, Heidelberg, Germany) and resuspended at a concentration of 5 × 106 cells/ml in complete medium, consisting of RPMI 1640 (Boehringer Ingelheim Bioproducts) supplemented with 2 mml-glutamine, penicillin 100 U/ml, streptomycin 100 μg/ml, and 10% bovine calf serum (Hyclone, Logan, UT). T cells were isolated as reported [28]. Lymphokwik-T (One Lambda Inc., Los Angeles, CA) was used to deplete monocytes and B cells. A MoAb mixture, consisting of complement-fixing anti-Leu-11b (anti-CD16; Becton Dickinson, Erembodegem, Belgium) and anti-NKH-1A (anti-CD56; Coulter, Hialeah, FL) MoAb, was then added for 30 min at 4°C followed by a second Lymphokwik-T treatment; resulting T cells were > 98% CD3+ and < 1% CD64+. To isolate adult CD4+ T cells, the second Lymphokwik treatment was replaced by treatment with Lymphokwik-Th (One Lambda Inc.). T cells were then incubated with either a mouse anti-human anti-CD45RO MoAb, UCHL-1 (a gift from Dr P. Beverley, E. Jenner Institute for Vaccine Research, Compton, UK) or a mouse anti-human anti-CD45RA MoAb 2H4 (Coulter), washed, and goat anti-mouse IgG Dynabeads (Dynal, Oslo, Norway) were added, according to the instructions of the manufacturers. Negatively selected T cells or CD4+ T cells were > 97% CD3+/CD45RA+, > 95% CD4+/CD45RA+, > 97% CD3+/CD45RO+ or > 95% CD4+/CD45RO+ T cells, respectively. CD45RO+ and CD45RA+ double-positive cells were < 5% in both isolated cell fractions.
To isolate cord blood mononuclear cells, heparinized cord blood was centrifuged on Ficoll–Hypaque density gradients. Interphase cells were washed three times with Dulbecco's PBS (Boehringer Ingelheim Bioproducts) and resuspended at a concentration of 5 × 106 cells/ml in complete medium. To isolate CD4+CD45RA+ T cells, monocytes and B cells were first removed by the use of Lymphokwik-T (One Lambda Inc.). Anti-CD4-coated Dynabeads (Dynal) were then used to isolate CD4+ T cells by positive magnetic immunoselection. Beads were detached from the cells by the use of DETACHaBEAD CD4/CD8 (Dynal), according to the guidelines provided by the manufacturers. CD45RO− cells were negatively selected as mentioned for adult T cells. Resulting cord blood cells were > 97% CD4+CD45RA+. Significant basophil contamination was excluded since CD123+ cells were < 0.07%. In some experiments T cells were positively selected by a mixture of anti-CD4- and anti-CD8-coated Dynabeads by magnetic immunoselection and detached as described, followed by negative selection of CD45RO− cells, as mentioned.
Anti-IL-4R monoclonal antibody
Mouse anti-human IL-4R MoAb (clone 25463.11) was bought from R&D (Abingdon, UK). To produce this MoAb, mice were immunized with recombinant soluble IL-4Rα. The MoAb blocks the bioactivity of both IL-4 and IL-13. According to the manufacturer, 3–6 ng of MoAb neutralizes 50% of the bioactivity of 200 pg of IL-4 on 105 TF-1 cells in a 1-ml volume. One milligram contains < 10 ng endotoxin and the MoAb is sold azide-free. We have also demonstrated that this MoAb has efficient blocking effect on IL-4 activity at the level of both B cells and T cells. Moreover, the MoAb by itself has no stimulating effect on B or T cell proliferation. In all experiments the MoAb was used at a concentration of 2.5 μg/ml [27].
Other monoclonal antibodies
MoAb UCHT-1 (mouse anti-human CD3) was a gift from Dr P. Beverley. Murine neutralizing anti-IL-2 MoAb (clone B-G5) was purchased from Diaclone (Besançon, France). Humanized mouse anti-human IL-2Rα (anti-CD25) MoAb anti-Tac (Zenapax), was purchased from Hoffman-La Roche (Nutley, NJ). Mouse anti-human anti-CD122 (mik β2) was bought from PharMingen (San Diego, CA).
Cytokine measurements
The concentrations of IL-4, IL-5, IL-10, IL-13 and interferon-gamma (IFN-γ) in culture supernatants were measured with a sandwich ELISA technique using combinations of unlabelled and biotin-coupled MoAb to different epitopes of each cytokine. Coating MoAbs for these five assays, respectively, were 860A4B3 (Medgenix Diagnostics, Fleurus, Belgium), TRFK5 (PharMingen), JES3–9D7 (PharMingen), JES10–5A2 (PharMingen) and 350B10G6 (Medgenix Diagnostics). Biotinylated detection MoAbs were 860F10H12 (Medgenix Diagnostics), JES1-SA10 (PharMingen), JES3–12G8 (PharMingen), B69–2 (PharMingen) and 67F12A8 (Medgenix Diagnostics). The concentrations of IL-2 in culture supernatants were detected by human IL-2 Duoset ELISA (Genzyme, Cambridge, MA). To prevent IL-2 consumption, anti-IL-2Rα (5 μg/ml) and anti-IL-2Rβ (2 μg/ml) MoAbs were added in the conditions in which IL-2 production had to be measured. Human recombinant IL-4, IL-5, IL-10, IL-13, IFN-γ and IL-2 were used as standards. The lower detection limit for all cytokines was < 20 pg/ml.
Cell lines
The P815 cell line is an NK-resistant DBA/2-derived mouse mastocytoma cell line that expresses mouse FcγRII and FcγRIII. The P815 cell line was derived from ATCC. P815 cells transfected with human CD80 or CD58 were a gift from L. L. Lanier (DNAX Research Institute of Molecular and Cellular Biology, Palo Alto, CA). The cells were cultured in complete medium supplemented with gentamycin 50 μg/ml, sodium pyruvate 1 mm, non-essential amino acids 1:100, 2-mercaptoethanol 50 μm and 10% fetal calf serum (Boehringer Ingelheim Bioproducts). Geneticin (400 μg/ml) was added every week for selecting transfected cells.
Activation of T cells for cytokine production
Purified T cells (5 × 105) were cultured in 24-well culture plates in a 1-ml volume at 37°C in 5% CO2/95% air and supernatants were collected after different periods of time as indicated in Results. T cells were stimulated with soluble anti-CD3 MoAb (UCHT-1) captured by mouse P815 or P815/CD80 or P815/CD58 mastocytoma cells, at a T/P815 ratio of 1:1. P815 cells were mitomycin-C-treated and washed five times before use.
Statistical analysis
Results were compared with non-parametric Wilcoxon paired tests or Mann–Whitney tests, where appropriate.
RESULTS
IL-4 production by human memory and naive T cells from adult non-atopic donors
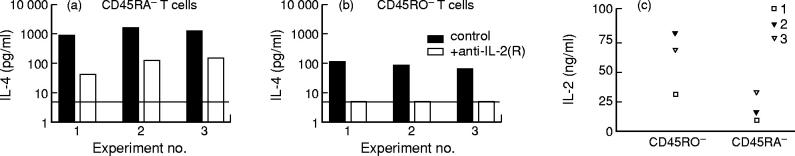
It has been reported previously that upon stimulation through the TCR/CD3 complex, ligation of CD28 on resting T cells by its ligand CD80 results in the induction of IL-4 production by total T cells [29]. To study the IL-4 production by separated human memory CD45RA− and naive CD45RO− T cells, we used a similar in vitro culture system. Memory T cells or CD4+ T cells of the memory phenotype indeed produced high amounts of IL-4 upon in vitro stimulation. As indicated in Fig. 1a, no difference in IL-4 production was observed whether adult memory T cells were cultured in the presence or absence of the blocking anti-IL-4Rα MoAb. In contrast, IL-4 levels in cultures of naive T cells stimulated under the same conditions could only be detected when IL-4 consumption had been blocked by the use of the anti-IL-4Rα MoAb (Fig. 1b). Naive T cells (mean production 104 pg/ml) produced two to 10 times less IL-4 than their memory T cell counterpart (mean production 1784 pg/ml) (P < 0.001) (Fig. 1a,b).
Fig. 1.
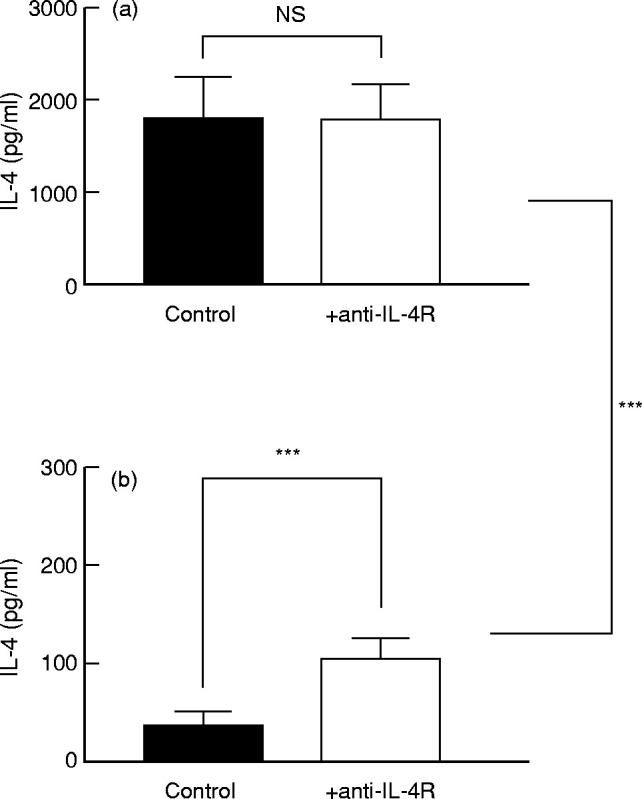
Effect of anti-IL-4R MoAb on IL-4 levels in cultures of human memory and naive T cells. Resting memory (CD45RA−) peripheral blood T cells (CD3+ T cells in five experiments or CD4+ T cells in seven experiments) (a) and paired resting naive (CD45RO−) CD3+ or CD4+ T cells (b) were cultured with P815/CD80 cells (5 × 105/ml) in the presence or absence of anti-IL-4R MoAb (2.5 μg/ml). Anti-CD3 MoAb (UCHT1) was added at a concentration of 2 μg/ml. Supernatants were collected after 72 h of culture and the IL-4 concentrations in the supernatants were determined by ELISA. The results (mean ± s.e.m.) of independent paired experiments with cells from 12 non-atopic adult donors are shown. NS, Not significant. ***P < 0.001.
No significant IL-4 production could be induced by either memory or naive CD8+ T cells upon stimulation under the same conditions (data not shown).
IL-4 production by naive cord blood CD4+ T cells
It has been debated whether all CD45RO− cells are naive T cells, because memory T cells could lose their CD45RO expression, and so be falsely considered as ‘naive’ cells [30]. To be more certain about the naive status of the T cells, we isolated CD45RO− cord blood CD4+ T cells, and stimulated them for 3 days with anti-CD3 and P815/CD80 transfected cells, in the presence or absence of anti-IL-4Rα MoAb. As shown in Fig. 2, we found IL-4 levels in the supernatants of T cell cultures performed in the presence of anti-IL-4Rα MoAb, but not in those without anti-IL-4Rα MoAb (P < 0.01). Thus, cord blood naive T cells also produce IL-4, although less than memory T cells from adults (P < 0.05).
Fig. 2.
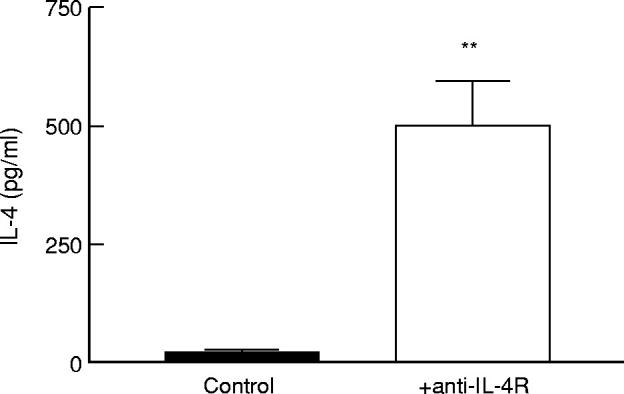
Effect of anti-IL-4R MoAb on IL-4 production by human neonatal naive CD4+ T cells. Resting CD4+CD45RO− cord blood T cells (5 × 105/ml) were cultured with P815/CD80 cells (5 × 105/ml) in the presence or absence of anti-IL-4R MoAb (2.5 μg/ml). Anti-CD3 MoAb (UCHT1) was added at a concentration of 2 μg/ml. Supernatants were collected after 72 h of culture and the IL-4 concentrations in the supernatants were determined by ELISA. The results (mean ± s.e.m.) of seven independent experiments are shown. **P < 0.01.
Only marginal amounts of IL-4 could be detected in cord blood CD4+ T cell cultures when stimulated with anti-CD3 and untransfected P815 cells. Furthermore, costimulation of cord blood CD4+ T cells by CD58 on P815 transfected cells could not induce IL-4 production (data not shown).
All further experiments on IL-4 production were performed in the presence of the anti-IL-4R MoAb.
IL-4 production by naive and memory T cells is differentially dependent upon IL-2
IL-4 production in naive murine T cells is dependent upon IL-2 production [31]. We wanted to study the role of IL-2 in IL-4 production by naive human T cells. To that aim, we quantified IL-4 production in the presence or absence of a MoAb combination that blocks all IL-2 activity [32]: a neutralizing anti-IL-2 MoAb in combination with an anti-IL-2Rα and an anti-IL-2Rβ MoAb (anti-IL-2(R)). Naive adult T cells did not produce IL-4 when IL-2 activity had been blocked (see Fig. 3b). For memory CD45RA− T cells, the effect of blocking IL-2 strongly varied from donor to donor, as shown in Fig. 3a. Figure 3c shows the IL-2 production by purified memory and naive adult T cells in the same experiments. Naive T cells produced higher amounts of IL-2 than their memory counterpart. It is therefore unlikely that inability to neutralize IL-2 in memory T cell cultures would account for their residual IL-4 production in the presence of anti-IL-2(R).
Fig. 3.
Importance of IL-2 for IL-4 production by naive and memory T cells. CD45RA− (a,c) or CD45RO− (b,c) adult T cells (5 × 105/ml) from three adult non-atopic donors were cultured with P815/CD80 cells (5 × 105/ml) and anti-CD3 MoAb (UCHT1) at a concentration of 2 μg/ml, in the presence of anti-IL-4R MoAb (2.5 μg/ml). To block all IL-2 activity, a neutralizing anti-IL-2 antibody (1 μg/ml) together with anti-IL-2Rα (5 μg/ml) and anti-IL-2Rβ (2 μg/ml) MoAbs were added as indicated (a,b). Supernatants were collected after 72 h and the IL-4 (a,b) and IL-2 (c) concentrations in the supernatants were determined by ELISA. The detection limit for IL-4 in these experiments (a) is represented by a horizontal black line.
We also repeated the same experiments with cord blood naive CD4+ T cells. When IL-2 activity was blocked, IL-4 was not detectable in cord blood CD4+ T cell supernatants (Fig. 4a). When kinetically analysed, IL-2 production by naive T cells was detectable as early as 5 h after the start of stimulation, whereas IL-4 production occurred later (see Fig. 4b). We can conclude that IL-4 production in naive adult and cord blood T cells is totally IL-2-dependent, whereas it is only partially dependent upon IL-2 in memory T cells. It is likely that the T cells which produce IL-4 independently of IL-2 are those T cells that have differentiated into Th2 cells [33].
Fig. 4.
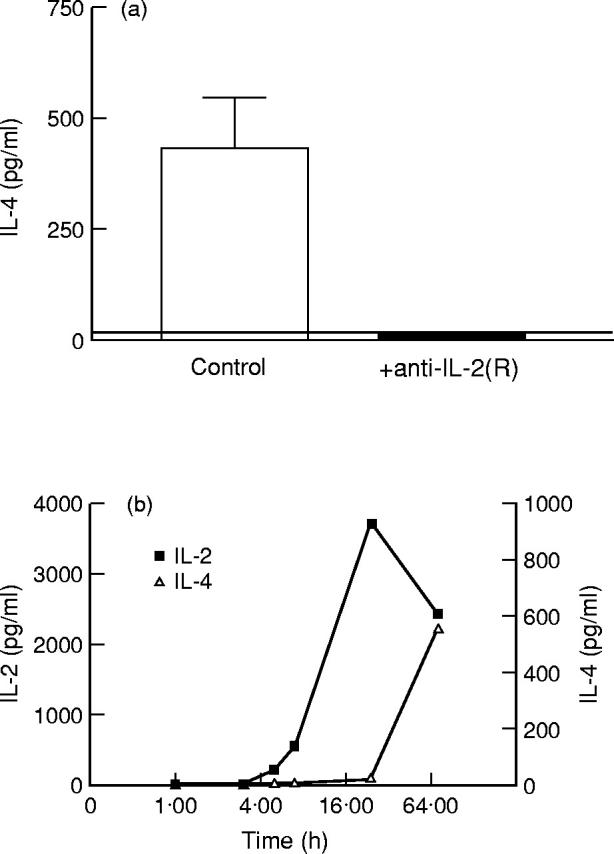
Importance of IL-2 for IL-4 production by naive cord blood T cells. CD4+CD45RO− cord blood T cells (5 × 105/ml) were cultured with P815/CD80 cells (5 × 105/ml) in the presence of anti-IL-4R MoAb (2.5 μg/ml) and anti-CD3 MoAb (UCHT1) at a concentration of 2 μg/ml. To block IL-2 activity, a neutralizing anti-IL-2 antibody (1 μg/ml) and anti-IL-2Rα (5 μg/ml) and anti-IL-2Rβ (2 μg/ml) MoAbs were added as indicated (a). Supernatants were collected after 72 h (a) or after 1, 3, 5, 7, 24 and 72 h (b) of culture and the IL-2 (b) and IL-4 (a,b) concentrations in the supernatants were determined by ELISA. The detection limit for IL-4 in these experiments (a) is represented by a horizontal black line. The results (mean ± s.e.m.) of three independent experiments (a) or one experiment (b) with different donors are shown.
IFN-γ/IL-4 balance in cultures of naive CD4+ T cells
In the next set of experiments we tried to show the balance between IFN-γ and IL-4 expressed by adult or neonatal naive CD4+ T cells (see Table 1). We also included memory CD4+ T cells in this comparison. IL-4/IFN-γ and IFN-γ/IL-4 ratios were calculated. From these ratios it can be concluded that adult naive CD4+ T cells preferentially produce IFN-γ, whereas cord blood naive CD4+ T cells preferentially produce IL-4. Cord blood neonatal T cells have the highest IL-4/IFN-γ balance when compared with either naive (P < 0.001) or memory adult T cells (P < 0.001). The difference in the IL-4/IFN-γ balance between adult naive CD4+ T cells and neonatal naive CD4+ T cells is mainly due to the difference in IFN-γ production, whereas in this regard the difference in IL-4 production is less important. Although we can not exclude that some of the adult CD45RO− T cells were not naive T cells, their low IL-4 production in contrast to the high IL-4 production by CD45RA− memory T cells argues against contamination by memory T cells. Furthermore, it has been described recently that although primed CD45RO−RA+ T cells might exist, they are not prevalent in peripheral blood and are rather sequestered within lymphoid tissue [34].
Table 1.
Comparison of IL-4 and IFN-γ production by adult and cord blood CD4+ T cells*
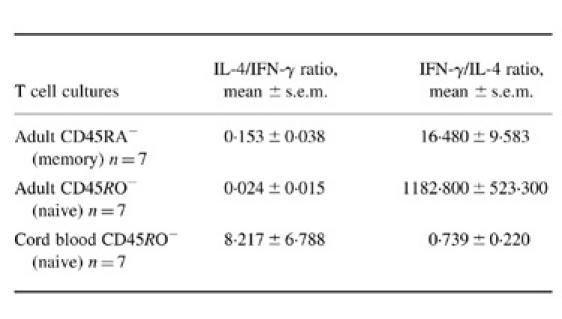
*Resting CD4+CD45RA−, CD4+CD45RO− adult T cells and CD4+CD45RO− cord blood T cells (5 × 105/ml) were cultured with P815/CD80 cells (5 × 105/ml). Anti-CD3 MoAb (UCHT1) was added at a concentration of 2 µg/ml. Anti-IL-4R MoAb (2.5 µg/ml) was added to the cultures for IL-4 production, and not to the cultures for IFN-γ production. Supernatants were collected after 72 h of culture and the IL-4 and IFN-γ concentrations in the supernatants were determined by ELISA.
Influence of endogenously produced IL-4 on the production of other cytokines by memory and naive T cells
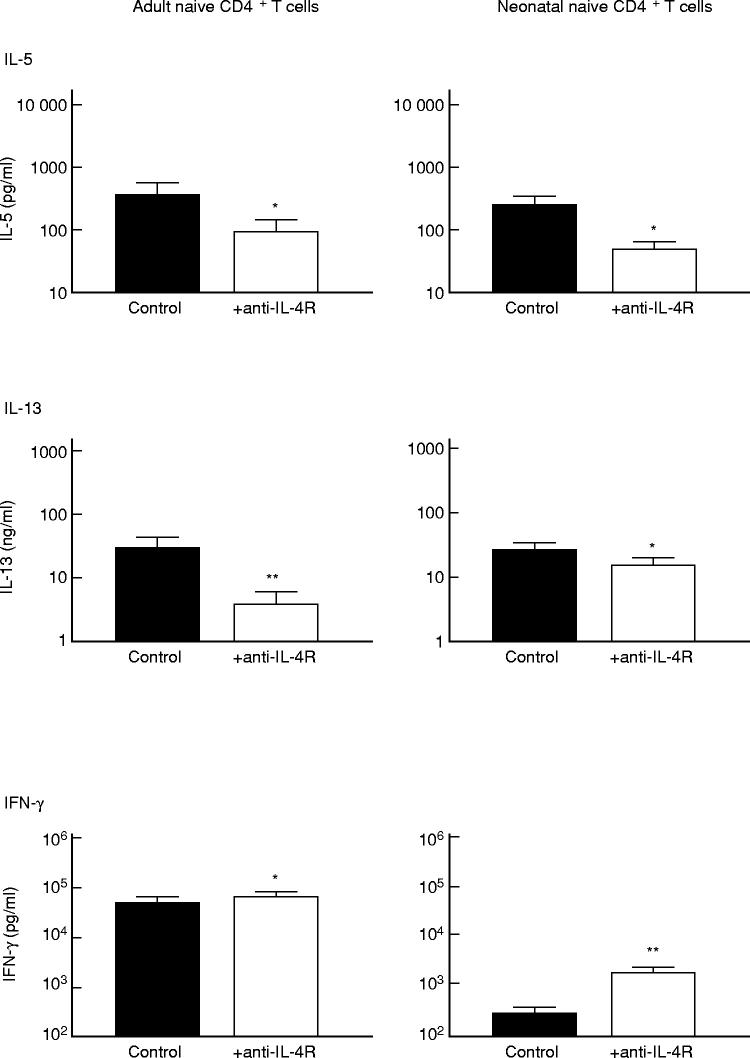
IL-4 is known to inhibit IFN-γ production [35,36] both in vitro and in vivo [37,38] and to enhance IL-5 and IL-10 production by T cells [18,39]. As we have shown that neonatal naive CD4+ T cells produce IL-4 but little IFN-γ, we wanted to study the effect of this IL-4 on the production of other cytokines. We used anti-IL-4Rα MoAb to block the potential effect of IL-4 on the production of other T cell cytokines (Fig. 5). First, the production of the Th1-type cytokine, IFN-γ, by neonatal CD4+ T cells was significantly increased as a result of blocking the IL-4Rα chain (P < 0.01) (Fig. 5). The inhibitory effect of endogenously produced IL-4 on in vitro IFN-γ production by neonatal T cells can contribute to the low IFN-γ production by these cells, as also reported in previous studies [40–42]. In contrast, IFN-γ production by adult naive CD4+ T cells, stimulated with anti-CD3 and CD80-transfected P815 cells, was only slightly enhanced after IL-4 signalling had been blocked (Fig. 5). Blocking the IL-4R in cultures of CD4+ naive T cells stimulated with captured anti-CD3 MoAb and P815/CD80-transfected cells significantly decreased IL-5 and IL-13 production (P < 0.05) (Fig. 5). Mean IL-10 production by both adult naive and cord blood naive T cells was below 100 pg/ml and not altered after IL-4 signalling had been blocked (data not shown).
Fig. 5.
Effect of endogenous IL-4 on cytokine production by human naive CD4+ T cells. Resting CD4+CD45RO− adult T cells or CD4+CD45RO− cord blood T cells at a concentration of 5 × 105 T cells in a 1-ml volume were cultured with P815/CD80 cells (5 × 105/ml), in the presence or absence of anti-IL-4R MoAb (2.5 μg/ml). Anti-CD3 MoAb (UCHT1) was added at a concentration of 2 μg/ml. Supernatants were collected after 72 h of culture and the IFN-γ, IL-5 and IL-13 concentrations in the supernatants were determined by ELISA. The results (mean ± s.e.m.) of seven independent experiments on different adult non-atopic donors and seven independent experiments with different cord blood samples are shown. *P < 0.05; **P < 0.01.
These results prove that IL-4 produced by naive T cells modulates their own cytokine production at least in in vitro experiments. Furthermore, IFN-γ production by neonatal CD4+ T cells is shown to be highly sensitive to the inhibitory effect of endogenous IL-4.
DISCUSSION
We here report that significant amounts of IL-4 are produced by naive CD45RO− adult T cells and by CD45RO−CD4+ neonatal T cells, when stimulated with anti-CD3- and CD80-transfected P815 cells. Detection of IL-4 in cultures of naive T cells requires blocking of the IL-4R with an anti-IL-4Rα MoAb. We have shown previously that IL-4 consumption interferes with attempts to quantify IL-4 production by human T cells [27]. Memory cells produced higher amounts of IL-4 than their naive counterparts in all donors. This suggests that at least part of the memory T cells have differentiated in vivo into Th2 cells [33]. Previous studies reporting low or undetectable IL-4 production by human peripheral blood naive T cells are probably biased by inadequate IL-4 evaluation [18,19]. Our findings that human naive CD4+ T cells produce IL-4 when stimulated with anti-CD3 captured by P815/CD80 cells are consistent with a limited set of results from other groups. Intracellular staining of IL-4 production in naive cord blood T cells revealed a small percentage of IL-4-producing lymphocytes (ranging from 1% to 2% of lymphocyte gated cells) [43]. However, even in total adult T cells, the mean percentage of IL-4-producing cells was not higher than 6% [43]. One other group also reported that human naive CD4+ T cells produce low levels of IL-4 during the first 3 days of anti-CD3/B7.1 activation, as evidenced by the observations that addition of anti-IL-4R neutralizing MoAb to primary T cell cultures reduced the differentiation into Th2 cells with IL-4/IL-5-producing capacity [24]; that IL-4 mRNA was detected after stimulation of naive CD4+ T cells with a mixture of soluble anti-CD3 and anti-CD28 MoAb [26] and that IL-4 protein could be measured in the supernatants of priming cultures when performed in the presence of anti-IL-4R blocking MoAb [26]. Although the primary source of IL-4 in primary immune responses is still debated, these and our results support the possibility that, in the human system, naive CD4+ T cells can provide the initial IL-4 required for differentiation into Th2 cells. Also in mice, naive CD4+ T cells themselves were reported to be responsible for the early production of IL-4 in the lymph node [21,22].
Costimulatory molecules are highly important for IL-4 induction. Indeed, naive T cells produced only very small amounts of IL-4 in the absence of CD80 costimulatory molecules. Previous studies in mice and humans also showed that IL-4 production at priming was critically dependent on CD28 costimulation [23,26,44,45]. In contrast, memory T cells, when stimulated with anti-CD3 alone, produced substantial levels of IL-4 and the amount of IL-4 strongly increased when they were costimulated by CD80 (our own unpublished observations). Costimulation by CD58 did not induce IL-4 production in naive T cell cultures.
We further have demonstrated, by the differences in the IL-4/IFN-γ ratios, that cord blood naive T cells produce relatively more IL-4, whereas adult naive T cells produce relatively more IFN-γ. This difference in IL-4/IFN-γ balance is mainly due to the low IFN-γ production by cord blood T cells, which is partly due to endogenous IL-4 and apparently to a higher sensitivity to IL-4. We indeed demonstrate in this study that the IL-4 produced by naive T cells influences the production of other cytokines by these cells. IL-4 produced in neonatal CD4+ T cell cultures decreased IFN-γ production and increased IL-5 and IL-13 production, as indicated by the significant changes found in the production of these cytokines after the IL-4R had been blocked. In neonatal T cell cultures, IFN-γ production is especially sensitive to inhibition by IL-4. This high sensitivity to IL-4 might be a contributing factor to the development of Th2 responses in the neonatal period. Recently Prescott et al. have similarly demonstrated that the cytokine/β-actin mRNA ratio in cord blood mononuclear cells upon allergen-specific stimulation was two to four times higher for IL-4 compared with the ratio for IFN-γ [46]. The high IL-4 production by neonatal T cells might be a residual effect of the pregnancy status [47,48]. Neonatal T cell responses after infection or vaccination are predominantly type 2 responses [49] and some groups have provided evidence that initial T cell responses are universally skewed towards the Th2 cytokine profile [50,51]. This bias can contribute to IgE production against common allergens and the development of atopic diseases in childhood. In our study, cord blood samples were taken without knowledge of the atopic state of the parents. To be more certain about preferential bias towards Th2 cytokine production by neonatal naive T cells, newborns should be followed to study whether they become atopics or not. Another cautionary note about the interpretation of our results is that we have focused on T cells only. However, besides T cells the dendritic cells also play an important role in the early immune responses, e.g. monocyte-derived dendritic cells were found to promote Th1 differentiation, whereas dendritic cells derived from plasmocytoid cells induced differentiation into Th2 cells [52]. Our in vitro system, however, did not allow us to study the role of the dendritic cell. Immune responses in vivo will therefore depend on characteristics of both T cells and dendritic cells.
In conclusion, our data indicate that both adult naive CD4+ T cells and neonatal naive CD4+ T cells produce IL-4 upon stimulation, without the need for differentiation into Th2 cells. Furthermore, IL-4 production by neonatal naive CD4+ T cells contributes to the low IFN-γ production by these cells. CD4+ naive T cells can be the initial source of IL-4 in primary immune responses, resulting in preferential T cell differentiation into Th2 cells when unbalanced by IL-12 effects.
Acknowledgments
We wish to thank Martine Adé for expert assistance with the ELISA techniques and Lieve Coorevits for general technical assistance. We are also grateful to the Obstetric Department of the ‘H. Hart Ziekenhuis’, Leuven, for providing cord blood samples. This work was supported by the ‘Onderzoeksfonds’ of the Catholic University of Leuven, grant OT 98/26. We also thank P. Beverley (E. Jenner Institute for Vaccine Research, Compton, UK) and L. L. Lanier (DNAX Research Institute of Molecular and Cellular Biology, Palo Alto, CA) for providing cells or reagents used in this study. D.M.A.B. is a research fellow supported by the Fund for Scientific Research (FWO), Vlaanderen, Belgium. S.W.v.G. is a postdoctoral fellow supported by the Fund for Scientific Research (FWO), Vlaanderen, Belgium.
REFERENCES
- 1.Paul WE. Interleukin 4: a prototypic immunoregulatory lymphokine. Blood. 1991;77:1859–70. [PubMed] [Google Scholar]
- 2.Keegan AD, Nelms K, Wang LM, Pierce JH, Paul WE. Interleukin 4 receptor: signaling mechanisms. Immunol Today. 1994;15:423–32. doi: 10.1016/0167-5699(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 3.Ryan JJ. Interleukin-4 and its receptor: essential mediators of the allergic response. J Allergy Clin Immunol. 1997;99:1–5. doi: 10.1016/s0091-6749(97)70293-8. [DOI] [PubMed] [Google Scholar]
- 4.Mosmann TR, Coffman RL. Heterogeneity of cytokine patterns and functions of helper T cells. Adv Immunol. 1989;46:111–47. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- 5.Romagnani S. Human Th1 and Th2 subsets: regulation of differentiation and role in protection and immunopathology. Int Arch Allergy Immunol. 1992;98:279–85. doi: 10.1159/000236199. [DOI] [PubMed] [Google Scholar]
- 6.Borish L, Rosenwasser LJ. Update on cytokines. J Allergy Clin Immunol. 1996;97:719–33. doi: 10.1016/s0091-6749(96)80146-1. [DOI] [PubMed] [Google Scholar]
- 7.Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990;172:921–9. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ricci M, Matucci A, Rossi O. Source of IL-4 able to induce the development of Th2-like cells. Clin Exp Allergy. 1997;27:488–500. [PubMed] [Google Scholar]
- 9.Yoshimoto T, Bendelac A, Watson C, Hu-Li J, Paul WE. Role of NK1.1+ T cells in a Th2 response and in immunoglobulin E production. Science. 1995;270:1845–7. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimoto T, Paul WE. CD4pos, NK.1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179:1285–95. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang MD, Ellison CA, Gartner JG, Hayglass KT. Natural killer cell depletion fails to influence initial CD4 T cell commitment in vivo in exogenous antigen-stimulated cytokine and antibody responses. J Immunol. 1998;160:1098–105. [PubMed] [Google Scholar]
- 12.Gollob KJ, Coffman RL. A minority subpopulation of CD4+ T cells directs the development of naive CD4+ T cells into IL-4-secreting cells. J Immunol. 1994;152:5180–8. [PubMed] [Google Scholar]
- 13.Vicari AP, Mocci S, Openshaw P, O'Garra A, Zlotnik A. Mouse γδ TCR+NK1.1+ thymocytes specifically produce interleukin-4, are major histocompatibility complex class I independent, and are developmentally related to αβ TCR+NK1.1+ thymocytes. Eur J Immunol. 1996;26:1424–9. doi: 10.1002/eji.1830260704. [DOI] [PubMed] [Google Scholar]
- 14.Brunner T, Heusser CH, Dahinden CA. Human peripheral blood basophils primed by interleukin 3 (IL-3) produce IL-4 in response to Immunoglobulin E receptor stimulation. J Exp Med. 1993;177:605–11. doi: 10.1084/jem.177.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moqbel R, Ying S, Barkans J, et al. Identification of messenger RNA for IL-4 in human eosinophils with granule localization and release of the translated product. J Immunol. 1995;155:4939–47. [PubMed] [Google Scholar]
- 16.Piccinni M-P, Macchia D, Parronchi P, et al. Human bone marrow non-B, non-T cells produce interleukin 4 in response to cross-linkage of Fcε and Fcγ receptors. Proc Natl Acad Sci USA. 1991;88:8656–60. doi: 10.1073/pnas.88.19.8656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitz J, Thiel A, Kuhn R, et al. Induction of interleukin-4 expression in T helper cells is not dependent on IL-4 from non-Th cells. J Exp Med. 1994;179:1349–53. doi: 10.1084/jem.179.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houssiau FA, Schandené L, Stevens M, et al. A cascade of cytokines is responsible for IL-9 expression in human T cells. Involvement of IL-2, IL-4 and IL-10. J Immunol. 1995;154:2624–30. [PubMed] [Google Scholar]
- 19.Lewis DB, Prickett KS, Larsen A, Grabstein K, Weaver M, Wilson CB. Restricted production of interleukin 4 by activated human T cells. Proc Natl Acad Sci USA. 1988;85:9743–7. doi: 10.1073/pnas.85.24.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi P, Reiser H. IL-4: role in disease and regulation of production. Clin Exp Immunol. 1998;113:317–9. doi: 10.1046/j.1365-2249.1998.00690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Croft M, Swain SL. Recently activated naive CD4 T cells can help resting B cells, and can produce sufficient autocrine IL-4 to drive differentiation to secretion of T helper 2-type cytokines. J Immunol. 1995;154:4269–82. [PubMed] [Google Scholar]
- 22.von der Weid T, Beebe AM, Roopenian DC, Coffman RL. Early production of IL-4 and induction of Th2 responses in the lymph node originate from an MHC class 1-independent CD4+ N.K.1.1− T cell population. J Immunol. 1996;157:4421–7. [PubMed] [Google Scholar]
- 23.Tao X, Constant S, Jorritsma P, Bottomly K. Strength of TCR signal determines the costimulatory requirements for Th1 and Th2 CD4+ T cell differentiation. J Immunol. 1997;159:5956–63. [PubMed] [Google Scholar]
- 24.Demeure CE, Yang LP, Byun DG, Ishihara H, Vezzio N, Delespesse G. Human naive CD4 T cells produce interleukin-4 at priming and acquire a Th2 phenotype upon repetitive stimulations in neutral conditions. Eur J Immunol. 1995;25:2722–5. doi: 10.1002/eji.1830250950. [DOI] [PubMed] [Google Scholar]
- 25.Yang L-P, Byun DG, Demeure CE, Vezzio N, Delespesse G. Default development of cloned human naive CD4 T cells into interleukin-4- and interleukin-5-producing effector cells. Eur J Immunol. 1995;25:3517–20. doi: 10.1002/eji.1830251247. [DOI] [PubMed] [Google Scholar]
- 26.Yang L-P, Demeure CE, Byun D-G, Vezzio N, Delespesse G. Maturation of neonatal human CD4+ T cells: role of B7 co-stimulation at priming. Int Immunol. 1995;7:1987–93. doi: 10.1093/intimm/7.12.1987. [DOI] [PubMed] [Google Scholar]
- 27.Bullens DMA, Kasran A, Peng X, Lorré K, Ceuppens JL. Effects of anti-interleukin-4-receptor monoclonal antibody on T cell cytokine levels in vitro: interleukin-4 production by T cells from non-atopic donors. Clin Exp Immunol. 1998;113:320–6. doi: 10.1046/j.1365-2249.1998.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ceuppens JL, Baroja ML, Lorre K, Van Damme J, Billiau A. Human T cell activation with phytohemagglutinin. The function of IL-6 as an accessory signal. J Immunol. 1988;141:3868–74. [PubMed] [Google Scholar]
- 29.Walter H, Schepens S, Van Wauwe J, de Boer M. Ligation of CD28 on resting T cells by its ligand B7 results in the induction of both Th1- and Th2-type cytokines. Eur Cytokine Netw. 1994;5:13–21. [PubMed] [Google Scholar]
- 30.Michie CA, McLean A, Alcock C, Beverley PCL. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992;360:264–5. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Sasson SZ, Le Gros G, Conrad DH, Finkelman FD, Paul WE. IL-4 production by T cells from naive donors. IL-2 is required for IL-4 production. J Immunol. 1990;145:1127–36. [PubMed] [Google Scholar]
- 32.Van Gool SW, Kasran A, Wallays G, de Boer M, Ceuppens JL. Accessory signalling by B7–1 for T cell activation induced by anti-CD2: evidence for IL-2-independent CTL generation and CsA-resistant cytokine production. Scand J Immunol. 1995;41:23–30. doi: 10.1111/j.1365-3083.1995.tb03529.x. [DOI] [PubMed] [Google Scholar]
- 33.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 34.Young YL, Ramage JM, Hill Gaston JS, Beverley PCL. In vitro responses of human CD45RObrightRA− and CD45RO−RAbright T cell subsets and their relationship to memory and naive T cells. Eur J Immunol. 1997;27:2383–9. doi: 10.1002/eji.1830270937. [DOI] [PubMed] [Google Scholar]
- 35.Peleman R, Wu J, Fargeas C, Delespesse G. Recombinant interleukin 4 suppresses the production of interferon γ by human mononuclear cells. J Exp Med. 1989;170:1751–6. doi: 10.1084/jem.170.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vercelli D, Jabara HH, Lauener RP, Geha RS. IL-4 inhibits the synthesis of IFN-γ and induces the synthesis of IgE in human mixed lymphocyte cultures. J Immunol. 1990;144:570–3. [PubMed] [Google Scholar]
- 37.Chatelain R, Varkila K, Coffman RL. IL-4 induces a Th2 response in Leishmania major-infected mice. J Immunol. 1992;148:1182–7. [PubMed] [Google Scholar]
- 38.Gross A, Ben-Sasson SZ, Paul WE. Anti-IL-4 diminishes in vivo priming for antigen-specific IL-4 production by T cells. J Immunol. 1993;150:2112–20. [PubMed] [Google Scholar]
- 39.Kasran A, Peng X, de Boer M, Ceuppens JL. Proceedings of the 12th European Immunology Meeting. Barcelona: 1994. B7 and IL-4 upregulate IL10 production in human T cells; p. 161. [Google Scholar]
- 40.Bryson YJ, Winter HS, Grad SE, Fischer TJ, Stiehm ER. Deficiency of immune interferon production by leukocytes of normal newborns. Cell Immunol. 1980;55:191–200. doi: 10.1016/0008-8749(80)90150-1. [DOI] [PubMed] [Google Scholar]
- 41.Roncarolo MG, Bigler M, Ciuti E, Martino S, Tovo PA. Immune responses by cord blood cells. Blood Cells. 1994;20:573–86. [PubMed] [Google Scholar]
- 42.Wakasugi N, Virelizier JL, Arenzana-Seisdedos F, et al. Defective IFN-γ production in the human neonate. II. Role of increased sensitivity to the suppressive effects of prostaglandin E. J Immunol. 1985;134:172–6. [PubMed] [Google Scholar]
- 43.Chalmers IMH, Janossy G, Contreras M, Navarrete C. Intracellular cytokine profile of cord and adult blood lymphocytes. Blood. 1998;92:11–18. [PubMed] [Google Scholar]
- 44.Rulifson IC, Sperling AI, Fields PE, Fitch FW, Bluestone JA. CD28 costimulation promotes the production of Th2 cytokines. J Immunol. 1997;158:658–65. [PubMed] [Google Scholar]
- 45.Webb LMC, Feldmann M. Critical role of CD28/B7 costimulation in the development of human Th2 cytokine-producing cells. Blood. 1995;86:3479–86. [PubMed] [Google Scholar]
- 46.Prescott SL, Macaubas C, Smallacombe T, Holt BJ, Sly PD, Holt PG. Development of allergen-specific T-cell memory in atopic and normal children. Lancet. 1999;353:196–200. doi: 10.1016/S0140-6736(98)05104-6. [DOI] [PubMed] [Google Scholar]
- 47.Krishnan L, Guilbert LJ, Russell AS, Wegmann TG, Mosmann TR, Belosevic M. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigen-specific IFN-γ responses and increased production of T helper 2 cytokines. J Immunol. 1996;156:644–52. [PubMed] [Google Scholar]
- 48.Krishnan L, Guilbert LJ, Wegmann TG, Belosevic M, Mosmann TR. T helper 1 response against Leishmania major in pregnant CD57BL/6 mice increases implantation failure and fetal resorptions. Correlation with increased IFN-γ and TNF and reduced IL-10 production by placental cells. J Immunol. 1996;156:653–62. [PubMed] [Google Scholar]
- 49.Barrios C, Brawand P, Berney M, Brandt C, Lambert P-H, Siegrist C-A. Neonatal and early life immune responses to various forms of vaccine antigens qualitatively differ from adult responses: predominance of a Th2-biased pattern which persists after adult boosting. Eur J Immunol. 1996;26:1489–96. doi: 10.1002/eji.1830260713. [DOI] [PubMed] [Google Scholar]
- 50.Prescott SL, Macaubas C, Holt BJ, et al. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T cell responses toward the Th2 cytokine profile. J Immunol. 1998;160:4730–7. [PubMed] [Google Scholar]
- 51.Wegmann TG, Hui L, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the fetal–maternal relationship: is successful pregnancy a Th2 phenomenon? Immunol Today. 1993;14:353–6. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 52.Rissoan M-C, Soumelis V, Kadowaki N, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]