Abstract
Autoantibodies to GAD, an important marker of the autoimmune process in type I or insulin-dependent diabetes mellitus (IDDM), are also found in non-diabetic individuals with autoimmune polyendocrine syndrome type 1 (APS1), APS2, and stiff man syndrome (SMS). Most IDDM sera contain two distinct GAD antibody specificities, one of which targets an epitope region in the middle-third of GAD65 (IDDM-E1; amino acids 221–359) and one of which targets the carboxy-third of GAD65 (IDDM-E2; amino acids 453–569). Using 11 chimeric GAD65/GAD67 proteins to maintain conformation-dependent epitopes of GAD65, we compared the humoral repertoire of IgG antibodies from an individual with APS2-like disease (b35, b78, and b96) and MoAbs from an IDDM patient (MICA-2, MICA-3, and MICA-4). Neither the APS2 IgG antibodies nor the IDDM MoAbs bind the amino-terminal third of GAD65, but instead target the carboxy-terminal two-thirds of GAD65. Amino acids 270–359 (IDDM-E1) are targeted by one APS2 IgG antibody and MICA-4, while two other APS2 IgG antibodies, MICA-2 and MICA-3, target amino acids 443–585 (IDDM-E2). Using GAD65/67 chimera that span the IDDM-E2 region, we found that MICA-2 binds amino acids 514–528 of GAD65, but two APS2 IgG antibodies require this region and amino acids 529–570. In contrast, the binding of MICA-3 requires two discontinuous amino acid segments of GAD65 (452–513 and 528–569), but not amino acids 514–528. These results indicate that there are both similarities and differences in the humoral response to GAD65 in APS2 and IDDM.
Keywords: diabetes, epitope, monoclonal antibody, glutamic acid decarboxylase
INTRODUCTION
Autoantibodies to GAD are an important marker for type 1 diabetes or insulin-dependent diabetes mellitus (IDDM) [1–8]. Individuals with other autoimmune diseases such as stiff man syndrome (SMS) and autoimmune polyglandular failure syndromes (APS) also have autoantibodies to GAD [9–17]. Diabetes is often a component of SMS and autoimmune polyendocrine syndrome type 2 (APS2), whereas it is not a usual feature of APS1 [18]. While there is an autoimmune response to GAD65 in all three diseases, a difference in the clinical frequency of diabetes suggests that the autoimmunity to GAD may differ in these diseases. GAD65 autoantibodies in SMS have both similarities to and differences with those found in IDDM [12,14–16,19]. For example, SMS sera contain an antibody which targets a linear epitope in the amino-terminus of GAD65, while this type of antibody is not present in IDDM sera [12–15]. GAD autoantibodies in APS1 and SMS also inhibit the enzymatic activity of GAD65, while this is not a property of IDDM autoantibodies [9,19]. Since GAD autoantibodies in IDDM bind conformation-dependent epitopes, chimeric proteins of GAD65 and GAD67 have been utilized to maintain protein conformation and allow identification of targeted regions of GAD65 [20]. GAD autoantibodies in IDDM serum target regions in the middle and carboxy-terminus of GAD65 ([20]; termed IDDM-E1 and IDDM-E2), but whether similar epitopes are targeted in polyglandular autoimmune syndromes is not known. A recent report suggested that GAD autoantibodies in polyglandular autoimmune syndromes are heterogeneous [21]. Definition of the epitopes targeted in IDDM and polyglandular syndromes has been assisted by panels of MoAbs derived from individuals with the disease [22–24], but the precise regions targeted by these MoAbs remain incompletely defined. The purpose of the current study was to define and compare GAD65 epitopes targeted in autoimmune polyglandular failure syndromes and IDDM using MoAbs derived from an individual with IDDM and an individual with APS2.
MATERIALS AND METHODS
Serum and antibody samples
The isolation of human MoAbs, MICA-2, MICA-3 and MICA-4, has been previously described [23]. The isolation of human IgG antibodies from the lines b35, b78, and b96, from an APS2-like patient, has been previously described [24]. Supernates of tissue culture media containing the IgG antibodies or MoAbs were diluted so as to immunoprecipitate approximately the same amount of labelled GAD65 or chimeric GAD protein.
Immunoprecipitation of GAD protein
The binding by MoAbs was assayed in duplicate using a binding assay as previously described [20]. The specificity of this GAD assay has been confirmed by the participation of our laboratory in interlaboratory GAD antibody workshops [25,26]. Background or non-specific binding (binding of labelled protein to protein A-Sepharose alone) was subtracted from the binding of each MoAb. This background binding varied with each chimeric protein but was < 5–10% of the total binding. MoAbs were defined as positive if after subtraction of background binding the amount of binding greatly exceeded the cut-off value of several GAD-negative MoAbs. In general, the binding of GAD-negative MoAbs was very similar to the background binding to protein A-Sepharose alone.
Creation of GAD65/GAD67 chimeric proteins
Chimeric GAD65/GAD67 cDNAs were created as previously described [20]. The chimeric proteins used for the current study are shown in Table 1. As reported previously [20], the following nomenclature for GAD chimera is used in this study: GAD65 or 67 (amino acid boundary of that GAD species in the GAD chimera)/GAD65 or 67 (amino acid boundary of that GAD species in the GAD chimera). As reported previously [20], the chimeric composition of the GAD cDNAs was confirmed by restriction enzyme digestion and/or dideoxy DNA sequencing. When assessed by SDS–PAGE the in vitro translated chimeric proteins were of expected molecular weight [20].
Table 1.
GAD65, GAD67 and chimeric GAD65/67 proteins used

As reported previously [20], the following nomenclature for GAD chimera is used in this study: GAD65 or 67 (amino acid boundary of that GAD species in the GAD chimera)/GAD65 or 67 (amino acid boundary of that GAD species in the GAD chimera).
RESULTS
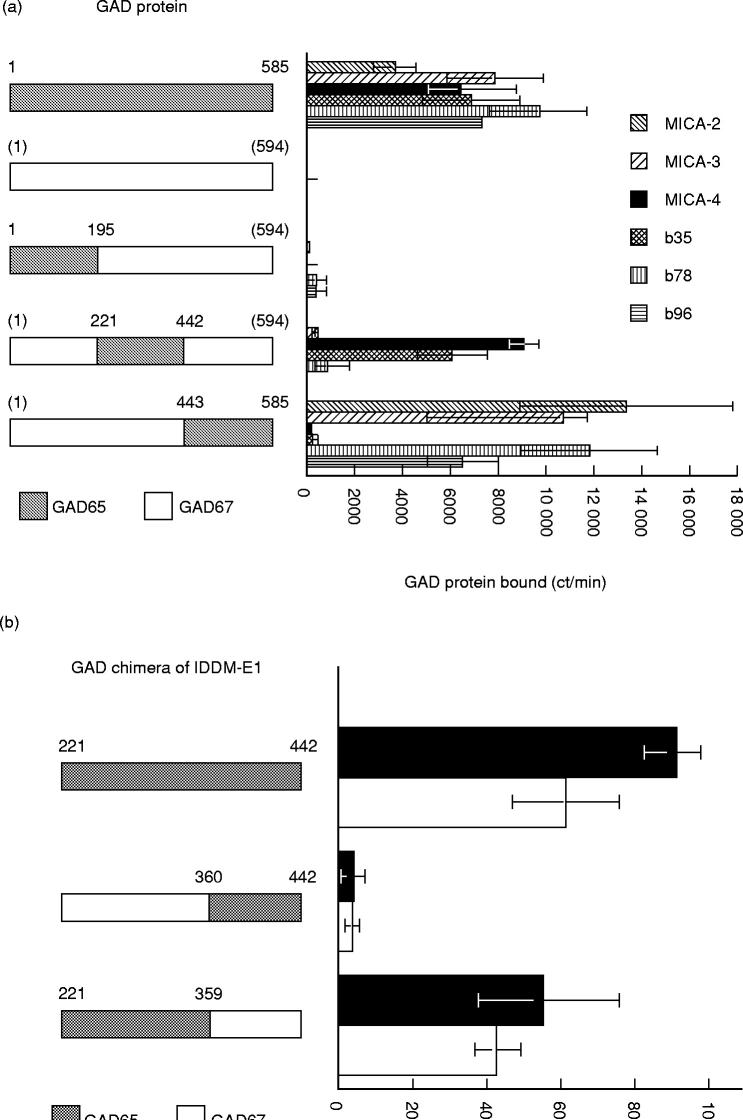
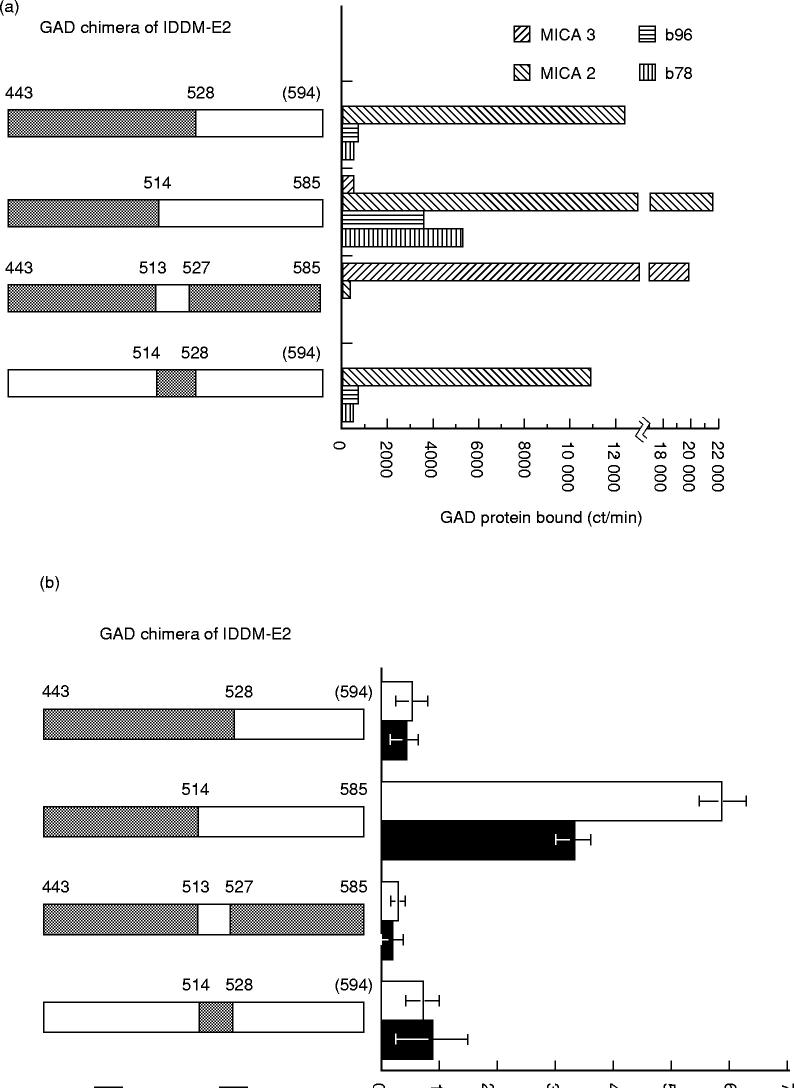
The human IgG antibodies b35, b78 and b96, and the MoAbs MICA-2, MICA-3, MICA-4, reacted with GAD65 but not GAD67 (Fig. 1a). MICA-3, MICA-4, b35, b78 and b96 did not bind a GAD chimeric protein containing the first 195 amino acids of GAD65 and in this way resemble most IDDM sera, which do not immunoprecipitate GAD67 or target the amino-terminus of GAD65 [8,20,27–29]. Both MICA-4 and b35 target the middle third of the GAD65 protein, while MICA-2, MICA-3, b78 and b96 target the carboxy terminal third of the protein (Fig. 1a). Using two chimeric proteins that span amino acids 221–442 of GAD65, we found that both b35 and MICA-4 target the chimeric protein containing amino acids 221–359 of GAD65 (Fig. 1b). IgG antibodies b78 and b96, MICA-2 and MICA-3 target the carboxy terminal third of GAD65, but differ in the epitope recognized (Fig. 2a). IgG antibodies b96 and b78 target a region of GAD65 which contains amino acids 514–585 (Fig. 2b). This contrasts with MICA-2, which targets amino acids 514–528 (Fig. 2a), and MICA-3, which requires two discontinuous sets of amino acids (amino acid 443–513 and 527–585) (Fig. 2a,c). Both regions are required for MICA-3 binding since chimeric proteins containing amino acids 443–528 or GAD65 amino acids 514–585 of GAD65 do not blocking binding to IDDM-E2 (Fig. 2c).
Fig. 1.
Mapping of GAD65 epitope regions targeted by insulin-dependent diabetes mellitus (IDDM)-derived or autoimmune polyendocrine syndrome type 2 (APS2)-derived MoAbs. Metabolically labelled GAD65, GAD67, or chimeric GAD65/67 protein was immunoprecipitated with MoAbs as reported previously [20]. Table 1 lists the chimeric GAD proteins. A schematic of the GAD chimeric protein used is shown on the left side of each figure. Above each GAD chimera is the amino acid number for GAD65 or the amino acid number for GAD67 (GAD67 numbers are in parentheses). Each chimeric protein is GAD67 (□) except for a small region of GAD65 (shaded). The dilution of MoAbs was chosen so that a similar amount of GAD65 was immunoprecipitated. The abscissa is the ct/min of GAD protein immunoprecipitated. The background binding (binding of protein A-Sepharose (PAS) to labelled GAD protein in absence of antibody) has been subtracted from each measurement. (a) Binding of MICA-2, MICA-3, MICA-4, b35, b78 and b96 to GAD65/67; the results with b35, b78 and b96 are adapted from [24] and are shown to facilitate comparison with MICA-2, MICA-3 and MICA-4. MICA-2 binding to GAD65 (1–195)/GAD67 (205–594) was not performed. In cases where binding was not different from background binding (for GAD67 and for GAD65 (1–195)/GAD67 (205–594)), the assay (duplicate determination) was performed only once or twice for certain antibodies. The error bars represent ± s.e.m. (b) Binding of b35 (□) and MICA-4 (▪) to chimeric proteins of IDDM-E1 region; a schematic of the region of GAD65 contained in the chimeric protein is shown on the left of the graph. Each chimeric protein is full-length GAD67 except for the small region of GAD65 shown by the shaded region. The bars represent mean ± s.e.m.
Fig. 2.
Carboxy terminus regions of GAD65 targeted by insulin-dependent diabetes mellitus (IDDM)-derived or autoimmune polyendocrine syndrome type 2 (APS2)-derived MoAbs. As described for Fig. 1, the chimeric GAD protein is full-length GAD67 except for the shaded areas, which are GAD65. (a) Binding of b78 and b96, MICA-2 and MICA-3 (mean of duplicate determination) to chimeric proteins of IDDM-E2 region; The background binding (binding of protein A-Sepharose (PAS) to labelled GAD protein in absence of antibody) has been subtracted from each measurement. (b) Binding of b78 (□) and b96 (▪) to chimeric proteins of IDDM-E2 region. (c) Binding of MICA-3 to GAD65 amino acids 443–585; MICA-3 was preincubated with either buffer or the unlabeled chimeric GAD protein shown on the left side of the graph. The entire chimeric protein (unlabelled) was GAD67 except for the region shown in the shaded pattern which represents the GAD65 portion of the chimeric protein. After MICA-3 incubation with buffer or the unlabelled chimeric GAD protein shown in the schematic to the left of the graph, the mixture was incubated with a labelled GAD67/65 chimeric protein containing GAD65 amino acids 443–585 (GAD67 (1–451)/GAD65 (443–585)). After the addition of PAS, the amount of labelled GAD protein immunoprecipitated was quantified.
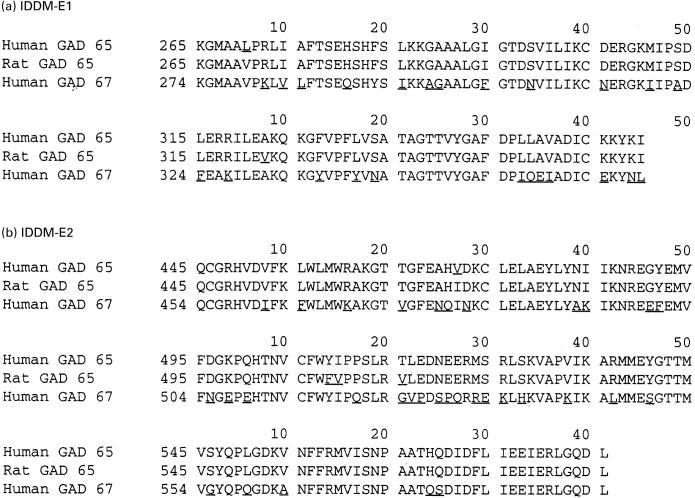
A comparison of GAD65 and GAD67 sequences in the IDDM-E1 and IDDM-E2 regions is shown in Fig. 3. The numbering of amino acids in the figures reflects where the chimeric cDNAs were spliced, but homology between GAD65 and GAD67 allows the amino acids responsible for the epitope to be narrowed further. After taking the amino acid homology of GAD65 and GAD67 into consideration, the region required for b35 and MICA-4 binding can be narrowed to amino acids 270–359 of GAD65. Based on the amino acid homology of GAD65 and GAD67, the epitope region targeted by b78 and b96 can be narrowed to 514–570 and the region required for MICA-3 binding to amino acids 452–512 and amino acids 528–570 (Fig. 3). GAD65 and GAD67 differ in 10 of 15 amino acids between 514 and 528, but binding of b78 and b96 requires additional amino acids in the carboxy terminus of GAD65 (Fig. 2b; Fig. 3). GAD65 and GAD67 differ in only 8/42 amino acids between amino acids 528–570, indicating that a small number of amino acids in this region, co-operating with amino acids 514–528, create the epitope targeted by b78 and b96 (Fig. 3). In the discontinuous segments of amino acids required for MICA-3 binding, GAD65 and GAD67 differ at only 22/104 amino acids, and these differences are scattered throughout these regions of GAD65 (Fig. 3). The greatest dissimilarity in GAD65 and GAD67 (amino acids 514–528) is not required for MICA-3 binding.
Fig. 3.
Amino acid sequence comparison between GAD65 and GAD67 in regions targeted by MoAbs. The amino acid sequence of GAD65 protein (human and rat) and GAD67 protein (human) is shown with amino acid differences underlined. (a) IDDM-E1. (b) IDDM-E2.
DISCUSSION
The current study demonstrates a similarity and difference in the GAD autoantibody repertoire in IDDM and APS2. Both MICA-4 and b35 antibodies target identical regions of GAD65 (amino acids 270–359). In contrast, other IDDM MoAbs and IgG autoantibodies from an APS2-like patient are directed at the carboxy terminus of GAD65, but target different epitopes. Amino acids 514–528 are required for binding by b78, b96 and MICA-2, but not MICA-3. However, amino acids 514–528 are not sufficient for b78 and b96 binding, as amino acids 529–570 are also required for the binding of b78 and b96. While MICA-2 requires amino acids 514–528, MICA-3 requires two discontinuous amino acid segments in the carboxy terminus of GAD65 (amino acids 443–513 and 528–570). Thus, MICA-2 and MICA-3 are distinct from each other and both differ from the binding of b78 and b96, in that MICA-2 binding requires only a subregion of that required by b78 and b96. The b78 and b96 IgG antibodies probably target a closely related, but different, sequence of GAD65, since b78 shows a different footprint from b96 and is the only IgG that reacts with GAD65 by Western blotting [24]. These studies identify regions of GAD65 important for creating the epitope targeted by the autoantibodies, but may or may not identify the actual contact points between antigen and antibody. It is possible that an identified region creates a protein conformation necessary for autoantibody binding, but that binding occurs in another region of the protein. A recent report found that a panel of MoAbs to GAD65 targeted much of the putative surface of the GAD protein [30].
The precise mapping of GAD epitopes targeted by human serum is difficult since a serum contains multiple antibody species. MoAbs derived from individuals with new-onset diabetes by Richter and colleagues have been extremely useful in mapping GAD epitopes in IDDM [22,31,32]. The two major epitope regions targeted by IDDM sera (IDDM-E1 and IDDM-E2) are also targeted by MICA-4 or MICA-3, respectively. Both MICA-2 and MICA-3 target IDDM-E2, but bind distinct epitopes within IDDM-E2. The current results extend these previously described differences in the IDDM-related MoAbs by narrowing the amino acids of GAD65 required for binding [22,23,33,34]. Recently, Schwartz and colleagues [30] described the epitope for a related b96 antibody produced after cloning of the b96 cell line. The epitope for the Moab from the cloned cells (known as b96.11) resides in the middle-third of GAD65 [30] and thus is different from the epitope of the b96 described here. The reason for this is not clear but may relate to the isolation of a cell line with a different specificity from the original cell line. Using MoAbs allows precise characterization of epitopes targeted by a single antibody species, but there are limitations to this approach. Since the humoral immune response is polyclonal, the specificity of one MoAb may or may not represent the total immune response profile in the serum of an affected individual. In addition, since the MoAbs are isolated from a single individual, it is important to confirm any findings in a population of affected individuals. Furthermore, the epitope profile may change during the natural history of the disease and MoAbs are isolated at a single point in time. Most sera from individuals with new-onset diabetes have antibodies which are similar to MICA-3 and MICA-4 in terms of epitope specificity, and do not have detectable titres of a MICA-2-type antibody (data not shown). Another limitation of using MoAbs to map diabetes-relevant epitopes is that some sera from individuals with type I diabetes have antibodies that target GAD67 while the monoclonals do not bind this isoform.
Despite the belief that IDDM is a T cell-mediated disease, investigation of T cell epitopes has produced conflicting results [35,36]. Some studies have detected cell-mediated immunity directed at a region of GAD65 (amino acids 250–273 of GAD65) which is homologous with the Coxsackie PC-2 protein [35–38], but this has not been a consistent finding [35,39,40]. Interestingly, IDDM-E1 overlaps this region of GAD65. Other studies in humans and non-obese diabetic (NOD) mice have detected T cell reactivity to the carboxy terminus of GAD65, including amino acids 524–541 and 521–535 [37,41–45]. The region of IDDM-E2 targeted by MICA-2 and MICA-3 overlaps this region of GAD65, and thus humoral and cellular autoimmunity to GAD65 appear to target similar epitope regions.
Comparisons of the autoimmune repertoires to GAD65 in APS1, APS2, IDDM and SMS may provide insight into why some individuals develop one disease and other individuals develop the other disease. GAD65 autoantibodies are not a common finding in individuals with APS2 [17]. The series of IgG antibodies derived from the individual with Graves' disease exhibit a similarity to and a difference from IDDM MoAbs. This difference may have disease implications, since the individual from whom the b78 and b96 were derived did not have IDDM. Neither IDDM- nor APS-2-derived MoAbs target the amino-terminus of GAD65 which is targeted in SMS [12–15]. Whether GAD is the primary autoantigen in any of these diseases is unknown, but the high frequency of GAD65 autoantibodies and the presence of cellular immunity in both human IDDM and animal models of the disease suggest that GAD65 is an important autoantigen in IDDM [46–48]. While IDDM is closely linked to certain HLA haplotypes, APS1 is not linked to the HLA region and the gene for this disorder has been localized to a nuclear factor on chromosome 21 [49]. The current study suggests that careful mapping and precise molecular characterization of the epitopes targeted by GAD autoantibodies may contribute to a better understanding of the pathogenesis of these diseases.
Acknowledgments
These studies were supported by a Merit Review Award from the Veterans Affairs Research Service, the Diabetes Research and Training Center at Vanderbilt University (NIH DK20593), and The Smith and Nephew Foundation (J.T.).
REFERENCES
- 1.Baekkeskov S, Aanstoot HJ, Christgau S, et al. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347:151–6. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- 2.Hagopian WA, Karlsen AE, Gottsater A, et al. Quantitative assay using recombinant human islet glutamic acid decarboxylase (GAD65) shows that 64K autoantibody positivity at onset predicts diabetes type. J Clin Invest. 1993;91:368–74. doi: 10.1172/JCI116195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson MA, Kaufman DL, Newman D, Tobin AJ, Maclaren NK. Islet cell cytoplasmic autoantibody reactivity to glutamate decarboxylase in insulin-dependent diabetes. J Clin Invest. 1993;91:350–6. doi: 10.1172/JCI116192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufman DL, Erlander MG, Clare-Salzler M, Atkinson MA, Maclaren NK, Tobin AJ. Autoimmunity to two forms of glutamate decarboxylase in insulin-dependent diabetes mellitus. J Clin Invest. 1992;89:283–92. doi: 10.1172/JCI115573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christie MR, Tun RY, Lo SS, et al. Antibodies to GAD and tryptic fragments of islet 64K antigen as distinct markers for development of IDDM. Studies with identical twins. Diabetes. 1992;41:782–7. doi: 10.2337/diab.41.7.782. [DOI] [PubMed] [Google Scholar]
- 6.Rowley MJ, Mackay IR, Chen QY, Knowles WJ, Zimmet PZ. Antibodies to glutamic acid decarboxylase discriminate major types of diabetes mellitus. Diabetes. 1992;41:548–51. doi: 10.2337/diab.41.4.548. [DOI] [PubMed] [Google Scholar]
- 7.Seissler J, Amann J, Mauch L, et al. Prevalence of autoantibodies to the 65- and 67-kD isoforms of glutamate decarboxylase in insulin-dependent diabetes mellitus. J Clin Invest. 1993;92:1394–9. doi: 10.1172/JCI116714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velloso LA, Kampe O, Hallberg A, Christmanson L, Betsholtz C, Karlsson FA. Demonstration of GAD-65 as the main immunogenic isoform of glutamate decarboxylase in type I diabetes and determination of autoantibodies using a radioligand produced by eukaryotic expression. J Clin Invest. 1993;91:2084–90. doi: 10.1172/JCI116431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjork E, Velloso LA, Kampe O, Karlsson FA. GAD autoantibodies in IDDM, stiff-man syndrome, and autoimmune polyendocrine syndrome type I recognize different epitopes. Diabetes. 1994;43:161–5. doi: 10.2337/diab.43.1.161. [DOI] [PubMed] [Google Scholar]
- 10.Tuomi T, Bjorses P, Falorni A, et al. Antibodies to glutamic acid decarboxylase and insulin-dependent diabetes in patients with autoimmune polyendocrine syndrome type I. J Clin Endocrinol Metab. 1996;81:1488–94. doi: 10.1210/jcem.81.4.8636356. [DOI] [PubMed] [Google Scholar]
- 11.Solimena M, De Camilli P. Autoimmunity to glutamic acid decarboxylase (GAD) in stiff-man syndrome and insulin-dependent diabetes mellitus. Neurochem Res. 1991;14:452–7. doi: 10.1016/0166-2236(91)90044-u. [DOI] [PubMed] [Google Scholar]
- 12.Butler MH, Solimena M, Dirkx R, Hayday A, De Camilli P. Identification of a dominant epitope of glutamic acid decarboxylase (GAD-65) recognized by autoantibodies in stiff-man syndrome. J Exp Med. 1993;178:2097–106. doi: 10.1084/jem.178.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Hagopian WA, Brashear R, Daniels T, Lernmark A. Identification of autoantibody epitopes of glutamic acid decarboxylase in stiff-man syndrome patients. J Immunol. 1993;152:930–4. [PubMed] [Google Scholar]
- 14.Kim J, Manchuk M, Bugawan T, et al. Higher titer autoantibody levels and recognition of a linear NH2-terminal epitope in the autoantigen, GAD65, distinguish stiff-man syndrome from insulin-dependent diabetes mellitus. J Exp Med. 1994;180:595–606. doi: 10.1084/jem.180.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daw K, Ujihara N, Atkinson M, Powers AC. Glutamic acid decarboxylase autoantibodies in stiff-man syndrome and insulin-dependent diabetes mellitus exhibit similarities and differences in epitope recognition. J Immunol. 1996;156:818–25. [PubMed] [Google Scholar]
- 16.Grimaldi LM, Martino G, Braghi S, et al. Heterogeneity of autoantibodies in stiff-man syndrome. Ann Neurol. 1993;34:57–64. doi: 10.1002/ana.410340111. [DOI] [PubMed] [Google Scholar]
- 17.Seissler J, Bieg S, Yassin N, et al. Association between antibodies to the MR 67,000 isoform of glutamate decarboxylase (GAD) and type 1 (insulin-dependent) diabetes mellitus with coexisting autoimmune polyendocrine syndrome type II. Autoimmunity. 1994;19:231–8. doi: 10.3109/08916939409071348. [DOI] [PubMed] [Google Scholar]
- 18.Riley WJ. Autoimmune polyglandular syndromes. Hormone Res. 1992;38(Suppl. 2):9–15. doi: 10.1159/000182585. [DOI] [PubMed] [Google Scholar]
- 19.Dinkel K, Meinck HM, Jury KM, Karges W, Richter W. Inhibition of gamma-aminobutyric acid synthesis by glutamic acid autoantibodies in stiff-man syndrome. Ann Neurol. 1998;44:194–201. doi: 10.1002/ana.410440209. [DOI] [PubMed] [Google Scholar]
- 20.Daw K, Powers AC. Two distinct glutamic acid decarboxylase autoantibody specificities in IDDM target different epitopes. Diabetes. 1995;44:216–20. doi: 10.2337/diab.44.2.216. [DOI] [PubMed] [Google Scholar]
- 21.Davenport C, Radford PM, Al-Bukhari TA, Lai M, Bottazzo GF, Todd I. Heterogeneity in the occurrence of a subset of autoantibodies to glutamic acid decarboxylase in autoimmune polyendocrine patients with islet cell antibodies. Clin Exp Immunol. 1998;111:497–505. doi: 10.1046/j.1365-2249.1998.00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richter W, Shi Y, Baekkeskov S. Autoreactive epitopes defined by diabetes-associated human monoclonal antibodies are localized in the middle and C-terminal domains of the smaller form of glutamate decarboxylase. Proc Natl Acad Sci USA. 1993;90:2832–6. doi: 10.1073/pnas.90.7.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richter W, Endl J, Eiermann TH, et al. Human monoclonal islet cell antibodies from a patient with insulin-dependent diabetes mellitus reveal glutamate decarboxylase as the target antigen. Proc Natl Acad Sci USA. 1992;89:8467–71. doi: 10.1073/pnas.89.18.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tremble J, Morgenthaler NG, Vlug A, et al. Human B cells secreting immunoglobulin G to glutamic acid decarboxylase-65 from a non-diabetic patient with multiple autoantibodies and Graves disease: a comparison with those present in type I diabetes. J Clin Endocrinol Metab. 1997;82:2664–70. doi: 10.1210/jcem.82.8.4171. [DOI] [PubMed] [Google Scholar]
- 25.Schmidli RS, Colman PG, Bonifacio E, Bottazzo GF, Harrison LC, Participating Laboratories. High level of concordance between assays for glutamic acid decarboxylase antibodies: the first international glutamic acid decarboxylase antibody workshop. Diabetes. 1994;43:1005–9. doi: 10.2337/diab.43.8.1005. [DOI] [PubMed] [Google Scholar]
- 26.Verge CF, Stenger D, Bonifacio E, et al. Combined use of autoantibodies (IA-2 autoantibody, GAD autoantibody, insulin autoantibody, cytoplasmic islet cell antibodies) in type I diabetes: combinatorial islet autoantibody workshop. Diabetes. 1998;47:1857–66. doi: 10.2337/diabetes.47.12.1857. [DOI] [PubMed] [Google Scholar]
- 27.Hagopian WA, Michelsen B, Karlsen AE, et al. Autoantibodies in IDDM primarily recognize the 65,000-M(r) rather than the 67,000-M(r) isoform of glutamic acid decarboxylase. Diabetes. 1993;42:631–6. doi: 10.2337/diab.42.4.631. [DOI] [PubMed] [Google Scholar]
- 28.Ujihara N, Daw K, Gianani R, Boel E, Yu L, Powers AC. Identification of glutamic acid decarboxylase autoantibody heterogeneity and epitope regions in type I diabetes. Diabetes. 1994;43:968–75. doi: 10.2337/diab.43.8.968. [DOI] [PubMed] [Google Scholar]
- 29.Falorni A, Ackefors M, Carlberg C, et al. Diagnostic sensitivity of immunodominant epitopes of glutamic acid decarboxylase (GAD65) autoantibodies in childhood IDDM. Diabetologia. 1996;39:1091–8. doi: 10.1007/BF00400659. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz HL, Chandonia JM, Kash SF, et al. High-resolution autoreactive epitope mapping and structural modeling of the 65 kDa form of human glutamic acid decarboxylase. J Mol Biol. 1999;287:983–99. doi: 10.1006/jmbi.1999.2655. [DOI] [PubMed] [Google Scholar]
- 31.Richter W, Seissler J, Northemann W, Wolfahrt S, Meinck HM, Scherbaum WA. Cytoplasmic islet cell antibodies recognize distinct islet antigens in IDDM but not in stiff man syndrome. Diabetes. 1993;42:1642–8. doi: 10.2337/diab.42.11.1642. [DOI] [PubMed] [Google Scholar]
- 32.Syren K, Lindsay L, Stoehrer B, et al. Immune reactivity of diabetes-associated human monoclonal autoantibodies defines multiple epitopes and detects two domain boundaries in glutamate decarboxylase. J Immunol. 1996;157:5208–14. [PubMed] [Google Scholar]
- 33.Richter W, Eiermann TH, Endl J, et al. Human monoclonal islet specific autoantibodies share features of islet cell and 64 kDa antibodies. Diabetologia. 1993;36:785–90. doi: 10.1007/BF00401152. [DOI] [PubMed] [Google Scholar]
- 34.Richter W, Northemann W, Muller M, Bohm BO. Mapping of an autoreactive epitope within glutamate decarboxylase using a diabetes-associated human monoclonal autoantibody and an epitope cDNA library. Hybridoma. 1996;15:103–8. doi: 10.1089/hyb.1996.15.103. [DOI] [PubMed] [Google Scholar]
- 35.Ellis TM, Atkinson MA. The clinical significance of an autoimmune response against glutamic acid decarboxylase. Nature Med. 1996;2:148–53. doi: 10.1038/nm0296-148. [DOI] [PubMed] [Google Scholar]
- 36.Maclaren NK, Alkinson MA. Insulin-dependent diabetes mellitus: the hypothesis of molecular mimicry between islet cell antigens and microorganisms. Mol Med Today. 1997;3:76–83. doi: 10.1016/s1357-4310(96)10056-3. [DOI] [PubMed] [Google Scholar]
- 37.Schloot NC, Roep BO, Wegmann DR, Yu L, Wang TB, Eisenbarth GS. T-cell reactivity to GAD65 peptide sequences shared with coxsackie virus protein in recent-onset IDDM, post-onset IDDM patients and control subjects. Diabetologia. 1997;40:332–8. doi: 10.1007/s001250050683. [DOI] [PubMed] [Google Scholar]
- 38.Atkinson MA, Bowman MA, Campbell L, Darrow BL, Kaufman DL, Maclaren NK. Cellular immunity to a determinant common to glutamate decarboxylase and coxsackie virus in insulin-dependent diabetes. J Clin Invest. 1994;94:2125–9. doi: 10.1172/JCI117567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Endl J, Otto H, Jung G, et al. Identification of naturally processed T cell epitopes from glutamic acid decarboxylase presented in the context of HLA-DR alleles by T lymphocytes of recent onset IDDM patients. J Clin Invest. 1997;99:2405–15. doi: 10.1172/JCI119423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wicker LS, Chen SL, Nepom GT, et al. Naturally processed T cell epitopes from human glutamic acid decarboxylase identified using mice transgenic for the type 1 diabetes-associated human MHC class II allele, DRB1*0401. J Clin Invest. 1996;98:2597–603. doi: 10.1172/JCI119079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel SD, Cope AP, Congia M, et al. Identification of immunodominant T cell epitopes of human glutamic acid decarboxylase 65 by using HLA-DR (alpha1*0101,beta1*0401) transgenic mice. Proc Natl Acad Sci USA. 1997;94:8082–7. doi: 10.1073/pnas.94.15.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chao CC, McDevitt HO. Identification of immunogenic epitopes of GAD 65 presented by Ag7 in non-obese diabetic mice. Immunogenetics. 1997;46:29–34. doi: 10.1007/s002510050238. [DOI] [PubMed] [Google Scholar]
- 43.Lohmann T, Leslie RD, Londei M. T cell clones to epitopes of glutamic acid decarboxylase 65 raised from normal subjects and patients with insulin-dependent diabetes. J Autoimmun. 1996;9:385–9. doi: 10.1006/jaut.1996.0052. [DOI] [PubMed] [Google Scholar]
- 44.Quinn A, Sercarz EE. T cells with multiple fine specificities are used by non-obese diabetic (NOD) mice in the response to GAD (524–543) J Autoimmun. 1996;9:365–70. doi: 10.1006/jaut.1996.0049. [DOI] [PubMed] [Google Scholar]
- 45.Kaufman DL, Clare-Salzler MJ, Tian J, et al. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature. 1993;366:69–72. doi: 10.1038/366069a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clare-Salzler MJ, Tobin AJ, Kaufman DL. Glutamate decarboxylase: an autoantigen in IDDM. Diabetes Care. 1992;15:132–5. doi: 10.2337/diacare.15.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eisenbarth GS. Mouse or man. Is GAD the cause of type I diabetes? Diabetes Care. 1994;17:605–7. doi: 10.2337/diacare.17.6.605. [DOI] [PubMed] [Google Scholar]
- 48.Atkinson MA, Maclaren NK. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994;331:1428–36. doi: 10.1056/NEJM199411243312107. [DOI] [PubMed] [Google Scholar]
- 49.Nagamine K, Peterson P, Scott HS, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–8. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]