Abstract
The immunopathogenesis of BD is believed to be T cell-mediated. The objective of this study was to characterize the activation stage and cytokine profile of peripheral blood lymphocytes (PBL), with particular emphasis on γδ T cells. Venous blood was collected from 20 patients with BD, and for comparison, from 11 patients with RAS and from 15 healthy controls. Both the expression of activation markers (CD25, CD29, CD40 ligand, CD69 and HLA-DR) on freshly isolated PBL and T cell subsets, and the expression of intracellular cytokines (IL-4, IL-10, interferon-gamma (IFN-γ) and tumour necrosis factor-alpha (TNF-α)) on mitogen-stimulated PBL and T cell subsets were analysed by double immunofluorescent staining and flow cytometry. Significantly decreased proportion of αβ T cells and increased proportion of γδ T cells, CD56+ cells and CD8+γδ T cells were found in BD patients compared with healthy controls. This was also seen to a lesser extent in patients with RAS. Furthermore, in BD a significantly increased proportion of the γδ T cell population expressed CD69 and high levels of CD29 and were induced to produce IFN-γ and TNF-α compared with healthy controls. In contrast, an increased percentage of γδ T cells from RAS patients was induced to produce IFN-γ, but not TNF-α. These results indicate that in BD, activated γδ T cells, capable of producing IFN-γ and TNF-α, are present in peripheral blood, suggesting that γδ T cells are dynamic and may be regulating immunopathogenic events.
Keywords: γδ T cells, Behçet's disease, recurrent aphthous stomatitis, cytokines, activation
INTRODUCTION
BD is a multisystemic disease with recurrent ulceration affecting both the oral and genital mucosa. Furthermore, patients with BD suffer from lesions affecting the eye, skin, joints, gastrointestinal tract, central nervous, vascular and respiratory systems. However, the aetiopathogenesis of BD remains unknown. Unlike BD, RAS is the most common oral mucosal disease, affecting about 15–20% of the population [1,2], with the oral mucosa being the prime and only site of lesions.
A major part of the immunopathogenesis in BD and RAS is believed to be a T cell-mediated immune response. We and others have shown a depressed number of CD4+ T cells and elevated numbers of CD8+ T cells in peripheral blood in both patient groups [3–5]. The proportion of γδ T cells has also been found to be increased in peripheral blood from patients with BD compared with controls [3], whereas the proportion of γδ T cells in peripheral blood from patients with RAS has been reported to be unchanged or raised [6,7]. γδ T cells are uncommon in a healthy oral mucosa ([8], Freysdottir et al., manuscript in preparation). However, γδ T cells are observed within the oral lesions of patients with BD and RAS (Freysdottir et al., manuscript in preparation). Immune regulation has been suggested as a role for γδ T cells in the immune system [9], with the γδ T cells acting as a first line of defence. The γδ T cells have also been associated with controlling epithelial growth, thus participating in maintaining epithelial integrity [10–12]. Furthermore, it is postulated that they recognize structures presented by microorganisms as well as by stressed cells but not normal cells [13,14], thus enabling them to prevent entrance of pathogens into the subepithelial layer by cytotoxicity against infected and stressed epithelial cells. γδ T cells have also been reported to produce several cytokines, with the cytokine profile dependent on the nature of antigen, enabling the γδ T cells to influence the nature of the immune response [15]. They also produce a panel of chemokines which may attract inflammatory cells within damaged epithelium [16].
In order to investigate the role of γδ T cells in the immunopathogenesis of BD, we analysed the cytokine production and activation profile of T cell subsets in peripheral blood from patients with BD. For comparison we also analysed peripheral blood from patients with RAS and from healthy individuals. An increased percentage of γδ T cells in peripheral blood from patients with BD compared with both RAS and healthy controls was found. An increased proportion of the γδ T cells from BD patients expressed CD25 (the α-chain of the IL-2 receptor), CD69 (an early activation marker) and high levels of CD29 (a βl-integrin), indicating that the cells were at an activated stage. Furthermore, a raised percentage of γδ T cells from BD patients produced the inflammatory cytokines interferon-gamma (IFN-γ) and tumour necrosis factor-alpha (TNF-α).
PATIENTS AND METHODS
Patients and controls
Twenty patients with BD and 11 patients with RAS were recruited from out-patient clinics at Guy's Hospital, London. All the BD patients (nine females and 11 males; mean age 40 years; range 22–62 years) fulfilled the International Criteria [17] and had clinically active disease, i.e. with active oral ulceration plus evidence of clinical signs of having more than one other organ system involvement, at the time of analysis (Fig. 1). All the RAS patients (eight females and three males; mean age 35 years; range 23–44 years) had more than one ulcer present for not more than 24 h at the time of analysis. For comparison, 15 healthy individuals (10 females and five males; mean age 40 years; range 26–45 years) were included as controls. Care was taken to match the ethnic origin of the BD patients, since it has been reported that the frequency of γδ T cells in peripheral blood differs in individuals from different areas of the world [18]. Patients with RAS and controls were excluded from the study if they had evidence of anaemia or systemic disease. Patients were venesected at their initial clinic visit before high doses of either topical or systemic immunosuppressives were prescribed. None of the patients with RAS were taking any medication, whereas four patients with ocular BD were already taking either prednisolone 5 mg (n = 2), azathioprine 100 mg (n = 1) or cyclosporin 100 mg (n = 1). Ethical approval for the blood samples was obtained from Guy's Hospital Ethics Committee.
Fig. 1.
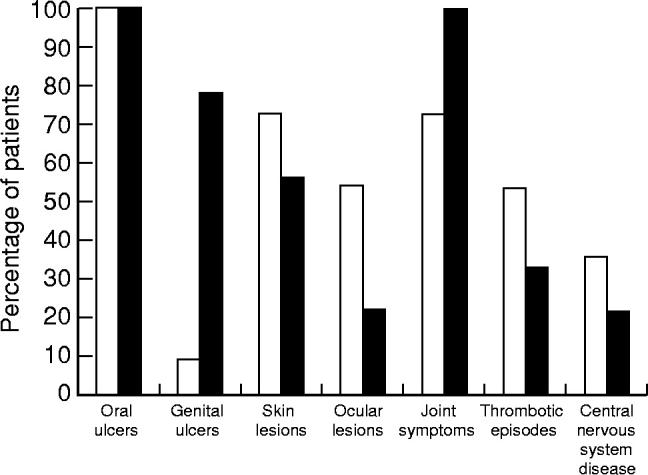
Clinical pattern of disease activity of the BD patients. □, Male; ▪, female.
Antibodies
Mouse MoAbs against human CD29 (clone K20), CD69 (clone TP1.55.3), CD40 ligand (CD40L; CD154, clone TRAP1) and γδ TCR (clone IMMU510) were all from Coulter Electronics (Luton, UK). FITC-labelled and unlabelled MoAbs against human αβ TCR (clone WT31), FITC-labelled MoAbs against human γδ TCR (clone 11F2), PE-labelled MoAbs against human CD4 (clone SK3) and CD8 (clone SK1) were all purchased from Becton Dickinson (Oxford, UK). For intracellular cytokine measurements, PE-labelled MoAbs against human IL-4 (clone 8D4-8), IL-10 (clone JES3-9D7), TNF-α (clone Mab11), IFN-γ (clone 4S.B3) and control mouse IgG1 were obtained from Cambridge BioScience (Cambridge, UK). MoAbs against human CD25 (clone M-A251) and FITC-labelled MoAbs against human CD56 (clone C5.9) were purchased from Serotec (Oxford, UK). PE- or FITC-labelled goat anti-mouse immunoglobulin, the F(ab)2 fragments, were purchased from Dako (High Wycombe, UK). MoAbs against human HLA-DR (clone DA6.231) were tissue culture supernatant produced in our own laboratory. FITC- or PE-labelled control mouse IgG1 were obtained from Sigma (Poole, UK).
Cell separation and culture
Peripheral blood mononuclear cells (PBMC) were obtained from each individual by separating heparinized venous blood on Histopaque (Sigma). The cells were washed in RPMI medium (Gibco, Paisley, UK) and either stained immediately or cultured before further analysis. The cells were cultured in 24-well plates at a concentration of 1 × 106 cells/ml in RPMI medium with 10% fetal calf serum (FCS; Gibco) for 4 h at 37°C, 5% CO2 and 100% humidity.
Initially, in order to determine optimal culture conditions for induction of cytokine expression, PBMC were cultured for 2, 4, 6, 9 and 12 h in medium alone, in the presence of phytohaemagglutinin (PHA; Sigma) at concentrations of 2.5, 4.5 or 7.5 μg/ml, or in the presence of phorbol mysitrate acetate (PMA; Sigma) at concentrations of 1, 5 or 50 ng/ml in combination with 1 μmol/ml of ionomycin (Sigma). In order to prevent secretion of the cytokines, brefeldin A (Sigma) at 10 μg/ml was added during the last 4 h of the incubation. Maximum production of IFN-γ and TNF-α was observed after stimulation with PMA in combination with ionomycin, when used at either 5 or 50 ng/ml; whereas PHA was more effective in inducing production of IL-4 and IL-10, with maximum production observed at 7.5 μg/ml. All the cytokines were detectable after 2 h and maximum production was observed between 4 h and 9 h. Therefore, in this study the PBMC were cultured for 4 h in the absence or presence of 7.5 μg/ml of PHA for detection of IL-4 and IL-10, and in the absence or presence of 10 ng/ml of PMA in combination with 1 μmol/ml of ionomycin for detection of IFN-γ and TNF-α. Brefeldin A at 10 μg/ml was present from the beginning.
Double immunofluorescence staining for activation markers or co-receptors and T cell receptors
In order to analyse expression of activation markers on peripheral blood lymphocytes (PBL) and T cell subsets, 5 × 105 freshly isolated PBMC in 100 μl of staining buffer (PBS with 1% FCS and 0.02% NaN3) were incubated with 5 μl of MoAbs against CD25 or CD40L, or 10 μl of MoAbs against CD29 or CD69, or 100 μl of MoAbs against MHC class II for 45 min on ice. Then the cells were washed twice with 1 ml of staining buffer at 180 g for 5 min at 4°C and subsequently incubated with 100 μl of PE-labelled goat anti-mouse immunoglobulin, diluted 1:100, for 30 min on ice, followed by two washes as before. Unbound goat antibody sites were blocked by incubating the cells with 100 μl of PBS containing 5% normal human serum (NHS) and 5% normal mouse serum (NMS) for 10 min on ice before the cells were incubated with 10 μl of FITC-labelled MoAbs against αβ or γδ TCR or FITC-labelled control mouse IgG1. The cells were washed as before and fixed in 400 μl of 1% paraformaldehyde in staining buffer. For co-receptor measurements, 5 × 105 cells were incubated for 45 min on ice with 10 μl of PE-labelled MoAbs against CD4 or CD8 or PE-labelled control mouse IgG1 and 10 μl of FITC-labelled antibodies against CD56, αβ TCR, or γδ TCR or FITC-labelled control mouse IgG1.
Double immunofluorescence staining for intracellular cytokines and TCR
Stimulated and unstimulated cells were stained in parallel by incubating 5 × 105 cells in 100 μl of staining buffer for 45 min on ice with 10 μl of MoAbs against αβ TCR, diluted 1:5, or γδ TCR, diluted 1:10, followed by 100 μl of FITC-labelled goat anti-mouse immunoglobulin, diluted 1:100, for 30 min on ice. Staining buffer was used for washing and dilutions. Before the second staining, the cells were fixed in 500 μl of 4% paraformaldehyde in PBS for 10 min on ice and then unbound goat antibody sites were blocked by incubating the cells with 100 μl of PBS containing 5% NHS and 5% NMS for 10 min on ice. In order to permeabilize the cells, 0.1% saponin was included in the staining buffer used for subsequent incubations and washing. The cells were incubated with 10 μl of PE-labelled antibodies against cytokines (IL-4, IL-10, TNF-α or IFN-γ) or PE-labelled control mouse IgG1, diluted 1:16, for 45 min on ice, washed and then fixed in 400 μl of 1% paraformaldehyde in staining buffer.
Flow cytometric analysis
Fluorescent labelled cells were analysed by a flow cytometer (Epics XL; Coulter). Before each set of data analysis the machine was standardized for data reproducibility using fluorescent labelled beads (Coulter). Ten thousand cells were collected. Plots showing size and granularity were used to select the lymphocyte population (PBL) for further analysis. Cells stained with isotype-matched antibody or second antibody alone were used as controls with positive cells set at 0.5%. For intracellular cytokine analysis, unstimulated cells were used as negative controls.
Statistical analysis
Results were expressed as mean ± s.e.m. for each study group. Significance was evaluated using Student's t-test for non-paired samples with unequal variance, with P < 0.05 being regarded as significant.
RESULTS
Expression of αβ TCR, γδ TCR, CD56, CD4 and CD8 on lymphocytes and T cell subsets in peripheral blood from patients with BD or RAS and healthy controls
The expression of αβ TCR, γδ TCR, CD56, CD4 and CD8 on lymphocytes and the expression of CD4 and CD8 on T cell subsets was analysed in peripheral blood from patients with BD or RAS and healthy controls. A significant decrease in the proportion of lymphocytes expressing αβ TCR and a significant increase in the proportion of lymphocytes expressing γδ TCR or CD56 was found in BD patients (63.2 ± 2.7%, 3.7 ± 0.5%, and 17.0 ± 2.5%, for αβ TCR, γδ TCR and CD56, respectively) compared with healthy controls (70.0 ± 1.7%, P < 0.05, 1.8 ± 0.2%, P < 0.005, and 8.6 ± 0.6%, P < 0.005, for αβ TCR, γδ TCR and CD56, respectively), as shown in Fig. 2. Furthermore, BD patients had a significantly decreased proportion of αβ T cells and a significantly increased percentage of γδ T cells compared with patients with RAS (71.9 ± 2.1%, P < 0.05, and 2.3 ± 0.4%, P < 0.05, for αβ TCR and γδ TCR, respectively). The proportion of lymphocytes expressing CD56 was also significantly raised in RAS patients (13.3 ± 1.3%) compared with controls (8.6 ± 0.6%, P < 0.01).
Fig. 2.
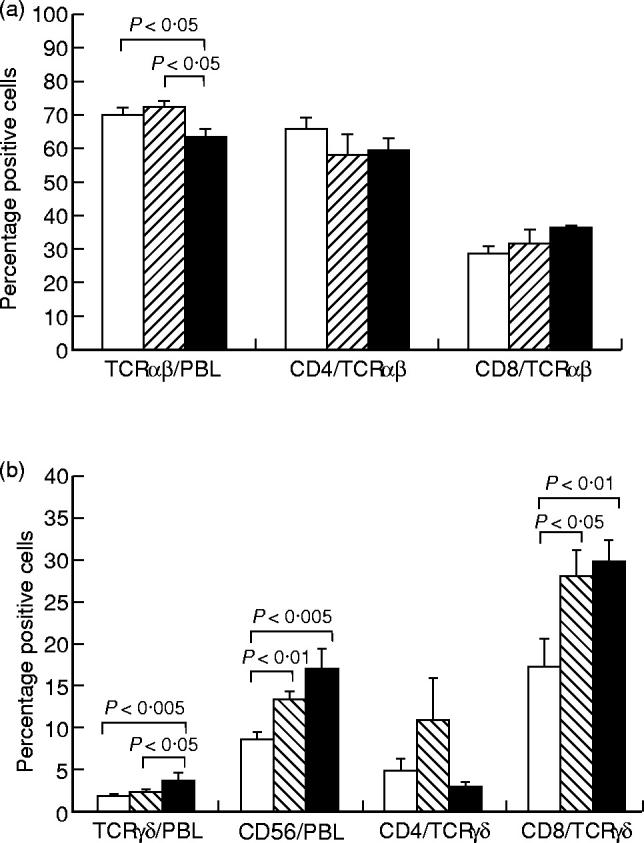
The expression of αβ T cell receptor (TCR) on peripheral blood lymphocytes (PBL) and expression of CD4 or CD8 on αβ T cells (a) and the expression of γδ TCR or CD56 on PBL and expression of CD4 or CD8 on γδ T cells (b), analysed by double immunofluorescent staining and flow cytometry. Results are expressed as percentage positive cells, shown as mean ± s.e.m. for 20 BD patients (▪), 11 RAS patients (hatched bars) and 20 healthy controls (□). Statistically significant differences between BD patients and controls, BD patients and RAS patients, and RAS patients and controls are indicated.
The proportion of CD8+ lymphocytes was raised in both patient groups (31.7 ± 4.1% and 30.5 ± 2.1%, for RAS and BD, respectively) compared with controls (25.5 ± 1.9%), although the difference did not reach significance. However, there was no difference in the percentage of CD4+ lymphocytes between either patient group and the controls (data not shown).
When the T cell subsets were analysed separately, a significantly raised proportion of γδ T cells from both BD patients (29.6 ± 2.6%) and RAS patients (27.9 ± 3.1%) co-expressed CD8 compared with γδ T cells from healthy controls (17.3 ± 3.3%, P < 0.01 for BD and P < 0.05 for RAS) (Fig. 2b). This was not observed for αβ T cells (Fig. 2a).
Expression of activation markers on lymphocytes and T cell subsets in peripheral blood from patients with BD or RAS and healthy controls
The expression of CD40L, CD69, CD29, CD25 and HLA-DR on lymphocytes and T cell subsets was analysed in peripheral blood from patients with BD or RAS and healthy controls.
A significantly increased percentage of CD69+ lymphocytes was observed in the BD patients (14.6 ± 1.1%) compared with the controls (10.2 ± 1.3%, P < 0.05) (Fig. 3a). This was reflected in a significantly raised proportion of αβ and γδ T cells expressing CD69 in BD patients (5.5 ± 1.3% and 14.2 ± 2.1%, for αβ and γδ T cells, respectively) compared with controls (2.6 ± 0.5%, P < 0.05, and 8.0 ± 1.9%, P < 0.05, for αβ and γδ T cells, respectively) (Fig. 3a). Furthermore, BD patients had a significantly increased proportion of lymphocytes expressing the CD40L (2.4 ± 0.7%) compared with healthy controls (1.3 ± 0.2%, P < 0.05) (Fig. 3a). Because a very low percentage of lymphocytes expressed CD40L, reliable results could not be obtained when the γδ T cell subset was analysed for co-expression of the CD40L.
Fig. 3.
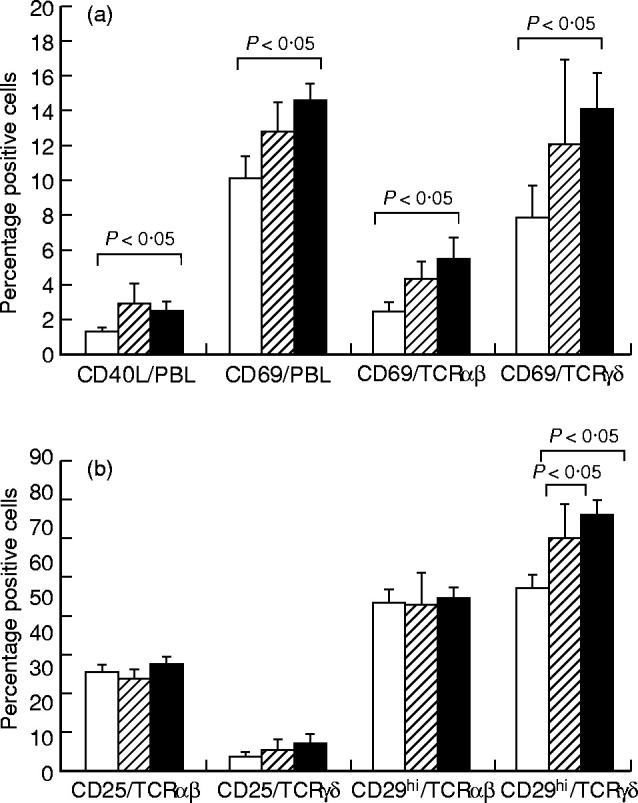
The expression of CD40 ligand (CD40L) or CD69 on peripheral blood lymphocytes (PBL) and expression of CD69 on αβ T cells or γδ T cells (a) and the expression of CD25 and CD29hi on αβ T cells and γδ T cells (b), analysed by double immunofluorescent staining and flow cytometry. Results are expressed as percentage positive cells, shown as the mean ± s.e.m. for 20 BD patients (▪), 11 RAS patients (hatched bars) and 20 healthy controls (□). Statistically significant differences between BD patients and controls, BD patients and RAS patients, and RAS patients and controls are indicated.
CD29 was expressed on all lymphocytes. Histogram analysis showed a biphasic population of cells with ‘low’ and ‘high’ expression of CD29. Significantly raised proportions of γδ T cells from BD patients (66.5 ± 3.9%) or RAS patients (60.5 ± 5.0%) expressed high levels of CD29 (CD29hi) when compared with healthy controls (47.8 ± 3.7%, P < 0.005 for BD and P < 0.05 for RAS) (Fig. 3b)
There was no significant difference in the proportion of lymphocytes or T cell subsets expressing CD25 or HLA-DR for either patient group compared with healthy controls (data not shown). However, an increase was observed for the proportion of γδ T cells expressing CD25 in patients with BD (7.4 ± 2.9%) or RAS (5.6 ± 3.0%) compared with healthy controls (4.2 ± 1.3%) (Fig. 3b). This was not observed for the αβ T cells (Fig. 3b).
Expression of intracellular cytokines on lymphocytes and T cell subsets in peripheral blood from patients with BD or RAS and healthy controls
The expression of intracellular IL-4, IL-10, TNF-α and IFN-γ in lymphocytes and T cell subsets was analysed in peripheral blood from patients with BD or RAS and healthy controls.
Before stimulation, lymphocytes did not express any of the cytokines analysed (data not shown). However, after mitogen stimulation of lymphocytes for 4 h, there was a remarkable up-regulation of TNF-α and IFN-γ, and a low but consistent IL-4 and IL-10 expression. Maximal expression of IL-4 and IL-10 was observed when the PBMC were stimulated with PHA, whereas TNF-α and IFN-γ expression was preferably induced by stimulation with PMA and ionomycin (see Patients and Methods).
As seen in Fig. 4a, a significantly raised proportion of lymphocytes from BD patients could be induced to express TNF-α after mitogen stimulation for 4 h (25.3 ± 2.3%) compared with controls (18.9 ± 2.2%, P < 0.05). This was reflected in a significantly increased frequency of γδ T cells expressing TNF-α in PBL from BD patients (48.2 ± 6.1%) compared with controls (28.3 ± 6.3%, P < 0.05). Interestingly, this was not seen for RAS patients (Fig. 4a).
Fig. 4.
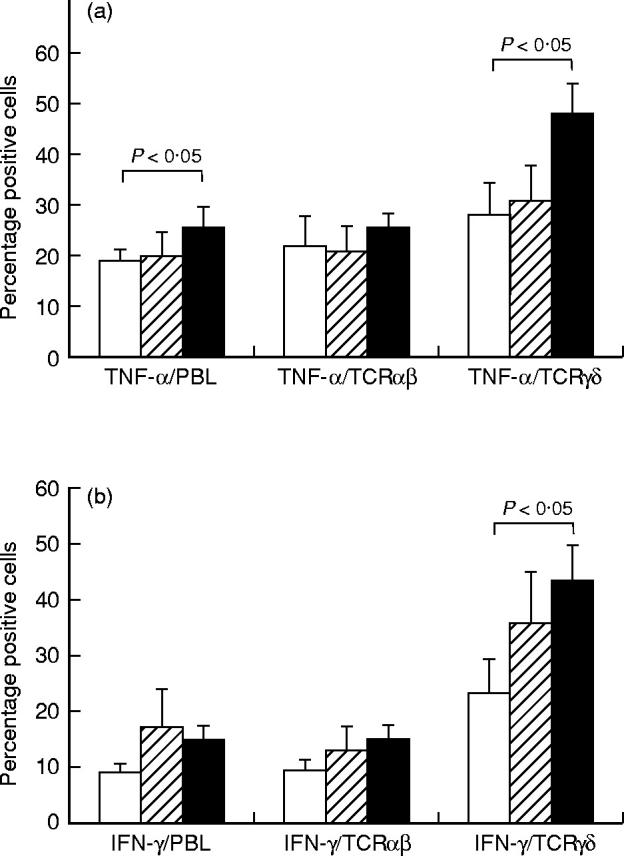
The expression of tumour necrosis factor-alpha (TNF-α) on peripheral blood lymphocytes (PBL), αβ T cells or γδ T cells (a) and the expression of IFN-γ on PBL, αβ T cells or γδ T cells (b), analysed by double immunofluorescent staining and flow cytometry. Results are expressed as percentage positive cells, shown as the mean ± s.e.m. for 20 BD patients (▪), 11 RAS patients (hatched bars) and 20 healthy controls (□). Statistically significant differences between BD patients and controls, BD patients and RAS patients, and RAS patients and controls are indicated.
An increased proportion of lymphocytes expressing IFN-γ was observed for the BD patients (14.6 ± 2.9%) compared with the controls (9.2 ± 1.5%), although the difference did not reach significance (Fig. 4b). This increase was reflected in a significantly increased proportion of γδ T cells expressing IFN-γ from BD patients (43.3 ± 6.6%) compared with controls (23.5 ± 6.1%, P < 0.05). In contrast to results obtained for TNF-α, RAS patients had an increased frequency of lymphocytes, as well as γδ T cells, that expressed IFN-γ compared with healthy controls, although the difference did not reach significance (Fig. 4b).
Because of the low frequency of lymphocytes expressing IL-4 or IL-10 (< 4%) it was not possible to obtain reliable results for IL-4 and IL-10 expression on γδ T cells. However, results obtained did not indicate a difference between either patient group and controls (data not shown).
DISCUSSION
Healthy oral mucosa does not contain many γδ T cells ([8], Freysdottir et al., manuscript in preparation). However, we have observed an increase in the number of γδ T cells within the oral ulcers, extending deep within the oral mucosa (Freysdottir et al., manuscript in preparation). It is still not known whether these cells accumulate at the site of oral ulceration or whether they actively participate in the pathogenesis of mucosal ulceration. If non-specific ulceration was the only reason for accumulation of γδ T cells, it could be postulated that there should be no difference between γδ T cells in peripheral blood from patients with RAS and patients with BD. We therefore analysed the activation and cytokine profiles of γδ T cells in peripheral blood from patients with either BD or RAS and compared the results with those found in a healthy control population.
The proportion of γδ T cells in peripheral blood from patients with BD was significantly higher than in RAS patients (P < 0.05) and in healthy controls (P < 0.05), as has been shown by us previously [3]. There was also an increase in the proportion of γδ T cells in peripheral blood from patients with RAS. Although this increase did not reach significance, it supports the findings of Pedersen & Ryder [7], who reported a significant increase in the proportion of γδ T cells in blood from RAS patients.
Both patient groups had a raised percentage of CD8+ cells amongst the cells expressing the γδ TCR. This suggests a role for CD8+ cells in both these diseases, with the γδ T cells being more prominent in BD. Furthermore, there was a significant increase in the proportion of CD56+ cells in peripheral blood from BD and RAS patients compared with controls. CD56 is one of the markers of natural killer (NK) cells. Although CD56 has also been found on γδ T cells [19], the large increase observed in the proportion of CD56+ lymphocytes far exceeded γδ T cells, indicating a genuine increase in the proportion of NK cells. The role of cytotoxicity in both RAS and BD has been proposed by the finding of peripheral blood leucocytes from the patients displaying a greater degree of cytotoxicity towards target epithelial cells than leucocytes from healthy controls [20,21]. This mechanism is further supported by the findings in this study of an increased percentage of lymphocytes expressing CD56, and CD8+γδ T cells.
A classical way of analysing T cell activation is to measure the up-regulation of CD25, the α-chain of the IL-2 receptor. In BD patients it was clearly the γδ T cell population that was in an activated stage, whereas the αβ T cell subset showed no increase in CD25 expression compared with controls. This observation indicates that γδ T cells in peripheral blood from BD patients had already been activated. Whether the activation occurs as a result of the disease or whether the activated γδ T cells play a direct role in the pathogenesis needs to be elucidated.
It was of interest to investigate expression of other activation and adhesion molecules on the T cells that could interact with the various cells and extracellular matrix (ECM) proteins in the oral mucosa. Therefore, we analysed the expression of CD40L, CD29 and CD69.
An increased percentage of lymphocytes expressing CD40L was observed in patients with BD compared with controls. CD40L is primarily expressed on activated T cells, including γδ T cells [22]. Its ligand, CD40, is expressed on B cells, endothelium, epithelium and some dendritic cells [23–25]. Interaction between CD40 and its ligand plays an important costimulatory role for B cells [23]. Whether interaction between CD40L on T cells and CD40 on the oral epithelium plays a role in maintaining healthy mucosa needs to be elucidated. The low frequency of CD40L+ lymphocytes in peripheral blood of BD patients suggest that CD40L does not play a significant role in ulcer formation.
This study found a raised proportion of γδ T cells expressing high levels of CD29 in both BD and RAS patients compared with controls. CD29, in combination with one of the α-integrin chains, comprises the very late activation (VLA) molecules. The VLA molecules interact with many of the ECM molecules, such as collagen, vimentin, laminin, fibronectin [26]. The major ligand for VLA-4 is the vascular cell adhesion molecule-1 (VCAM-1) molecule, which is expressed on activated endothelium [27]. Infiltrating T cells in oral lesional biopsies from BD patients are found surrounding blood vessels (Freysdottir et al., manuscript in preparation). Expression of raised levels of CD29 enables the γδ T cells to interact with the endothelial cells, as well as with the ECM proteins that are present in the oral mucosa.
A raised percentage of γδ T cells expressing CD69 was observed in BD patients compared with controls. CD69, an early activation marker on activated T and B cells, is involved in lymphocyte proliferation, and functions as a signal-transmitting receptor in T and B lymphocytes, NK cells and platelets [28]. The ligand for CD69 is still unknown, which makes it very interesting to analyse the distribution of the CD69+ T cells at the lesional sites. This might cast light on to what extent CD69+ T cells play a role in BD.
Unbalanced expression of Th1 or Th2 cytokines has been associated with inflammation in different animal disease models and may play a role in human inflammatory diseases. Previous studies analysing cytokines in BD and RAS have not been directed against individual cells, but have analysed cytokines in serum or in supernatants from bulk populations of PBMC [29–36]. These bulk populations consist of T cells and other lymphoid and non-lymphoid cells and secrete various cytokines, such as IL-1β, IL-4, IL-6, IL-8, IL-10, IL-12, IL-13, IFN-γ, TNF-α, granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF), with the specific origin of the cytokines being unknown. The development of a method of detecting intracellular cytokines by flow cytometry [37] meant that it was possible to study the contribution of individual cells, in a heterogeneous cell population, to specific cytokine production.
Both patient groups had an increased proportion of γδ T cells secreting IFN-γ when compared with healthy controls. Sugi-Ikai et al. [38] demonstrated an increased frequency of IFN-γ and IL-2 cytokine-producing CD4+ and CD8+ cells in peripheral blood from BD patients with active disease. Taken together, these two sets of data show that Th1 cytokine-producing cells, of both the αβ and the γδ TCR type, are present in BD and RAS patients. These Th1 cytokine-producing cells may play a specific role in the ulceration of both BD and RAS; or they may be up-regulated as part of the general inflammatory response.
TNF-α is another cytokine involved in inflammation which plays a major role in induction of adhesion molecules and inflammatory cytokine production. It is mainly produced by activated monocytes and macrophages. Our results show however that in BD a substantial proportion of γδ T cells is able to participate in TNF-α production. Previous studies have also shown that the production of TNF-α is increased in patients with BD [30,31]. However, none have demonstrated which cell types participated in the TNF-α production.
If one assumes that mucosal ulceration causes an increase in TNF-α-producing γδ T cells, no difference should be observed between patients with RAS or with BD. In this study however the BD patients had significantly raised percentages of TNF-α-producing γδ T cells, indicating that this high percentage may be disease-specific. BD has been strongly associated with the HLA-B51 antigen, although it has been suggested that the important gene for BD is not the HLA-B51 gene but a gene located near the HLA-B gene [39,40]. One such gene is the gene encoding for TNF-α. Recent studies have shown that the levels of gene expression of TNF-α are related to the clinical activity of BD [41]. Whether there is a link between raised TNF-α production and any polymorphism within the TNF-α gene in BD needs to be investigated. Interestingly, thalidomide, which is known to suppress TNF-α production [42], causes healing of oral ulceration in BD ([43], personal observations).
The significant findings in this study were the increased percentage of γδ T cells in peripheral blood of patients with BD, and that a greater proportion of these cells was in an activated stage and could be induced to secrete the inflammatory cytokines IFN-γ and TNF-α. These findings suggest that γδ T cells may play an important role in the immunopathogenesis of BD. In order to confirm this, the presence of γδ T cells in oral lesions from BD patients is being analysed with emphasis on their cytokine production and activation stage. The observation of an increased proportion of γδ T cells which secrete TNF-α provides an interesting therapeutic strategy for the development of novel treatments for BD.
Acknowledgments
We would like to thank Ms P. Shirlaw and Dr E. Graham for providing patients for this study, and all the staff at the Consultants Clinic at Guy's Hospital for assisting in collecting samples from both patients and healthy volunteers.
REFERENCES
- 1.Embil JA, Stephens RG, Manuel FR. Prevalence of recurrent herpes labialis and aphthous ulcers among young adults on six continents. Can Med Assoc J. 1975;113:627–30. [PMC free article] [PubMed] [Google Scholar]
- 2.Axell T, Henricsson V. The occurrence of recurrent aphthous ulcers in an adult Swedish population. Acta Odontol Scand. 1985;43:121–5. doi: 10.3109/00016358509046497. [DOI] [PubMed] [Google Scholar]
- 3.Fortune F, Walker J, Lehner T. The expression of γδ T cell receptor and the prevalence of primed, activated and IgA-bound T cells in Behçet's syndrome. Clin Exp Immunol. 1990;82:326–32. doi: 10.1111/j.1365-2249.1990.tb05447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valesini G, Pivetti-Pezzi P, Mastrandrea F, et al. Evaluation of T cell subsets in Behçet's syndrome using anti-T cell monoclonal antibodies. Clin Exp Immunol. 1985;60:55–60. [PMC free article] [PubMed] [Google Scholar]
- 5.Savage NW, Mahanonda R, Seymour GJ, et al. The proportion of suppressor-inducer T-lymphocytes is reduced in recurrent aphthous stomatitis. J Oral Pathol. 1988;17:293–7. doi: 10.1111/j.1600-0714.1988.tb01539.x. [DOI] [PubMed] [Google Scholar]
- 6.Hamzaoui K, Hamzaoui A, Hentati F, et al. Phenotype and functional profile of T cells expressing γδ receptor from patients with active Behçet's disease. J Rheumatol. 1994;21:2301–6. [PubMed] [Google Scholar]
- 7.Pedersen A, Ryder LP. γδ T-cell fraction of peripheral blood is increased in recurrent aphthous ulceration. Clin Immunol Immunopathol. 1994;72:98–104. doi: 10.1006/clin.1994.1112. [DOI] [PubMed] [Google Scholar]
- 8.Bramanti TE, Dekker NP, Lozada-Nur F, et al. Heat shock (stress) proteins and γδ T lymphocytes in oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Oral Endontol. 1995;80:698–704. doi: 10.1016/s1079-2104(05)80254-9. [DOI] [PubMed] [Google Scholar]
- 9.Janeway CA, Jr, Jones B, Hayday A. Specificity and function of T cells bearing γδ receptors. Immunol Today. 1988;9:73–76. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]
- 10.Boismenu R, Havran WL. An innate view of γδ T cells. Curr Op Immunol. 1997;9:57–63. doi: 10.1016/s0952-7915(97)80159-8. [DOI] [PubMed] [Google Scholar]
- 11.Komano H, Fujiura Y, Kawaguchi M, et al. Homeostatic regulation of intestinal epithelia by intraepithelial γδ T cells. Proc Natl Acad Sci USA. 1995;92:6147–51. doi: 10.1073/pnas.92.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chien Y-H, Jores R, Crowley MP. Recognition by γ/δ T cells. Annu Rev Immunol. 1996;14:511–32. doi: 10.1146/annurev.immunol.14.1.511. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka Y, Morita CT, Tanaka Y, et al. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–8. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 14.Porcelli SA, Morita CT, Modlin RL. T-cell recognition of non-peptide antigens. Curr Opin Immunol. 1996;8:510–6. doi: 10.1016/s0952-7915(96)80039-2. [DOI] [PubMed] [Google Scholar]
- 15.Ferrick DA, Schrenzel MD, Mulvania T, et al. Differential production of interferon-γ and interleukin-4 in response to Th1- and Th2-stimulating pathogens by γδ T cells in vivo. Nature. 1995;373:255–7. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 16.Boismenu R, Feng L, Xia YY, et al. Chemokine expression by intraepithelial γδ T cells. Implications for recruitment of inflammatory cells to damaged epithelia. J Immunol. 1996;157:985–92. [PubMed] [Google Scholar]
- 17.International study group for Behçet's disease. Criteria for diagnosis of Behçet's disease. Lancet. 1990;335:1078–80. [PubMed] [Google Scholar]
- 18.Esin S, Shigematsu M, Nagai S, et al. Different percentages of peripheral blood γδ+ T cells in healthy individuals from different areas of the world. Scand J Immunol. 1996;43:593–6. doi: 10.1046/j.1365-3083.1996.d01-79.x. [DOI] [PubMed] [Google Scholar]
- 19.Musha N, Yoshida Y, Sugahara S, et al. Expansion of CD56+ NK T and γδ T cells from cord blood of human neonates. Clin Exp Immunol. 1998;113:220–8. doi: 10.1046/j.1365-2249.1998.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reimer G, Steinkohl S, Djawari D, et al. Lytic effect of cytotoxic lymphocytes on oral epithelial cells in Behçet's disease. Br J Dermatol. 1982;107:529–36. doi: 10.1111/j.1365-2133.1982.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 21.Thomas DW, Bagg J, Walker DM. Characterisation of the effector cells responsible for the in vitro cytotoxicity of blood leucocytes from aphthous ulcer patients for oral epithelial cells. Gut. 1990;31:294–9. doi: 10.1136/gut.31.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horner AA, Jabara H, Ramesh N, et al. γ/δ T lymphocytes express CD40 ligand and induce isotype switching in B lymphocytes. J Exp Med. 1995;181:1239–44. doi: 10.1084/jem.181.3.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banchereau J, Bazan F, Blanchard D, et al. The CD40 antigen and its ligand. Ann Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 24.Pammer J, Weninger W, Mazal PR, et al. Expression of the CD40 antigen on normal endothelial cells and in benign and malignant tumours of vascular origin. Histopathol. 1996;29:517–24. doi: 10.1046/j.1365-2559.1996.d01-531.x. [DOI] [PubMed] [Google Scholar]
- 25.Peguet-Navarro J, Dalbiez-Gauthier C, Moulon C, et al. CD40 ligation of human keratinocytes inhibits their proliferation and induces their differentiation. J Immunol. 1997;158:144–52. [PubMed] [Google Scholar]
- 26.Hemler ME. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Ann Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- 27.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 28.Testi R, D'Ambrosio D, De Maria R, et al. The CD69 receptor: a multipurpose cell-surface trigger for hematopoietic cells. Immunol Today. 1994;15:479–83. doi: 10.1016/0167-5699(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 29.Ohno S, Kato F, Matsuda H, et al. Detection of γ-interferon in the sera of patients with Behçet's disease. Infect Immunol. 1982;36:202–8. doi: 10.1128/iai.36.1.202-208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akoglu TF, Direskeneli H, Yazici H, et al. TNF, soluble IL-2R and soluble CD-8 in Behçet's disease. J Rheumatol. 1990;17:1107–8. [PubMed] [Google Scholar]
- 31.Hamzaoui K, Hamza M, Ayed K. Production of TNF-α and IL-1 in active Behçet's disease. J Rheumatol. 1990;17:1428–9. [PubMed] [Google Scholar]
- 32.Taylor LJ, Bagg J, Walker DM, et al. Increased production of tumour necrosis factor by peripheral blood leukocytes in patients with recurrent oral aphthous ulceration. J Oral Pathol Med. 1992;21:21–25. doi: 10.1111/j.1600-0714.1992.tb00963.x. [DOI] [PubMed] [Google Scholar]
- 33.Mege J-L, Dilsen N, Sanguedolce V, et al. Overproduction of monocyte derived tumour necrosis factor α, interleukin (IL) 6, IL-8 and increased neutrophil superoxide generation in Behçet's disease. A comparative study with familial Mediterranean fever and healthy subjects. J Rheumatol. 1993;20:1544–9. [PubMed] [Google Scholar]
- 34.Takahama H, Itoh R, Inoue-Komatsu C, et al. Granulocyte colony-stimulating factor (G-CSF) and granulocyte macrophage colony stimulating factor (GM-CSF) in Behçet's disease. J Dermatol. 1994;21:546–52. doi: 10.1111/j.1346-8138.1994.tb01792.x. [DOI] [PubMed] [Google Scholar]
- 35.Turan B, Gallati H, Erdi H, et al. Systemic levels of the T cell regulatory cytokines IL-10 and IL-12 in Behçet's disease; soluble TNFR-75 as a biological marker of disease activity. J Rheumatol. 1997;24:128–32. [PubMed] [Google Scholar]
- 36.Raziuddin S, Al-Dalaan A, Bahabri S, et al. Divergent cytokine production in Behçet's disease. Altered Th1/Th2 cell cytokine pattern. J Rheumatol. 1998;25:329–33. [PubMed] [Google Scholar]
- 37.Jung T, Schauer U, Heusser C, et al. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993;159:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- 38.Sugi-Ikai N, Nakazawa M, Nakamura S, et al. Increased frequencies of interleukin-2- and interferon-γ-producing T cells in patients with active Behçet's disease. Invest Ophthalmol Vis Sci. 1998;39:996–1004. [PubMed] [Google Scholar]
- 39.Mizuki N, Inoko H, Ohno S. Pathogenic gene responsible for the predisposition of Behçet's disease. Int Rev Immunol. 1997;14:33–48. doi: 10.3109/08830189709116843. [DOI] [PubMed] [Google Scholar]
- 40.Mizuki N, Ohno S, Sato T, et al. Microsatellite polymorphism between the tumour necrosis factor and HLA-B genes in Behçet's disease. Human Immunol. 1995;43:129–35. doi: 10.1016/0198-8859(94)00159-n. [DOI] [PubMed] [Google Scholar]
- 41.Yamakawa Y, Sugita Y, Takahashi Y, et al. Gene expression of tumour necrosis factor-α (TNF-α) and heat-shock protein (HSP) 70 in patients with Behçet's disease. Arc Dermatol Res. 1993;285:505–8. doi: 10.1007/BF00376825. [DOI] [PubMed] [Google Scholar]
- 42.Tavares JL, Wangoo A, Dilworth P, et al. Thalidomide reduces tumour necrosis factor-alpha production by human alveolar macrophages. Resp Med. 1997;91:31–39. doi: 10.1016/s0954-6111(97)90134-7. [DOI] [PubMed] [Google Scholar]
- 43.Hamuryudan V, Mat C, Saip S, et al. Thalidomide in the treatment of the mucocutaneous lesions of the Behçet syndrome. A randomized, double-blind, placebo-controlled trail. Ann Intern Med. 1998;128:443–50. doi: 10.7326/0003-4819-128-6-199803150-00004. [DOI] [PubMed] [Google Scholar]


