Abstract
Two amyloidogenic Bence Jones proteins (Am37 VκIV and NIG1 VκI) and one non-amyloidogenic protein (NIG26 VκIII) were characterized. The protein Am37 had four deletions when compared with the translated germ-line gene sequence: two Ser residues following position 27 (27e, 27f) in CDR1 and two amino acids Pro-44, and Tyr-49 in FR2 were deleted. A strictly conserved salt-bridge-forming amino acid, Asp-82, was replaced by the hydrophobic residue Leu. In a comparative study of amyloidogenic and non-amyloidogenic proteins, five amino acids (Ser-10, Ala-13, Ser-65, Gln-90, and Ile-106) were found to be unique to NIG1 and several other amyloidogenic proteins. Additional substitutions also occur within these proteins. These substitutions might be significant in altering protein folding as well as in contributing to their aggregation as amyloid fibrils.
Keywords: Bence Jones protein, primary structure, amyloidogenicity, multiple myeloma, amyloidosis
INTRODUCTION
The major fibril protein found deposited in the tissues of individuals with primary amyloidosis and amyloidosis associated with multiple myeloma, i.e. light chain (AL) amyloidosis, is related to a monoclonal immunoglobulin light chain [1]. The deposits are composed mainly of one protein that possesses a β-pleated sheet structure and is stacked upon itself into long unbranched strands or fibrils of intermediate length. The subunits for AL amyloidosis can vary in length from the entire light chain to only a portion of the variable region [1,2]. It has been proposed that certain immunoglobulin light chains are predisposed to amyloid formation by their secondary and tertiary structure [3], and this may be the result of specific amino acid substitutions in their primary structure.
The specific chemical and structural features which determine the divergent accumulation properties of light chains may be factors in the pathological deposition of fibril protein in different sites of various organs. In this study, we examined the amino acid sequences of two amyloidogenic and one non-amyloidogenic κ-type light chain proteins and compared them with other proteins belonging to the same subgroups. We also attempted to identify the structural features controlling amyloid formation.
MATERIALS AND METHODS
Purification of proteins
The urine samples containing the Bence Jones proteins Am37 and NIG1 were obtained from individuals with multiple myeloma-associated AL amyloidosis, and another sample containing NIG26 was obtained from a patient with Bence Jones proteinaemia. The proteins were precipitated by ammonium sulphate fractionation and were purified using a conventional two-dimensional high performance liquid chromatography (HPLC) system. Next, the proteins were treated using an anion exchange HPLC column loaded with DEAE cellulose (2.16 × 16 cm), followed by a reversed phase HPLC column loaded with phenyl-5PWRP (4.6 × 75 mm). The purity was checked by MALDI-TOF/MS, SDS–PAGE, and immunoblot analyses.
Reduction and alkylation
The purified protein was dissolved in 6 m guanidine-chloride, 0.001 m EDTA and 0.25 m Tris–HCl pH 8.5, followed by addition of dithiothreitol. The reaction vessel was wrapped in aluminium foil and the mixture was held at 25°C for 2 h in a nitrogen atmosphere. The reduced protein was alkylated by addition of 4-vinylpyridine. After 30 min, the resultant mixture was subjected to reversed phase HPLC.
Peptide preparation
Proteolytic digestion of the reduced and S-pyridylethylated protein was carried out with lysylendopeptidase, followed by a secondary digestion with trypsin TPCK. The digest was separated and purified using reversed phase HPLC. A VYDAC Protein C4 (4.6 × 150 mm) column was used for the separation, and a Super Sphere RP100-C18 (4 × 250 mm) column was used for the purification. A flow rate of 1 ml/min was used with a linear gradient of acetonitrile (5–60%, 40 min) in 0.1% trifluoroacetic acid (TFA).
Amino acid analysis
The resultant peptides were dissolved in 6 n HCl containing 5% (v/v) phenol. They were hydrolysed in evacuated tubes in a constantly boiling solution of HCl for 24 h at 110°C. The solution was then evaporated. The hydrolysates were dissolved in 200 μl of 0.02 m Tris–HCl buffer pH 8.0, separated, and identified using a Jasco 800 series HPLC system.
Peptide sequence analysis
Sequence analysis was carried out by the Edman degradation method as described previously [4]. Approximately 60–100 picomol of peptide were loaded on polybrene-treated glass fibre disks and placed in an ABI model 473A gas-phase sequencer. The resultant phenylthiohydanated amino acids were identified by an on-line ABI HPLC system.
RESULTS AND DISCUSSION
Immunoglobulin-related polypeptides represent a class of proteins that have been implicated in the pathogenesis of one type of amyloidosis that occurs in patients with monoclonal B cell proliferative disorders of idiopathic or neoplastic origin, i.e. immunoglobulin or multiple myeloma-associated amyloidosis [5]. The precise chemical structure or conformational properties of such proteins which influence the process of deposition as amyloid fibrils, or other forms, or as non-nephrotoxic Bence Jones proteins, are unknown. In this study, we characterized two amyloidogenic proteins (BJP), Am37 and NIG1, and one non-amyloidogenic protein, NIG26, and compared them with other reported amyloidogenic and non-amyloidogenic proteins in order to determine the key sequence(s) relating to disease.
The purified monomeric fractions of Am37, NIG1, and NIG26 had molecular masses (M + H)+ of 24 362.60, 23 350.89, and 23 512.90 D, respectively. During immunoblot analyses all three reacted with anti-κ antiserum. Am37 contained 216 residues and its primary structure was comparable to the VκIV subgroup (90% homology with BJP LEN). NIG1 consisted of 213 residues and belonged to the VκI subgroup, and NIG26 consisted of 215 residues and its sequence was homologous to the VκIII subgroup. All three proteins possessed a Km(3) allotypic CL domain. No significant carbohydrate moiety or other post-translational contaminants were detected in the fractions.
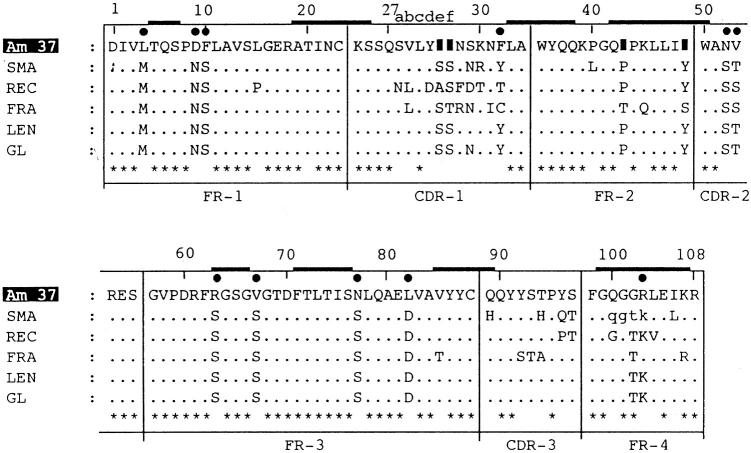
Multiple deletions in Am37
In 1973, κIV was isolated from κ-type light chains and reported as the so-called cold agglutinins [6]. It was fully sequenced from the protein LEN [7]. It has been reported that VκIV is encoded by a single κIV germ-line V-segment exon [8–10]. All members of this subgroup have six extra amino acids in the CDR1 region in comparison with other subgroups such as VκI. The V domain of Am37 has been aligned with the translated germ-line gene sequence [8] in Fig. 1. In order to get maximum homology, several deletions were introduced into the sequence of Am37. Multiple deletions occurred in its V region. The protein contained 15 residues instead of the 17 normally found in the CDR1 region. Two amino acids were deleted from positions 27e and 27f of this domain. In addition to this, the amino acids Pro-44 and Tyr-49 were deleted from the FR2 region. These changes in the primary structure of Am37 strongly support the possibility of deletion, insertion and somatic mutation in the restricted germ-line gene.
Fig. 1.
Amino acid sequences of the variable regions of the monoclonal κIV proteins. The translated germ-line (GL) gene sequence, the non-amyloidogenic and non-nephrotoxic protein LEN, the non-amyloidogenic protein FRA, the amyloidogenic protein SMA, and REC are shown relative to the amyloidogenic protein Am37 sequence. Dots indicate an amino acid residue identical to that in the GL, and solid boxes indicate deletion of an amino acid from that position. Stars represent common amino acids in all sequences. Solid circles represent novel substitutions, and thick bars indicate the β-sheet region for those positions. Small letters for residues 100–103 of SMA are not unambiguously determined. The numbering system follows Kabat et al. [4], and the β-sheet locations are superimposed from the three-dimensional structure of REI [14].
Novel substitutions in Am37
In addition to the four deletions, Am37 also had several novel substitutions, all in the V domain. In the FR1 region, Met-4 and Ser-10 were replaced by the hydrophobic residues Leu and Phe, respectively. Significant alterations occurred in the third frame work (FR3) region. The amino acids Ser-63 and Ser-77 were replaced by Arg and Asn, respectively. The positively charged Asp-82 was substituted with the hydrophobic amino acid Leu. Asp-82 is important because it stabilizes the domain by making a key conserved salt bridge with Arg-61 located on the adjacent loop. Replacement of one member of a salt bridge may be particularly destabilizing, because it leaves behind a potentially unpaired charge [11]. In fact, the replaced salt bridge partners are also present in the κ-type VL proteins MCM (D82I) [12] and BAN (R61N), both pathogenic proteins associated with protein deposition [13]. These replacements were introduced as point mutations into the κ VL domain REI [14] and their susceptibility to fibril formation has been extensively studied [15,16]. A correlation between salt bridge disruption and pathogenesis was observed. In another study the Asp-82 replacement was found to occur in the FR region, which determines the overall folding of the immunoglobulin domain conformation and holds the complementary region in place [17]. This change may result in the variable region structure being more prone to fibril formation.
In the Jκ [18] joining region (96–108) Lys-103 is replaced by Arg. Two replacements occurred in the CDR1 region. The amino acids Asn-29 and Tyr-32 were substituted by Ser and Phe, respectively.
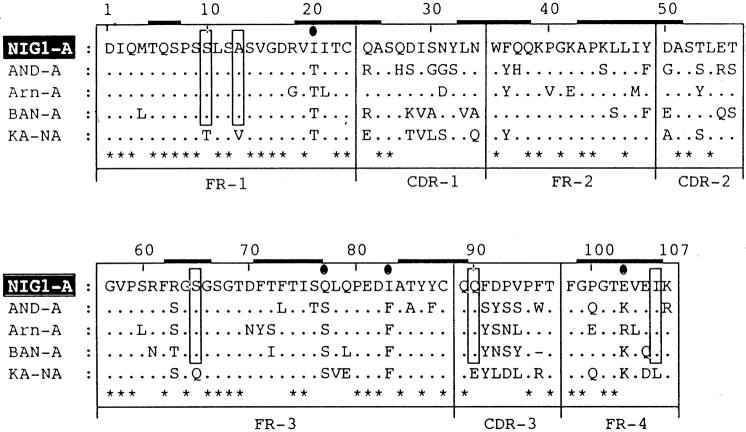
Unique substitutions in NIG1
The primary structure of the amyloidogenic light chain protein NIG1 was aligned with three amyloidogenic Bence Jones proteins, AND [19], ARN [20], and BAN [13], and one non-amyloidogenic protein, KA [21] (Fig. 2). All are κI light chains. Five amino acids, Ser-10, Ala-13, Se-65, Gln-90, and Ile-106 were found to be unique to the amyloidogenic light chains. The NIG1 protein also contained two special substitutions: Phe-36 (FR2) and Arg-63 (FR3). These two components are 0.95% invariant in κ-type proteins. NIG1 also contained Gln-77 (FR3), Ile-83 (FR3), and Glu-103 (FR4), which have not been previously reported.
Fig. 2.
Comparison of the V region sequences of VκI proteins. The V region sequence of the amyloidogenic protein NIG1 is aligned with the sequences of three amyloidogenic (A) proteins AND, ARN, and BAN, and one non-amyloidogenic (NA) protein KA. The unique residues are boxed. The numbering and other symbols are as in Fig. 1. The β-sheet locations are superimposed from the three-dimensional structure of REI [14] protein.
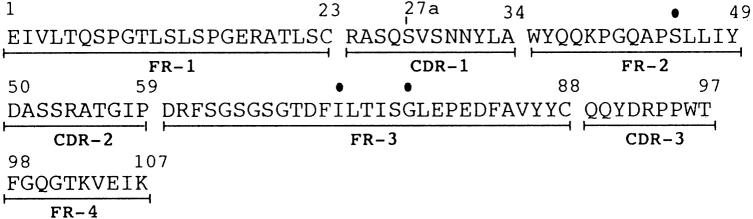
Substitutions in NIG26
In comparison with other κ-type human immunoglobulin light chains, NIG26 has five substitutions (Fig. 3) not previously reported. They are as follows: Ser-45 in the FR2 region; Ile-72 in the FR3 region; and an Asp-Arg-Pro sequence in the CDR3 region, a position generally occupied by a Gly-Ser-Ser sequence in most VκIII proteins. NIG26 also contained Asn-30 and Asn-31 in the CDR1 region, Asp-50 in the FR2 region, and Trp-96 in the CDR3 region. These substitutions are not as commonly found in other members of the same subgroup.
Fig. 3.
Amino acid sequence of the variable regions of NIG26. The solid circles on single-letter codes of amino acids represent novel substitutions in the sequence.
Structural relationships with amyloidogenecity
κ-related amyloidosis is less frequent than that from λ-related proteins, and the κIV subgroup is very poorly represented, accounting for only about 8% of the monoclonal κ-chains [22]. In vivo and in vitro analyses of certain VκIV light chains have revealed their high nephrotoxic potential which leads to their pathological deposition in tissues such as renal tubular casts [23], basement membrane precipitates [24], and in particular, amyloid fibrils [25]. Some are also reported as non-amyloidogenic [26], and non-nephrotoxic [26,27]. The alignment results of the variable region sequences of the amyloidogenic proteins Am37, SMA [25], and REC [25], the non-amyloidogenic FRA [25], and the non-amyloidogenic non-nephrotoxic protein LEN [7] with the translated germ-line protein sequence [8] have shown no common amino acids in the amyloidogenic or non-amyloidogenic protein sequences. The exchange rates for all amino acids between the germ-line sequence and other compared sequences are different. In comparison with the germ-line gene, the LEN protein had only one replacement (Ser-29), but this substitution was non-amyloidogenic and non-nephrotoxic. Thus, when the amyloidogenic proteins are compared with LEN, every amino acid variation could potentially play a significant role in amyloid formation.
In this pilot study, five amino acids (Ser-10, Ala-13, Ser-65, Gln-90, and Ile-106) were found to be common in several amyloidogenic light chain proteins (Fig. 2). When these substitutions are tentatively superimposed on the three-dimensional structure of the human κ protein REI [14], two amino acids, Ser-65 and Ile-106, correspond to the β-sheet domain. In Am37 (Fig. 1), Arg-63 and Arg-100 also correspond to the β-sheet region of the REI protein. These local substitutions may be the cause of the alteration in the protein conformation, which in turn might lead to their being more prone to association with amyloid processes. Alternatively, the changes in NIG26 may be insufficient to render it amyloidogenic. In order to confirm these findings we are now undertaking an attempt to clone cDNA from an individual (NIG26 patient) and trying to determine, by site-directed mutagenesis, which substituent(s) mentioned above are necessary for the amyloidogenicity of the protein.
Acknowledgments
This work was supported in part by grants-in-aid from the Ministry of Education, Science and Culture of Japan and the Ministry of Health (Welfare Primary Amyloidosis Research Committee).
REFERENCES
- 1.Glenner GG, Terry W, Harada M, Isersky C, Page D. Amyloid fibril proteins: proof of homology with immunoglobulin light chains by sequence analysis. Science (Wash DC) 1971;172:1150–1. doi: 10.1126/science.172.3988.1150. [DOI] [PubMed] [Google Scholar]
- 2.Glenner GG. Amyloid deposits and amyloidosis. The β-fibrillosis. N Engl J Med. 1980;302:1283–92. 1333–43. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- 3.Solomon A, Frangione B, Franklin EC. Preferential association of the VλVI subgroup of human light chains with amyloidosis AL (l) J Clin Invest. 1982;70:453–60. doi: 10.1172/JCI110635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kabat EA, Wu TT, Reid-Miller M, Perry HM, Gottesman KS, Foeller C. Sequences of proteins of immunological interest. Bethesda, MD: Natl Inst Health; 1994. pp. 165–462. [Google Scholar]
- 5.Stone MJ. Amyloidosis: a final common pathway for protein deposition in tissues. Blood. 1990;75:531–45. [PubMed] [Google Scholar]
- 6.Wang AC, Fudenberg HH, Wells JV, Roelcke D. A new subgroup of the kappa chain variable region associated with anti-pr cold agglutinins. Nature New Biol. 1973;243:126–7. [PubMed] [Google Scholar]
- 7.Schneider M, Hilschmann N. Die Primarrstruktur einer monoklonalen immunglobulin-L-Kette der Subgruppe IV vom κ-typ (Bence-Jones-Protein Len.): eine neue Subgruppe der L-Kitten vom κ-typ. Hoppe-Seyler's Z Physiol Chem. 1974;355:1164–8. [PubMed] [Google Scholar]
- 8.Klobeck HG, Bornkamm GW, Combriato G, Mocikat R, Pohlenz HD, Zachu HG. Subgroup IV of human immunoglobulin κ light chain is encoded by a single germ line gene. Nucl Acids Res. 1985;13:6515–29. doi: 10.1093/nar/13.18.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khamlichi AA, Aucouturier P, Silvain C, Bauwens M, Touchard G, Preud'Homme J-L, Nau F, Cogne M. Primary structure of a variable monoclonal κ chain in myeloma with light chain deposition disease. Clin Exp Immunol. 1992;87:122–6. doi: 10.1111/j.1365-2249.1992.tb06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurth JH, Cavalli-Sforza LL. Notes on individual sequence variation in humans: immunoglobulin kappa light chain. Am J Human Genet. 1994;54:1037. [PMC free article] [PubMed] [Google Scholar]
- 11.Waldburger CD, Schildbach JF, Sauer RT. Are buried salt bridges important for protein stability and conformational specificity? Nature Struct Biol. 1995;2:122–8. doi: 10.1038/nsb0295-122. [DOI] [PubMed] [Google Scholar]
- 12.Gallo G, Boctor F, Frangonie B, Ghiso J. Light chains deposition disease: biochemical characterization of tissue deposits. In: Kisilevsky R, Benson MD, Frangione B, et al., editors. Amyloid and amyloidosis. New York: Parthenon; 1994. pp. 280–3. [Google Scholar]
- 13.Liepnieks JJ, Benson MD, Dwulet FE. Comparison of the amino acid sequences of ten kappa-I amyloid proteins for amyloidogenic sequences. In: Natvig JB, Forre O, Husby G, Husebekk A, Skogen B, Sletten K, Westermark P, editors. Amyloid and amyloidosis. Dordrecht: Kluwer Academic Publishers; 1990. pp. 53–156. [Google Scholar]
- 14.Huang DB, Chang CH, Ainsworth C, Brunger AT, Eulitz M, Solomon A, Stevens FJ, Schiffer M. Comparison of crystal structures of two homologous proteins: structural origin of altered domain interactions in immunoglobulin light chain dimers. Biochemistry. 1994;33:14848–57. doi: 10.1021/bi00253a024. [DOI] [PubMed] [Google Scholar]
- 15.Helms LR, Wetzel R. Destabilizing loop swaps in the CDRs of an immunoglobulin VL domain. Protein Sci. 1995;4:2073–81. doi: 10.1002/pro.5560041012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helms LR, Wetzel R. Specificity of abnormal assembly in immunoglobulin light chain deposition disease and amyloidosis. J Mol Biol. 1996;257:77–86. doi: 10.1006/jmbi.1996.0148. [DOI] [PubMed] [Google Scholar]
- 17.Epp O, Tattman EE, Schiffer M, Huber R, Palm W. The molecular structure of a dimer composed of the variable portion of the Bence-Jones protein REI refined at 2.0 Å resolution. Biochemistry. 1975;14:4943–52. doi: 10.1021/bi00693a025. [DOI] [PubMed] [Google Scholar]
- 18.Hieter PA, Maizel JV, Leder P. Evolution of human κ region genes. J Biol Chem. 1982;257:1516–22. [PubMed] [Google Scholar]
- 19.Liepnieks JJ, Dwulet FE, Benson MD. Amino acid sequence of a kappa-I primary (AL) amyloid protein (AND) Mol Immunol. 1990;27:481–5. doi: 10.1016/0161-5890(90)90066-9. [DOI] [PubMed] [Google Scholar]
- 20.Aucouturier P, Khamlichi AA, Preud'Homme JL, Bauwens M, Touchard G, Cogne M. Complementary DNA sequence of human amyloidogenic immunoglobulin light-chain precursors. Biochem J. 1992;285:149–52. doi: 10.1042/bj2850149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shinoda T. Comparative structural studies on the light chains of human immunoglobulins. J Biochem. 1975;77:1277–96. [PubMed] [Google Scholar]
- 22.Solomon A, Weiss DT. A perspective of plasma cell dyscrasias: clinical implications of monoclonal light chains in renal disease. In: Minetti L, D'Amico G, Ponticelli C, editors. The kidney in plasma cell dyscrasias. Dordrecht: Academic Publishers; 1988. pp. 3–18. [Google Scholar]
- 23.Khamlichi AA, Aucouturier P, Silvain C, Bauwens M, Touchard G, Preud'Homme J-L, Nau F, Cogne M. Primary structure of a monoclonal κ chain in myeloma with light chain deposition disease. Clin Exp Immunol. 1992;87:122–6. doi: 10.1111/j.1365-2249.1992.tb06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denoroy L, Deret S, Aucouturier P. Overrepresentation of the VκIV subgroup in L chain deposition disease. Immunol Letters. 1994;42:63–66. doi: 10.1016/0165-2478(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 25.Stevens PW, Raffen R, Hanson DK. Recombinant immunoglobulin variable domains generated from synthetic genes provide a system for in vitro characterization of light-chain amyloid proteins. Protein Sci. 1995;4:421–32. doi: 10.1002/pro.5560040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solomon A, Weiss DT, Kattine AA. Nephrotoxic potential of Bence Jones proteins. N Eng J Med. 1991;324:1845–51. doi: 10.1056/NEJM199106273242603. [DOI] [PubMed] [Google Scholar]
- 27.Solomon A, Weiss DT, Pepys MB. Induction in mice of human light-chain associated amyloidosis. Am J Pathol. 1992;140:629–37. [PMC free article] [PubMed] [Google Scholar]