Abstract
In a longitudinal observational study of 94 patients (39M:55F, mean age 69) at elevated risk for developing post herpetic neuralgia (PHN), the natural history of pain during the first 6 months after herpes zoster (HZ) rash onset was determined. Pain severity and impact were rated using pain-VAS, SF-MPQ, and MPI.
Applying a definition of PHN of average daily pain >0/100 on the pain VAS during the last 48 hours, 30 subjects had PHN at 6 months. These 30 subjects reported more pain and a higher SF-MPQ score (p<0.01) at study inclusion than the 64 subjects whose pain completely resolved by 6 months. At 6 months, mean daily pain in the PHN group was 11/100 (95% CI 5,16) and only nine of these subjects were still taking prescription medication for HZ pain. The rate of recovery (pain severity over time) was the same in the PHN and no-pain groups. At study inclusion, the SF-MPQ and MPI scores in our PHN group were similar to historical controls with chronic severe PHN enrolled in clinical trials, but by 6 months the scores in our PHN subjects were significantly lower than historic controls. Only two subjects met the more stringent criteria for ‘clinically meaningful’ PHN at 6 months (≥30/100 on the pain VAS).
Defining PHN as average daily pain >0/100 at 6 months after rash onset appears to substantially overestimate the number of HZ patients negatively impacted by ongoing pain and disability.
Keywords: AHZ, post herpetic neuralgia, questionnaires, SFMPQ, MPI, diagnostic criteria
INTRODUCTION
Herpes zoster (HZ) is caused by reactivation of the varicella-zoster virus (VZV) in dorsal root ganglion neurons. Clinically, the disease manifests as a painful dermatomal, unilateral, vesicular rash (Dworkin and Portenoy 1996). Post-herpetic neuralgia (PHN), the most common complication of HZ in immunocompetent subjects, is characterized by persisting pain in the affected dermatome after rash healing and which lasts for years (Kost and Straus 1996; Rowbotham and Petersen 2001). Identifying those at highest risk for developing PHN as early as possible during HZ could optimize treatment and prevent chronicity in this population (Dworkin 1997; Dworkin and Schmader 2001; Johnson 2001). Longitudinal studies have identified age, initial pain severity, presence of prodrome, and rash severity as risk factors for persistence of pain after HZ (DeMoragas and Kierland 1957; Hope-Simpson 1975; Dworkin and Portenoy 1996; Choo et al. 1997; Dworkin and Schmader 2001; Nagasako et al. 2002; Jung et al. 2004). Other reported risk factors include sensory dysfunction in the affected dermatome (Nurmikko and Bowsher 1990; Bruxelle 1995), mechanical allodynia and prodromal pain(Choo et al. 1997; Whitley et al. 1998; Haanpää et al. 2000; Jung et al. 2004), and psychosocial variables (Dworkin et al. 1992; Rose et al. 1992; Engberg et al. 1995).
Definitions of PHN have varied. Most define PHN as ‘presence of pain’ without specifying a numerical threshold, but use different time points after rash onset or rash healing (Watson et al. 1991; Dworkin and Portenoy 1996; Helgason et al. 2000; Dworkin and Schmader 2001). Across a group of studies that includes those from before the introduction of antiviral drugs, the incidence of PHN defined as ‘presence of pain’ ranges between 7–25% at three months and 5–13% at six months (McKendrick et al. 1989, Crooks et al. 1991; Beutner et al. 1995; Bruxelle 1995; Haanpää et al. 2000; Helgason et al. 2000; Dworkin and Schmader 2001; Coplan et al. 2004, Scott et al. 2006). The term ‘clinically meaningful’ PHN, defined as pain of 30 or higher in the 0-100mm pain VAS to exclude those with mild pain, was employed in the recent shingles vaccine study (Coplan et al. 2004; Oxman et al. 2005).
The objective of this longitudinal observational study was to describe the natural history of pain severity and impact during the first 6 months after HZ onset in a cohort of patients whose age and pain severity conferred an elevated risk of developing PHN.
METHODS
Subjects
Immunocompetent subjects in stable health over the age of 50 with cervical, thoracic, lumbar, or sacral outbreaks of HZ were eligible if subject-reported average daily pain over the last 48 hours prior to the study inclusion visit was at least 20 on the 0–100 mm pain VAS. Subjects were excluded if they had other neurological dysfunction, significant cognitive impairment, psychiatric disorder severe enough to interfere with study procedures, another pain problem of equal or greater severity than HZ, or signs of stroke, myelopathy or progressive malignant disease. Subjects were not permitted to use medicated topical agents or receive nerve blocks or neurosurgical procedures for pain while enrolled, but were allowed to use oral medications prescribed by their treating physician. The diagnosis of HZ was based on physical exam by the study physician and review of available medical records. Subjects were recruited through newspaper ads, physician referrals, and community outreach efforts. All subjects provided written informed consent. The study was conducted in accordance with the Helsinki Declaration and approved by the Committee on Human Research at the University of California, San Francisco.
Study procedures and outcome measures
The study included a total of four visits over a 6 month period. The study inclusion visit was performed within 2–6 weeks after rash onset, visit 2 at 6–8 weeks, visit 3 at 3 months, and visit 4 at 6 months. The study inclusion visit included a detailed general medical history and a complete physical and neurological exam that was updated at subsequent visits. At every visit, subjects rated their ‘average daily pain over the last 48 hours’ using a handheld 0–100 mm pain VAS (0 mm=’no pain’ and 100 mm=’worst pain imaginable’). Rash severity was graded by the investigator at the study inclusion visit as mild (<25% of the linear dermatome affected with lesions), moderate (25–75%), or severe (>75%). Detailed sensory examination, quantitative assessments of thermal and touch sensation, capsaicin response test, and skin biopsies for assessment of cutaneous nerve density were also performed and will be reported separately. Subjects with facial or ophthalmic HZ were not eligible to avoid application of capsaicin and collection of skin biopsies in the face. Specific case examples from the study cohort have been previously reported (Berry et al. 2004).
Definition of PHN
PHN was defined as average daily pain ratings over the last 48 hours > 0/100 on the pain VAS at 6 months after rash onset. ‘Clinically meaningful’ PHN was defined as average daily pain ratings over the previous 48 hours ≥30/100 on the pain VAS at 6 months after rash onset (Coplan et al. 2004).
Questionnaires
Short-Form McGill Pain Questionnaire (SF-MPQ)
The SF-MPQ (Melzack 1987) was used to assess pain at all four visits. Fifteen pain descriptors (11 sensory, 4 affective) are rated by the subjects on a four-point intensity scale (0=none to 3=severe) regarding the preceeding week. For data analysis, three scores are derived: 1) sensory score (descriptors: ‘throbbing’, ‘shooting’, ‘stabbing’, ‘sharp’, ‘cramping’, ‘gnawing’, ‘hot-burning’, ‘aching’, ‘heavy’, ‘tender’, and ‘splitting’); 2) affective score (descriptors: ‘tiring-exhausting’, ‘sickening’, ‘fearful’, and ‘punishing-cruel’); and 3) total score (sensory plus affective score).
Multidimensional Pain Inventory (MPI)
The MPI (Kerns et al. 1985) was administered at all four visits. The MPI consists of 12 scales grouped into three domains: 1) the impact of pain on the subject’s life, 2) the responses of others to the subject’s communications of pain, and 3) the extent to which the subject participates in common daily activities. Five scales are used to assess pain impact (‘pain severity’, ‘interference’, ‘life control’, ‘affective distress’, and ‘support’). Three scales are used to assess the response by others to the subject’s pain complaint (‘negative’, ‘solicitous’, and ‘distracting responses’). Four scales are used to assess effect on daily activities (‘household chores’, ‘outdoor work’, ‘activities away from home’, and ‘social activities’). After completion, a ‘general activity level’ is calculated as a mean of the four activity scales. Each scale is comprised of between 3–11 statements (e.g., “How much has your pain interfered with your ability to get enough sleep?”), and each statement is rated on a seven-point scale (0=’no interference’ and 6=’extreme interference’). The mean score for each of the 12 scales is the sum of all the scale’s statement ratings divided by the number of statements in the scale.
Ways of Coping Questionnaire (WAYS)
As a measure of subject coping strategies, WAYS (Folkman and Lazarus 1988) was administered at study inclusion and 6 months after rash onset. The questionnaire is used to assess the thoughts and actions subjects employ in order to cope with stressful events in everyday living. In this study, coping was assessed as a trait and not specifically related to coping with the pain of HZ. Subjects were asked to think of a time or an event that was particularly stressful, but were not asked to specify the time or event. The questionnaire consists of 66 statements on ways of coping with stress (e.g., “I tried to keep my feelings to myself” or “I asked a relative or friend I respect for advice”). Statements are grouped into 8 overall coping strategies (between 4–8 statements per strategy): ‘Confrontive coping’, ‘distancing’, ‘seeking social support’, ‘accepting responsibility’, ‘planful problem solving’, ‘positive appraisal’, ‘self-controlling’, and ‘escape-avoidance’. The subjects rate to what extent they use each of the ways of coping described in the 66 statements on a four-point scale (0=“does not apply or not used” to 3=“used a great deal”). Two scores were calculated: A mean score for each strategy (the sum of the ratings on the statements within the strategy divided by the number of statements within the strategy), and a relative score for each strategy (the mean score for each strategy divided by the sum of mean scores for all 8 strategies). Relative scores reflect the proportion of the total coping effort represented by each type of coping strategy.
Life Events Checklist (LEC)
To assess the impact of stressful life events within the last 12 months on the natural history of pain, the LEC (Cohen et al. 1991) was administered at study entry and again at 6 months after rash onset. The checklist consists of 24 common potentially stressful events that are grouped into those that might have occurred in the life of the subject (‘self’ events) or in those close to the subject (‘other’ events). Events are grouped as ‘negative’ or ‘positive’ based on additional questions regarding the perception of each life event. Examples of events were moving, death, divorce, and broken relationship. A sum of all events (negative and positive ‘self’ and ‘other’ events) was calculated.
Data Analysis
Demographic and non-repeated measures were analyzed using a non-parametric two-tailed Fisher’s exact test for categorical variables and two-tailed Mann-Whitney rank-sum test for continuous variables. Because the interval from rash onset to the 4 study visits was variable between subjects, the repeated measures data were analyzed using a mixed effects regression model with predictors: PHN at 6 months (y/n), Visit Day (days since rash onset), and the interaction of PHN at 6 months and Visit Day. Data are presented as the slope across the four visit days for the PHN and no-pain groups with estimates for the following time points: 0, 30, 50, 90, and 180 days after rash onset. Analyses included comparisons of predicted slopes of PHN and no-pain groups along with comparisons at relevant time points. No adjustment was done for multiple comparisons (Katz 2003). A p-value less than 0.05 was considered statistically significant.
A post-hoc analysis compared the SF-MPQ data to historic data from 110 patients with chronic PHN from the placebo group (Rowbotham et al. 1998). In this study PHN was defined as average daily pain ratings ≥4 on a 0-10 numerical pain rating scale and median duration since zoster eruption was 27 months. Another post-hoc analysis compared the MPI data to historic data from 26 patients with chronic PHN (unpublished data from Rowbotham et al. 2003). In this study PHN was defined as average daily pain ≥25 on the 0-100 VAS and duration of pain was > 11 months). The p-values for post-hoc analyses were based on a one-way ANOVA. The data-analysis was performed in collaboration with Dr. Alan Bostrom, Department of Biostatistics and Epidemiology, UCSF.
RESULTS
Subjects
A total of 1003 subjects were screened on the telephone between December 1999 and December 2003. Of these, 870 did not come for a screening/inclusion visit for the following reasons: 156 already had PHN of long duration; 91 were already beyond the inclusion time window; 75 had pain that was too mild to qualify; 52 felt participation would be too time consuming; 69 had exclusionary medical conditions or medications; 65 had cranial zoster; 31 were too young; and in 11 there was uncertainty about the diagnosis. Another 320 either failed to attend a screening examination, were just seeking information, or were not interested in participating (often due to distance from the research center or lack of transportation). No potential subject cited their pain as being too severe to allow study participation.
Of the 133 subjects who came in for the screening/study inclusion visit, 103 were eligible and completed the study inclusion visit. Ninety-four subjects completed all four study visits and their data are included in the data analysis. Six subjects withdrew consent due to the time commitment involved in study participation, two subjects were dropped due to uncertainty about the diagnosis of HZ, and one was excluded due to progressive cognitive impairment. Demographic data and characteristics at the study inclusion visit are shown in Table 1. Twenty-nine of the 94 subjects had average daily pain over the preceeding 48 hours of 50 or higher prior to study inclusion.
Table 1.
Demographics at the study inclusion visit (n=94). PHN defined at average daily pain >0 on the 0–100 pain VAS.
| PHN at 6 months (n=30) | No-Pain at 6 months (n=64) | |
|---|---|---|
| Age (median [range]) | 70.5 [46*–89] | 67 [51–89] |
| Gender (% female) | 57 | 59 |
| Race (% Caucasian) | 87 | 92 |
| Work status (% employed) | 37 | 44 |
| Marital status (% married, S/O) | 57 | 66 |
| Alcohol use (% high): | 3 | 4 |
| Substance abuse (%) | 7 | 8 |
| Tobacco use (%) | 7 | 9 |
| History of psychiatric disease (%) | 20 | 13 |
| History of depression (%) | 23 | 16 |
: One subject was 46 years of age, but met all other inclusion criteria.
Resolution of pain over the study observation period is shown in Figure 1. At 3 months, 47 subjects (50%) met criteria for PHN (average daily pain >0/100), and of these, three subjects (3%) met the more stringent criteria for clinically meaningful PHN (average daily pain ≥30/100). At 6 months, 30 subjects (32%) met criteria for PHN, with two subjects (2%) also meeting criteria for clinically meaningful PHN.
Figure 1.
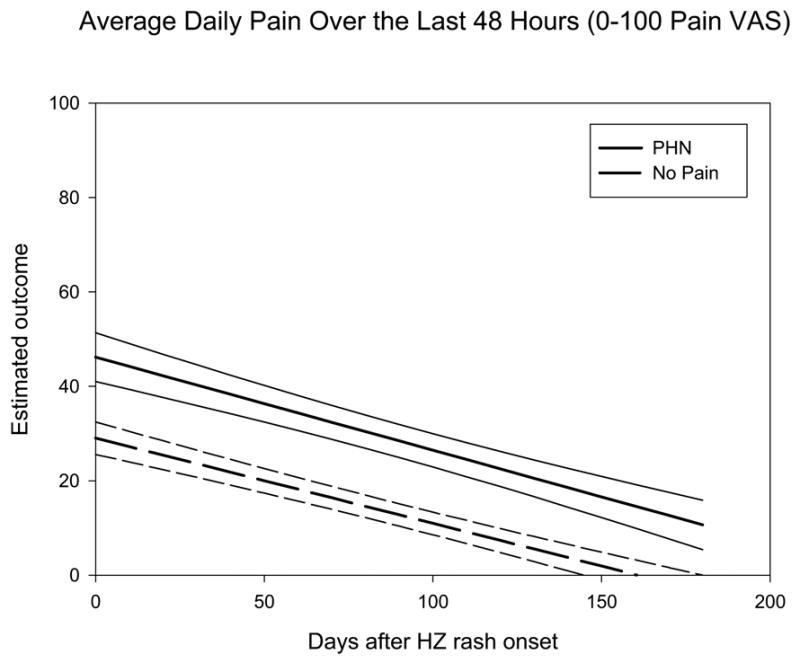
Estimates of Average Daily Pain in 30 subjects with PHN at 6 months (dashed line with 95% CI) and 64 subjects with no-pain at 6 months (solid line with 95% CI). PHN was defined as average daily pain over the last 48 hours > 0/100 on 100 mm pain VAS.
Comparison of subjects with and without PHN at 6 months
There was no difference in demographics at study inclusion between subjects with and without PHN at 6 months (Table 1). PHN subjects completed the study inclusion visit a median of 5 days later than subjects whose pain fully resolved (PHN group: 27 days (±13) (mean (SD)) vs. no-pain group: 22 (±10); p=0.05). The timing of the remainder of the visits was the same in the two groups.
Pain and medication use
At study inclusion, subjects with PHN at 6 months (referred to below as PHN subjects) reported higher average daily pain compared to subjects with complete resolution of pain (Figure 1, Table 2). However, the rate of pain resolution over time was not different between PHN subjects and those whose pain resolved fully (slopes analysis: PHN: −0.18 [95% CI: −0.20 to –0.15] vs no pain: −0.19 [−0.24 to –0.15], p=0.52). PHN subjects more often had outbreaks in the cervical or lumbar region, and were somewhat more likely to have had a ‘severe’ rash (p=0.07). The majority of subjects in both groups had received a course of antiviral medication and was taking an analgesic medication at the time of study inclusion. PHN subjects were taking a greater number of analgesics and were more likely to be taking an antidepressant for pain. The majority of subjects in both groups reported that HZ affected their ability to sleep, work and participate in recreational activities.
Table 2.
Subject characteristics at study inclusion visit (n=94). PHN defined as average daily pain >0 on the 0–100 pain VAS over the last 48 hours.
| PHN at 6 months (n=30) | No-Pain at 6 months (n=64) | |
|---|---|---|
| Average daily pain VAS (median [range]) | 49 [20–82] | 34 [20–98]** |
| Prodromal pain (% present) | 83 | 77 |
| Duration, days (median [range]) | 3 [0–11] | 3 [0–20] |
| Dermatome affected (%) | ||
| Cervical | 33 | 15 * |
| Thoracic | 47 | 72 |
| Lumbar | 20 | 8 * |
| Sacral | 0 | 5 |
| Rash severity (%) | ||
| Mild | 27 | 33 |
| Moderate | 17 | 36 |
| Severe | 53 | 31 |
| Antiviral treatment (%) | 93 | 91 |
| Taking any analgesic | 100% | 97% |
| Number of analgesics taken (median [range]) | 4 (1–8) | 3 (0–6)** |
| Acetaminophen (% of subjects) | 97 | 84 |
| Opioid | 83 | 78 |
| NSAID | 47 | 47 |
| Gabapentin | 37 | 23 |
| Antidepressant | 30 | 11* |
: p<0.05,
: P< 0.001.
The characteristics of the 30 PHN subjects at 6 months are presented in Table 3. From the slopes analysis, the mean pain rating in this group was estimated to be 10.6 on the 0–100 pain VAS (95% CI: 5,16). At the 6-month study visit, 18 of the 30 PHN subjects rated their average daily pain during the last 48 hours as 10 on the pain VAS and 13 subjects chose the category ‘no pain’ on the Present Pain Intensity Scale of the SF-MPQ. Only 2 PHN subjects rated their average daily pain over the last 48 hours as ≥30 on the pain VAS. Only 9 PHN subjects were still using prescription medications for their pain, and of these, 5 subjects rated their average daily pain ≤10 on the pain VAS.
Table 3.
Characteristics of the 30 PHN subjects at 6 months. PHN was defined as average daily pain >0 on the 0–100 pain VAS over the last 48 hours.
| Subject | Average daily pain (48 hr pre visit) | Present Pain Intensity (SFMPQ) | Prescription Medication | Followed by MD for HZ pain |
|---|---|---|---|---|
| 0–100 VAS | (0–5) | (total daily dose) | yes/no | |
| 001 | 8 | no pain (0) | gabapentin 300 mg | yes |
| 002 | 2 | no pain (0) | none | no |
| 005 | 71 | n/a | oxycodone 40 mg
amitriptyline 50 mg |
yes |
| 014 | 1 | no pain (0) | none | no |
| 016 | 2 | mild (1) | none | no |
| 019 | 18 | mild (1) | none | no |
| 020 | 25 | distressing (3) | gabapentin 1200 mg
nortriptyline 25 mg |
yes |
| 025 | 5 | no pain (0) | none | no |
| 026 | 20 | no pain (0) | gabapentin 1800 mg
hydrocodone 22.5 mg |
no |
| 039 | 12 | discomforting (2) | none | no |
| 043 | 28 | no pain (0) | none | no |
| 046 | 2 | no pain (0) | none | no |
| 047 | 12 | discomforting (2) | none | no |
| 048 | 1 | no pain (0) | gabapentin 600 mg | yes |
| 059 | 37 | distressing/ horrible (3/4) | none | no |
| 060 | 7 | no pain (0) | gabapentin 600 mg | yes |
| 062 | 5 | no pain (0) | none | no |
| 066 | 2 | no pain (0) | none | no |
| 067 | 7 | mild (1) | none | no |
| 069 | 6 | mild (1) | none | no |
| 070 | 9 | mild (1) | gabapentin 1500 mg | yes |
| 072 | 18 | discomforting (2) | hydrocodone 5 mg at most 1/d | no |
| 075 | 23 | mild (1) | none | no |
| 080 | 8 | no pain (0) | none | no |
| 081 | 5 | mild (1) | none | no |
| 087 | 8 | mild (1) | none | no |
| 095 | 8 | no pain (0) | none | no |
| 096 | 6 | n/a | none | no |
| 098 | 10 | mild (1) | gabapentin 900 mg | no |
| 102 | 13 | discomforting (2) | none | yes |
Questionnaires
SF-MPQ
Descriptors used by more than 70% of subjects at study inclusion were: ‘tender’, ‘hot-burning’, aching’, stabbing’, ‘throbbing’, ‘shooting’, ‘sharp’, and ‘tiring-exhausting’. At the 6 month visit, the only descriptor used significantly less often was ‘hot-burning’. PHN subjects had significantly higher SF-MPQ scores at study inclusion and at 6 months after rash onset (Table 4), but the only descriptor used more frequently at study inclusion by PHN subjects was ‘sharp’ (90% vs. 64%, p=0.042, Fisher’s exact test). At the time of study inclusion, the SF-MPQ scores in PHN subjects were similar to the scores in historic control patients with chronic PHN (Table 4). At 6 months after rash onset, the scores in PHN subjects were significantly lower than the historic controls. For subjects whose pain fully resolved by 6 months, their SF-MPQ scores were lower than historic control PHN patients at study inclusion and at 6 months.
Table 4.
Short Form McGill Pain Questionnaire.
| Study inclusion | 6 months after rash onset | Historic PHN controls [Rowbotham et al., 1998] | |||
|---|---|---|---|---|---|
| SF-MPQ | PHN (n=30) | No-Pain (n=64) | PHN (n=30) | No-Pain (n=64) | PHN‡ (placebo group) (n=110) |
| Sensory score (range 0–33) | 14.8 (7.6)* | 10.3 (6.3) | 4.2 (4.2)** | 0.2 (0.7) | 14.5 (6.4) |
| Affective score (range 0–1) | 4.0 (3.5)* | 2.3 (2.5) | 0.6 (1.2)** | 0 (0) | 4.1 (3.2) |
| Total score (range 0–45) | 18.5(10.4)** | 12.3(8.3) | 4.8 (5.0)** | 0.2 (0.7) | 18.7 (8.5) |
|
| |||||
| Comparison with historic PHN controls | No difference | P<0.02 | P<0.0001 | P<0.0001 | |
Total, sensory, and affective scores (mean (SD)) on the SF-MPQ at study inclusion and 6 months after rash onset for subjects with and without PHN at 6 months. For reference historic PHN controls were included from Rowbotham et al., 1998.
: In this study PHN was defined as average daily pain ≥4 on the 0–10 numerical pain rating scale and median duration since last zoster eruption was 27 month. Comparison PHN vs no-pain groups at study inclusion and at 6 months:
: p<0.05,
: p<0.001
MPI
At study inclusion, MPI ratings of ‘pain severity’ and ‘affective distress’ were higher in PHN subjects than subjects whose pain resolved by 6 months (Table 5). Six months after rash onset, PHN subjects rated higher on ‘pain severity’, ‘interference’, and ‘affective distress’ and lower on ‘life control’ and ‘general activity level’ than subjects without pain. At study inclusion, ratings on ‘affective distress’ were higher in PHN subjects than in historic PHN controls, while the remaining scores were similar. However, at 6 months, PHN subjects’ scores on ‘pain intensity’, ‘interference’ and ‘negative response’ were significantly lower than scores from historic control patients with severe PHN.
Table 5.
Multidimensional Pain Inventory at study inclusion and 6 months for subjects with and without PHN 6 months after rash onset (mean (SD)). For reference historic PHN controls were included from Rowbotham et al., 2003 (previously unpublished data).
| MPI Scales | Study Inclusion | 6 Months After Rash Onset | Historic PHN controls [Rowbotham et al., 2003] | ||
|---|---|---|---|---|---|
| PHN (n=30) | No-pain (n=64) | PHN (n=30) | No-pain (n=64) | PHN¥ (n=26) | |
| Pain severity | 3.6 (1.2)** | 2.6 (1.3) | 1.3 (1.2)**,‡‡ | 0.09 (0.3) | 3.77 (1.1) |
| Interference | 3.2 (1.4) | 2.6 (1.5) | 1.2 (1.2)**,‡‡ | 0.3 (0.6) | 2.66 (1.6) |
| Life control | 3.2 (0.8) | 3.3 (1.0) | 3.7 (1.3)** | 4.5 (1.3) | 3.65 (1.2) |
| Affective distress | 3.1 (1.0)*,‡ | 2.6 (1.0) | 2.1 (1.2)* | 1.5 (1.1) | 2.28 (1.2) |
| Support | 4.3 (1.1) | 4.2 (1.1) | 3.4 (1.6) | 2.6 (1.9) | 4.29 (1.6) |
| Negative responses | 1.6 (1.3) | 0.9 (0.8) | 1.1 (1.1)‡ | 0.9 (0.8) | 1.74 (1.1) |
| Solicitous responses | 3.3 (1.4) | 3.1 (1.7) | 3.1 (1.5) | 2.9 (1.6) | 2.95 (1.6) |
| Distracting responses | 2.0 (1.1) | 1.5 (1.3) | 2.1 (1.4) | 1.7 (1.1) | 1.93 (1.5) |
| Household chores | 3.5 (1.4) | 3.4 (1.4) | 3.4 (1.4) | 3.6 (1.6) | 3.18 (1.6) |
| Outdoor work | 1.4 (1.1) | 1.4 (1.3) | 1.1 (0.9) | 1.7 (1.2) | 1.68 (1.5) |
| Activities away from home | 2.8 (1.1) | 2.7 (1.2) | 2.7 (1.0) | 3.2 (1.1) | 3.13 (1.0) |
| Social activities | 2.3 (1.1) | 2.1 (1.3) | 2.3 (0.9) | 2.6 (1.2) | 2.15 (1.5) |
| General activity level | 2.5 (0.8) | 2.4 (1.0) | 2.4 (0.7)* | 2.8 (0.9) | |
In this study PHN was defined as average daily pain ≥25 on the 0–100 pain VAS and duration of pain was greater than 11 months. Comparison PHN vs no-pain groups at study inclusion and at 6 months:
: P<0.05,
: P<0.001. Comparison PHN/no-pain vs historic PHN controls:
P<0.05,
P<0.01.
WAYS and LEC
Coping strategies did not change between the two assessments. Coping strategies did not differ between subjects with and without PHN at study inclusion or at 6 months. The two most used coping strategies for both groups at study entry and 6 months were ‘seeking social support’ and ‘planful problem solving’, which are both active coping strategies. Subjects with PHN at 6 months reported the same number of negative and positive life events as those subjects whose pain resolved.
DISCUSSION
In this population of HZ subjects aged ≥50 with pain persisting at >2 weeks after rash onset, 30 subjects (32%) met criteria for PHN at 6 months (average daily pain >0/100 on the pain VAS over the last 48 hours), a percentage similar to that reported in antiviral trials (Whitley et al. 1998). Only two of our subjects met the additional criteria for clinically meaningful PHN at 6 months (average daily pain ≥30/100 on the pain VAS). Slopes analysis showed that the rate of pain resolution was the same in subjects with or without PHN at 6 months. Because the PHN subjects started at a higher average pain level, the projected time to full pain resolution would be greater. It still remains unknown what trajectory the slope of subjects with clinically meaningful PHN would follow in the course of 6 months following HZ.
Even though our group of PHN subjects met the criteria for PHN used most often in longitudinal studies, their pain at 6 months was very mild compared to the pain reported by patients with longstanding PHN entering clinical trials of pain therapies for PHN or studies of pain mechanisms. The total SF-MPQ score and MPI ratings at study inclusion among the PHN subjects in the present study were similar to the total score observed in patients with severe longstanding PHN seen previously in our research center (Table 4; Rowbotham et al. 1998) and elsewhere (Graff-Radford et al. 1986; Bhala et al. 1988; King 1993). However, at 6 months the SF-MPQ total score in the PHN subjects in the present study were dramatically lower than in the historic controls. Ratings of ‘pain intensity’, ‘interference’ and ‘negative response’ on the MPI were not only significantly lower than ratings from the historic PHN controls, but also lower than in other chronic pain conditions (Kerns et al. 1985; Reitsma and Meijler 1997).
Most longitudinal HZ studies, including studies of predictors of PHN, have defined PHN as ‘presence of pain’ at variable time points after rash onset or healing without specifying a numerical threshold (Wood 1995; Wood et al. 1995; Dworkin and Portenoy 1996; Dworkin and Schmader 2001). Lydick and colleagues demonstrated that pain ratings less than 3 on the 0–10 NRS were associated with minimal impact on quality of life and functional levels (Lydick et al. 1995). Coplan and colleagues (Coplan et al. 2004) recently demonstrated that numerical pain ratings ≥3/10 corresponded to ratings higher than ‘mild’ on the SFMPQ and suggested that average daily pain ratings of ≥3 on the 0–10 NRS is a clinically meaningful definition of PHN.
Long before the introduction of effective and safe antivirals, Hope-Simpson found that only 2% had protracted neuralgia after 5 years (Hope-Simpson 1975). Haanpää and colleagues studied 112 patients of all ages referred by primary care doctors in Finland (Haanpää et al. 2000). While 25% of her subjects ‘complained of pain’ at 3 months, only 4 had severe pain. In a prospective study of an unselected primary care cohort in Iceland interviewed about their pain (Helgason et al. 2000), 6 of 168 subjects older than 50 years of age contacted 3 months after rash outbreak reported moderate discomfort (25–75 on a 100 point numerical pain rating scale), and 2 reported severe discomfort (75–100 on the pain NPRS). By 12 months post-HZ, 2 reported moderate pain, and none severe, leading the authors to suggest that previous studies have overestimated the problem of PHN (Helgason et al. 2000). In the recent zoster vaccine trial, the definition of PHN proposed by Coplan and colleagues (numerical pain ratings ≥3/10) was used. The incidence of PHN was 5.1% at 6 months in placebo vaccinated subjects aged 60 or older (Oxman et al. 2005). In contrast, all clinical trials of new therapies for PHN and studies of mechanisms of PHN use a stringent definition of PHN. Most have been restricted to those with ‘moderate to severe pain’ or ratings ≥40 on the 0–100 pain VAS despite analgesics (Rowbotham et al. 1998; Raja et al. 2002; Hempenstall et al. 2005).
To link the results from risk factor and antiviral studies that recruit subjects shortly after HZ onset to clinical trials and mechanistic studies of established PHN, the definition of PHN needs to be standardized. HZ patients who go on to develop ‘clinically meaningful’ PHN constitute a very small (2% in our group) but distinct sub-population of zoster patients (Hope-Simpson 1975; McKendrick et al. 1989; Coplan et al. 2004). These are patients with moderate to severe pain who may find their lives devastated by pain and require daily medication and frequent physician visits. Scott and colleagues recently evaluated the burden of HZ (defined as presence of pain) on patients, health care system and society and estimated an average cost of £ 520 per patient aged 65 and older (Scott et al. 2006). This study did not include a cost estimate for patients with clinically meaningful PHN. Cost estimates in this group would be expected to be considerably higher due to involvement of secondary and tertiary health services. Moreover, it remains unknown if the mechanisms underlying pain in patients with severe PHN are different from the mechanisms underlying pain in subjects with PHN defined as any pain at 6 months after rash onset.
A standardized definition of PHN should also take into account the use of prescription medication for pain, since successful analgesic medication reduces pain severity. If the definition of clinically meaningful PHN described by Coplan and colleagues (Coplan et al 2004) was modified to include prescription medication use for pain at 6 months, the incidence of clinically meaningful PHN in our cohort would be 9%.
Given the low incidence of clinically meaningful PHN, a longitudinal study designed to understand predictors of development of clinically meaningful PHN would require a sample of 1000–5000 HZ patients to generate 50 subjects with clinically meaningful PHN. Hence, future studies should use a case-control design, or other more efficient designs.
The present study evaluated several other risk factors for pain persistence. Depression, as measured with the Beck Depression Inventory, has been suggested to be a risk factor for PHN (Dworkin et al. 1992). Affective distress, which has a high positive correlation with the Beck Depression Inventory (Kerns et al. 1985), was assessed in the present study. PHN subjects were in more affective distress at study inclusion and at 6 months, suggesting that affective distress at zoster onset may be a predictor and that the persisting pain was associated with affective distress. Likewise, life events in the preceding year did not appear to affect the likelihood of pain persistence, nor did the response of others in the subject’s environment to their communications of pain. In the literature, coping strategies have only been examined after PHN has been established (Haythornthwaite et al. 2003). Assessing ways of coping both at HZ onset and 6 months later, we did not find a change in coping style or significant differences between PHN subjects and those who recovered fully. Passive coping efforts have been associated with more perceived pain and maladaptive physical and psychological adjustment (Folkman and Lazarus 1988; Jensen et al. 1991; Rose et al. 1992; Snow-Turek et al. 1996). The active coping style used by our HZ cohort suggests a psychologically well-functioning elderly population.
The picture of HZ is continuously changing. As reviewed by Dworkin and Schmader (2001) the introduction of antivirals treatments have reduced the overall duration of pain and the incidence of PHN. Studies are ongoing to determine if more aggressive analgesic treatment of the acute HZ pain will further reduce the risk of PHN. Recent data cast doubt about the ability of a single epidural steroid injection to prevent PHN (van Wijck et al. 2006). It is clear from the shingles vaccine trial that the vaccine will reduce the burden associated with HZ, but the very low incidence of clinically meaningful PHN cases must be considered (Coplan et al. 2004; Oxman et al. 2005).
In conclusion, the present study shows that persistence of mild pain was common 6 months after rash onset, but clinically meaningful PHN was a rare outcome. The findings have important implications. First, they suggest that the definition of PHN should be revised and only include cases with clinically meaningful PHN, where pain is severe enough to produce disability or require medical treatment. Second, the rarity of severe longstanding PHN should be kept in mind when generalizing the results of treatment trials. As the slope of pain reduction was the same in both the PHN and no-pain groups, the higher starting level of pain in our PHN group implies that the majority of patients with persistent pain at 6 months simply need more time for pain resolution. Third, since only a very small sub-group of all zoster patients develop severe PHN, there may be important mechanistic differences that have yet to be elucidated.
Acknowledgments
Supported by NINDS grants K24 NS02164 (MCR) and RO-1 NS39521 and a grant from the VZV Research Foundation Inc (KLP). HGT received support from Familien Hede Nielsen’s Fond and Direktør Jakob Madsens og Hustru Olga Madsen’s Fond. We are grateful to Dr. Alan Bostrom for his invaluable assistance with the statistical analysis. HGT completed the Doris Duke Fellowship Program and is grateful to Dr. Joel Palefsky and Terry O’Donnell for their invaluable help.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arani RB, Soong SJ, Weiss HL, Wood MJ, Fiddian PA, Gnann JW, Whitley R. Phase specific analysis of herpes zoster associated pain data: a new statistical approach. Stat Med. 2001;20(16):2429–2439. doi: 10.1002/sim.851. [DOI] [PubMed] [Google Scholar]
- Berry JD, Rowbotham MC, Petersen KL. Complex regional pain syndrome-like symptoms during herpes zoster. Pain. 2004;110(1–2):e1–12. doi: 10.1016/j.pain.2003.12.038. [DOI] [PubMed] [Google Scholar]
- Beutner KR, Friedman DJ, Forszpaniak C, Andersen PL, Wood MJ. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother. 1995;39(7):1546–1553. doi: 10.1128/aac.39.7.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhala BB, Ramamoorthy C, Bowsher D, Yelnooker KN. Shingles and postherpetic neuralgia. Clin J Pain. 1988;4:169–174. [Google Scholar]
- Bruxelle J. Prospective epidemiologic study of painful and neurologic sequelae induced by herpes zoster in patients treated early with oral acyclovir. Neurology. 1995;45(12 Suppl 8):S78–79. doi: 10.1212/wnl.45.12_suppl_8.s78. [DOI] [PubMed] [Google Scholar]
- Choo PW, Galil K, Donahue JG, Walker AM, Spiegelman D, Platt R. Risk factors for postherpetic neuralgia. Arch Intern Med. 1997;157(11):1217–1224. [PubMed] [Google Scholar]
- Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325(9):606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- Coplan PM, Schmader K, Nikas A, Chan IS, Choo P, Levin MJ, Johnson G, Bauer M, Williams HM, Kaplan KM, Guess HA, Oxman MN. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain. 2004;5(6):344–356. doi: 10.1016/j.jpain.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Crooks RJ, Jones DA, Fiddian AP. Zoster-associated chronic pain: an overview of clinical trials with acyclovir. Scand J Infect Dis Suppl. 1991;80:62–68. [PubMed] [Google Scholar]
- De Moragas JM, Kierland RR. The outcome of patients with herpes zoster. AMA Arch Derm. 1957;75(2):193–196. doi: 10.1001/archderm.1957.01550140037006. [DOI] [PubMed] [Google Scholar]
- Desmond RA, Weiss HL, Arani RB, Soong SJ, Wood MJ, Fiddian PA, Gnann JW, Whitley RJ. Clinical applications for change-point analysis of herpes zoster pain. J Pain Symptom Manage. 2002;23(6):510–516. doi: 10.1016/s0885-3924(02)00393-7. [DOI] [PubMed] [Google Scholar]
- Dworkin RH. Which individuals with acute pain are most likely to develop a chronic pain syndrome? Pain Forum. 1997;6(2):127–136. [Google Scholar]
- Dworkin RH, Hartstein G, Rosner HL, Walther RR, Sweeney EW, Brand L. A high-risk method for studying psychosocial antecedents of chronic pain: the prospective investigation of herpes zoster. J Abnorm Psychol. 1992;101(1):200–205. doi: 10.1037//0021-843x.101.1.200. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Portenoy RK. Proposed classification of herpes zoster pain. Lancet. 1994;343(8913):1648. doi: 10.1016/s0140-6736(94)93106-2. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Portenoy RK. Pain and its persistence in herpes zoster. Pain. 1996;67(2–3):241–251. doi: 10.1016/0304-3959(96)03122-3. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Schmader KE. The epidemiology and natural history of herpes zoster and postherpetic neuralgia. In: Watson CPN, Gershon AA, editors. Herpes Zoster and Postherpetic Neuralgia. 2nd Revised and Enlarged Edition. Vol. 11. Amsterdam: Elsevier; 2001. pp. 39–64. [Google Scholar]
- Engberg IB, Grondahl GB, Thibom K. Patients' experiences of herpes zoster and postherpetic neuralgia. J Adv Nurs. 1995;21(3):427–433. doi: 10.1111/j.1365-2648.1995.tb02723.x. [DOI] [PubMed] [Google Scholar]
- Folkman S, Lazarus RS. Manual for the Ways of Coping Questionnaire (Research Edition) Palo Alto: Consulting Psychologists Press; 1988. [Google Scholar]
- Graff-Radford SB, Kames LD, Naliboff BD. Measures of psychological adjustment and perception of pain in postherpetic neuralgia and trigeminal neuralgia. Clin J Pain. 1986;2:55–58. [Google Scholar]
- Haanpää M, Laippala P, Nurmikko T. Allodynia and pinprick hypesthesia in acute herpes zoster, and the development of postherpetic neuralgia. J Pain Symptom Manage. 2000;20(1):50–58. doi: 10.1016/s0885-3924(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Haythornthwaite JA, Clark MR, Pappagallo M, Raja SN. Pain coping strategies play a role in the persistence of pain in post-herpetic neuralgia. Pain. 2003;106(3):453–460. doi: 10.1016/j.pain.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Helgason S, Petursson G, Gudmundsson S, Sigurdsson JA. Prevalence of postherpetic neuralgia after a first episode of herpes zoster: prospective study with long term follow up. Bmj. 2000;321(7264):794–796. doi: 10.1136/bmj.321.7264.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempenstall K, Nurmikko TJ, Johnson RW, A'Hern RP, Rice AS. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2(7):e164. doi: 10.1371/journal.pmed.0020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope-Simpson RE. Postherpetic neuralgia. J R Coll Gen Pract. 1975;25(157):571–575. [PMC free article] [PubMed] [Google Scholar]
- Jensen MP, Turner JA, Romano JM, Karoly P. Coping with chronic pain: a critical review of the literature. Pain. 1991;47(3):249–283. doi: 10.1016/0304-3959(91)90216-K. [DOI] [PubMed] [Google Scholar]
- Johnson R. Herpes zoster--predicting and minimizing the impact of post-herpetic neuralgia. J Antimicrob Chemother. 2001;47(Suppl T1):1–8. doi: 10.1093/jac/47.suppl_1.1. [DOI] [PubMed] [Google Scholar]
- Jung BF, Johnson RW, Griffin DR, Dworkin RH. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology. 2004;62(9):1545–1551. doi: 10.1212/01.wnl.0000123261.00004.29. [DOI] [PubMed] [Google Scholar]
- Katz JM. Multivariable Analysis: A Practical Guide for Clinicians. Cambridge, United Kingdom: Cambridge University Press; 2003. [Google Scholar]
- Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI) Pain. 1985;23(4):345–356. doi: 10.1016/0304-3959(85)90004-1. [DOI] [PubMed] [Google Scholar]
- King RB. Topical aspirin in chloroform and the relief of pain due to herpes zoster and postherpetic neuralgia. Arch Neurol. 1993;50(10):1046–1053. doi: 10.1001/archneur.1993.00540100041012. [DOI] [PubMed] [Google Scholar]
- Kost RG, Straus SE. Postherpetic neuralgia--pathogenesis, treatment, and prevention. N Engl J Med. 1996;335(1):32–42. doi: 10.1056/NEJM199607043350107. [DOI] [PubMed] [Google Scholar]
- Lydick E, Epstein RS, Himmelberger D, White CJ. Herpes zoster and quality of life: a self-limited disease with severe impact. Neurology. 1995;45(12 Suppl 8):S52–53. doi: 10.1212/wnl.45.12_suppl_8.s52. [DOI] [PubMed] [Google Scholar]
- McKendrick MW, McGill JI, Wood MJ. Lack of effect of acyclovir on postherpetic neuralgia. Bmj. 1989;298(6671):431. doi: 10.1136/bmj.298.6671.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30(2):191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- Nagasako EM, Johnson RW, Griffin DR, Dworkin RH. Rash severity in herpes zoster: correlates and relationship to postherpetic neuralgia. J Am Acad Dermatol. 2002;46(6):834–839. doi: 10.1067/mjd.2002.120924. [DOI] [PubMed] [Google Scholar]
- Nurmikko T, Bowsher D. Somatosensory findings in postherpetic neuralgia. J Neurol Neurosurg Psychiatry. 1990;53(2):135–141. doi: 10.1136/jnnp.53.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, Weinberg A, Boardman KD, Williams HM, Zhang JH, Peduzzi PN, Beisel CE, Morrison VA, Guatelli JC, Brooks PA, Kauffman CA, Pachucki CT, Neuzil KM, Betts RF, Wright PF, Griffin MR, Brunell P, Soto NE, Marques AR, Keay SK, Goodman RP, Cotton DJ, Gnann JW, Jr, Loutit J, Holodniy M, Keitel WA, Crawford GE, Yeh SS, Lobo Z, Toney JF, Greenberg RN, Keller PM, Harbecke R, Hayward AR, Irwin MR, Kyriakides TC, Chan CY, Chan IS, Wang WW, Annunziato PW, Silber JL. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- Raja SN, Haythornthwaite JA, Pappagallo M, Clark MR, Travison TG, Sabeen S, Royall RM, Max MB. Opioids versus antidepressants in postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2002;59(7):1015–1021. doi: 10.1212/wnl.59.7.1015. [DOI] [PubMed] [Google Scholar]
- Reitsma B, Meijler WJ. Pain and patienthood. Clin J Pain. 1997;13(1):9–21. doi: 10.1097/00002508-199703000-00004. [DOI] [PubMed] [Google Scholar]
- Rose MJ, Klenerman L, Atchison L, Slade PD. An application of the fear avoidance model to three chronic pain problems. Behav Res Ther. 1992;30(4):359–365. doi: 10.1016/0005-7967(92)90047-k. [DOI] [PubMed] [Google Scholar]
- Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. Jama. 1998;280(21):1837–1842. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- Rowbotham MC, Petersen KL. Zoster-associated pain and neural dysfunction. Pain. 2001;93(1):1–5. doi: 10.1016/S0304-3959(01)00328-1. [DOI] [PubMed] [Google Scholar]
- Rowbotham MC, Twilling L, Davies PS, Reisner L, Taylor K, Mohr D. Oral opioid therapy for chronic peripheral and central neuropathic pain. N Engl J Med. 2003;348(13):1223–1232. doi: 10.1056/NEJMoa021420. [DOI] [PubMed] [Google Scholar]
- Scott FT, Johnson RW, Leedham-Green M, Davies E, Edmunds WJ, Breuer J. The burden of herpes zoster: A prospective population based study. Vaccine. 2006;24:1308–1314. doi: 10.1016/j.vaccine.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Snow-Turek AL, Norris MP, Tan G. Active and passive coping strategies in chronic pain patients. Pain. 1996;64(3):455–462. doi: 10.1016/0304-3959(95)00190-5. [DOI] [PubMed] [Google Scholar]
- Watson CP, Watt VR, Chipman M, Birkett N, Evans RJ. The prognosis with postherpetic neuralgia. Pain. 1991;46(2):195–199. doi: 10.1016/0304-3959(91)90076-A. [DOI] [PubMed] [Google Scholar]
- Whitley RJ, Shukla S, Crooks RJ. The identification of risk factors associated with persistent pain following herpes zoster. J Infect Dis. 1998;178 (Suppl 1):S71–75. doi: 10.1086/514274. [DOI] [PubMed] [Google Scholar]
- Wood M. Understanding pain in herpes zoster: an essential for optimizing treatment. J Infect Dis. 2002;186 (Suppl 1):S78–82. doi: 10.1086/342958. [DOI] [PubMed] [Google Scholar]
- Wood MJ. How should we measure pain in herpes zoster? Neurology. 1995;45(12 Suppl 8):S61–62. doi: 10.1212/wnl.45.12_suppl_8.s61. [DOI] [PubMed] [Google Scholar]
- Wood MJ, Balfour H, Beutner K, Bruxelle J, Fiddian P, Johnson R, Kay R, Cubed S, Portnoy J, Rentier B. How should zoster trials be conducted? J Antimicrob Chemother. 1995;36(6):1089–1101. doi: 10.1093/jac/36.6.1089. [DOI] [PubMed] [Google Scholar]


