Abstract
In certain animal models of autoimmunity the isoxasol derivative leflunomide has been reported to exert a protective effect against autodestruction. In the present study, the immunomodulatory potential of the main metabolite of leflunomide, A77 1726, in experimentally induced autoimmune diabetes was investigated. The disease was induced in genetically susceptible CBA/H mice by multiple low doses of streptozotocin (MLD-SZ, 40 mg/kg per day, given intraperitoneally for 5 consecutive days). Effects of leflunomide were evaluated by two treatment protocols: mice treated with MLD-SZ were injected intraperitoneally with A77 1726 for 10 consecutive days, either during the first 10 days of the disease (early treatment), or starting from day 10 after disease induction (late treatment). Disease manifestations defined by hyperglycaemia, mononuclear infiltration into pancreas, expression of interferon-gamma (IFN-γ) and inducible nitric oxide synthase (iNOS) and destruction of the islets of Langerhans were reduced in a dose-dependent fashion after early treatment with A77 1726 (dose range of 5–35 mg/kg per day). Moreover, late treatment with the high dose of the drug (25 mg/kg per day), started when the autoimmune disease was already apparent, arrested progression of ongoing inflammatory response. Analysis of the effects of A77 1726 on the adhesive interactions of spleen-derived or peripheral blood-derived mononuclear cells from MLD-SZ-treated and normal mice demonstrated that the drug inhibits both ex vivo and in vitro spontaneous mononuclear cell aggregation, thus suggesting that an important component of leflunomide's immunomodulatory action is suppression of adhesive interactions. These results demonstrate both preventive and therapeutic effects of leflunomide in a model of MLD-SZ-induced diabetes and suggest that the drug may be considered a potent therapeutic tool for autoimmune inflammatory disorders, including diabetes.
Keywords: insulin-dependent diabetes mellitus, leflunomide, streptozotocin, hyperglycaemia, autoimmune disease
INTRODUCTION
In the search for new immunomodulatory drugs, recent preclinical and clinical trials revealed the potential of the isoxasol derivative leflunomide for the therapy of rheumatoid arthritis [1]. In addition, preclinical studies in animals have shown that leflunomide may favourably influence the course of several experimental autoimmune disorders such as tubulonephritis [2], glomerulonephritis [3], autoimmune uveitis [4], autoimmune encephalomyelitis [5], autoimmune manifestations in MRL/lpr mice [6] and autoimmune myocarditis [7]. Considering the effectiveness and good tolerability of the drug [1], these data predict a role for leflunomide in the treatment of autoimmune and other immunoinflammatory diseases. However, there is no evidence whether leflunomide and related compounds may be of interest in an important, presumably T cell triggered disease—insulin-dependent diabetes mellitus (IDDM). There is a consensus that experimental models of IDDM in mice and rats represent an autoimmune disorder in which T cells and macrophages play a major pathogenic role [8]. Therefore, the aim of our study was to examine whether leflunomide may also have a protective effect in autoimmune diabetes. To this end, we studied the influence of leflunomide's primary metabolite, A77 1726, which mediates disease-modifying effects of the parent drug [9], in a murine model of diabetes induced by multiple low doses of streptozotocin (MLD-SZ).
IDDM is a prototypic T cell-mediated autoimmune disease whose targets are the insulin-producing β cells located in the islets of Langerhans. Experimentally induced IDDM in rodents with MLD-SZ is a widely used model which has clinical and histoimmunological features similar to those of human disease, with T cells and macrophages playing a major pathogenic role [8]. When administered in animals at multiple low doses, β cell toxin streptozotocin is thought to induce initial cell damage by alkylating the DNA in islet cells [10] and/or by spontaneously releasing nitric oxide [11]. Subsequently, as a result of a novel β cell antigen expression, mononuclear cells that infiltrate the islets will start a multifactorial process [12] resulting in the expansion of pathological response from a ‘mini-autoimmune response’ into chronic immune-mediated disease. The main functional and histopathological defects in the MLD-SZ model of diabetes appear to be induced by T helper 1 (Th1) lymphocytes and T cytolytic 1 (Tc1) cells which secrete soluble molecules that promote autoimmune destruction of the islets [13, 14]. Therefore, in order to analyse the possible mechanism of A77 1726 effects on cell interactions in the development of inflammatory lesions in the pancreata we also analysed the capacity of the drug to modify spontaneous aggregation of normal murine mononuclear cells and cells derived from diabetic animals.
By using this model, we demonstrated that leflunomide's active compound A77 1726 suppresses disease manifestations, thus suggesting that the drug may have a role in the treatment of autoimmune reaction against pancreatic β cells.
MATERIALS AND METHODS
Mice
Genetically susceptible inbred male CBA/H mice, maintained from the colony at the Institute of Biological Research, Belgrade, Yugoslavia, were used from 8 to 10 weeks of age. Mice were housed under conventional conditions with laboratory chow and water ad libitum. Animals were randomly divided into groups of 5–10 to receive the different treatment protocols.
Reagents
Streptozotocin (SZ, S-0130) was purchased from Sigma (St Louis, MO). Leflunomide (N-(4-trifluoromethylphenyl)-5-methylisoxazol-4-carboxamide), in the form of its active metabolite A77 1726, was provided by Dr Kämmerer as the water-soluble sodium salt, prepared in the Chemistry Department, Hoechst AG (Wiesbaden, Germany). Mouse MoAb specific for mouse Thy-1,2 (F7D5), rat MoAbs specific for mouse CD4 (GK1.5), CD8 (YTS 169), and interferon-gamma (IFN-γ) (F3), as well as rabbit anti-iNOS antibody (anti-NO16; kindly provided by Dr C. Nathan, Cornell University Medical College, NY) were used for immunohistochemical stainings.
Induction of diabetes
Autoimmune diabetes with delayed onset was induced by MLD-SZ, as described by Like & Rossini [10]. SZ was dissolved in citrate buffer pH 4.5, and injected intraperitoneally within 10 min of dissolution, at doses of 40 mg/kg of body weight daily for 5 consecutive days. In all studies, day 0 was defined as the first day of MLD-SZ administration. In one series of experiments, ‘toxic’ diabetes, due to the direct toxic effect of the drug on β cells, was induced by the i.p. injection of a single high SZ dose of 200 mg/kg of body weight.
Quantification of glucose in the blood
Clinical diabetes was defined by hyperglycaemia (blood glucose > 11.8 mmol/l). Blood samples were obtained from the retroorbital plexus of non-fasted animals. Plasma glucose determination was performed at weekly intervals by a glucose oxidase method using a glucometer (Glucotronic C; Macherey-Nagel, Duren, Germany).
In vivo treatment with A77 1726
A77 1726 was diluted in PBS and injected intraperitoneally in a volume of 0.2 ml at daily doses ranging from 5 to 35 mg/kg per day, as indicated in Results. A77 1726 was given as continuous 10 days treatment starting either jointly with the MLD-SZ doses (the first injection of A77 1726 given at day 0), or after MLD-SZ (starting at day 10 in relation to the first SZ dose). Control, non-treated mice received injections of PBS. A modest but significant reduction in body weight was noticed in the mice treated with A77 1726 in comparison with control animals. Adverse effects were clearly dose-dependent, as most of the mice (12 out of 15) treated with the highest tested dose of the drug (35 mg/kg per day) displayed a progressive worsening of their general condition and became moribund within 50 days of the observation period.
Aggregation assay
In vitro analysis of spontaneous cell aggregation was performed according to modification of the protocol of Rothlein & Springer [15]. Briefly, mononuclear cells (MNC) were isolated from peripheral blood (PB) and spleen (SPL) obtained from A77 1726-treated (in vivo treatment) and/or MLD-SZ-treated mice 10 days after diabetes induction, as well as from normal untreated animals. Cells were incubated in triplicates for 2 h at 37°C in 96-well microtitre plates (1 × 105/well) in RPMI 1640 medium (Gibco, Paisley, UK) supplemented with 10% fetal calf serum (FCS) in the presence (in vitro treatment) or absence of 50 μm of A77 1726, as indicated in Results. For quantification of cells in aggregates, free cells were counted in a haemocytometer by two independent observers. The percentage of cells in aggregates was calculated as follows: 100 × (1 − (number of free cells in test/number of free cells in control)), where control represents the total number of free cells (1 × 106/ml). The percentage inhibition of aggregation by in vivo or in vitro treatment with A77 1726 was determined using the formula: inhibition of aggregation (%) = (1 − (total number of free cells − number of free cells in test/total number of free cells − number of free cells in control)). The total number of free cells was 1 × 106/ml, while control represents the number of free cells in medium. Cell viability was checked by trypan blue exclusion.
Histological examination of pancreas
Pancreata were prepared for histology by fixing in neutral buffered formalin and then embedding in paraffin. The fixed blocks were sectioned (7 μm in thickness) and haematoxylin and eosin (H–E), Maldonado or Masson's trichrome stainings were performed to assess the incidence of inflammatory changes, degree of islet cell destruction and proliferation of connective tissue. α1-antitrypsin (α1-AT) was used for detection of macrophages. Histological examination of the pancreatic islets, viewed by light microscopy of the slides, was undertaken in a blind fashion by two observers unaware of the status and/or treatment of the animals. The magnitude of insulitis and the degree of islet destruction were graded according to an arbitrary scale as follows: 0 = intact islet; 1 = area of MNC infiltration within an islet < 25%; 2 = 25–50%; 3 = > 50%, and 4 = small retracted islet with or without some residual infiltrates, cords of connective tissue, extensive destruction of islet, β cell necrosis. At least 20 islets were counted for each pancreas. The mean score for each animal (group) was calculated by dividing the total score by the number of islets examined.
Immunohistochemical analyses of pancreas
Tissues were quick frozen in liquid nitrogen with OCT compound. Cryomicrotome sections (7–9 μm thick) were prepared and dried onto gelatin-coated slides. Before staining, the slides were fixed in cold acetone for 5 min at −20°C, then rinsed in 0.1 m Tris buffer solution (TBS) and incubated with primary antibodies (F7D5, GK5.1, YTS 169, F3, anti-NO16) overnight at 4°C. After additional washings in TBS, sections were incubated for 1 h with either anti-mouse or anti-rat IgG peroxidase (for mouse and rat MoAbs, respectively) or with anti-rabbit immunoglobulin biotin followed by streptavidin biotinylated peroxidase complex (for rabbit anti-iNOS antibody). Peroxidase reaction was visualized with 0.05% diaminobenzidine (DAB) in 0.01% H2O2 for 7–8 min. The colour reaction was stopped by washing slides in running water. All slides were lightly counterstained with haematoxylin, mounted in gelatin/glycerol medium and assessed by light microscopy.
Statistical analysis
Results were expressed as means ± s.e.m. Student's t-test was used to determine the significance of differences between means.
RESULTS
Effects of A77 1726 treatment on hyperglycaemia
Preliminary experiments have shown that A77 1726 does not affect blood glucose level in normal untreated CBA/H mice (data not shown). In order to evaluate the ability of leflunomide to interfere with the autoimmune diabetogenic process, we used the murine model of autoimmune diabetes induced with MLD-SZ. In this model, sustained hyperglycaemia and insulitis occur over a 2-week period following administration of five daily low doses of SZ [16]. Three groups of MLD-SZ-treated mice received different doses of A77 1726 (ranging between 5 and 20 mg/kg per day) over a period of 10 days, starting in conjunction with SZ. The control group of mice received MLD-SZ plus 10 daily i.p. injections of equivalent volumes of saline. Figure 1 shows that after completion of MLD-SZ treatment elevated levels of glucose in blood were observed in control animals, which is in accordance with a previous report [16]. Treatment with A77 1726 significantly reduced MLD-SZ-induced hyperglycaemia in a dose-dependent way. The protective effect of A77 1726 on the development of diabetes did not appear to depend on continuous application of the drug, since after interruption of treatment with A77 1726 none of the mice developed hyperglycaemia for an overall 70-day observation period. An age- and sex-matched control group of mice treated similarly with A77 1726, but without MLD-SZ, showed no effect on basal normoglycaemia (not shown).
Fig. 1.
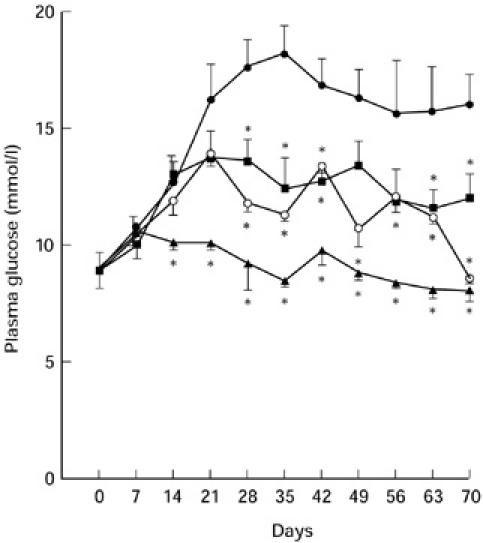
Dose-dependent suppression of MLD-SZ-induced hyperglycaemia in mice treated with A77 1726. Plasma glucose levels in mice (n = 6 in each group) receiving, respectively, MLD-SZ (40 mg/kg per day, for 5 consecutive days) in conjunction with 10 daily i.p. injections of saline only (•), or different doses of A77 1726: 5 mg/kg (▪), 10 mg/kg (○), or 20 mg/kg (▴). Significantly different from the value of MLD-SZ-treated but non-A77 1726-treated control mice: *P ≤ 0.01; Student's t-test.
In order to analyse further potentially therapeutic properties of leflunomide, the experiments were repeated with additional groups of mice to include the delayed treatment with A77 1726. In addition to previously described concomitant treatments, groups of mice were injected for 10 subsequent days with A77 1726 but starting 10 days after the first streptozotocin administration. This regimen was based on histological studies demonstrating the first cellular influx in the islets of Langerhans by day 7 [12]. As shown in Fig. 2, after the late treatment with A77 1726 at a lower dose (5 mg/kg per day) suppression of hyperglycaemia was not observed, which confirmed our preliminary report [17]. However, high doses of the drug (25 mg/kg per day) exhibited protective action, although less effective than early concomitant treatment. In addition, therapeutic effectiveness was not obtained in an acute form of diabetes induced by a single toxic dose of streptozotocin (‘toxic’ diabetes), when A77 1726 was administered at doses that were protective in an immune-mediated MLD-SZ-induced diabetes (data not shown).
Fig. 2.

Comparison of the early and late treatment of mice with A77 1726 on MLD-SZ-induced development of hyperglycaemia. Plasma glucose levels in mice receiving MLD-SZ only (40 mg/kg per day, for 5 consecutive days) (•), or mice treated with MLD-SZ in conjunction with A77 1726 (25 mg/kg per day) from day 0 to day 9 (○), or from day 10 to day 19 (▴) in relation to the first SZ injection (n = 10 in each group). Significantly different from the value of MLD-SZ-treated but non-A77 1726-treated control mice: *P ≤ 0.01; Student's t-test.
Histological and immunohistochemical analysis
To determine the effect of A77 1726 administration on the islets, we examined histological sections of the pancreata from control, MLD-SZ-treated and A77 1726-treated mice. Histological analysis were done at days 10, 20, 30 and 84 after the induction of the disease with MLD-SZ, as well as before any treatment. Representative examples of the light microscopical evaluation of the islets are presented in Fig. 3. Control mice displayed normal architecture without MNC infiltration and islet necrosis. Pancreatic islets in mice killed 10 days after receiving five daily injections of SZ revealed massive infiltrations accompanied with initial necrotic changes. However, numerous islets were well preserved at this stage of the disease evolution (mean histological grade 0.8), which is probably sufficient for the normoglycaemic status of the animals (Fig. 1). The majority of infiltrated MNC were Thy1.2+ and CD4+. Macrophages (α1-AT+) were detected at the periphery of the infiltrates (Fig. 3). Some of the cells in the islets were strongly IFN-γ+ and iNOS+. At day 20 (mean grade 1.7) and day 30 (mean grade 2.4) islet necrosis was more prominent, which correlated with the sustained hyperglycaemia (Fig. 1). From this period up to 84 days, the destructive process progressed (mean grade 3.6) and necrotic areas were replaced with fibrotic tissue (Fig. 3). In general, during the disease course, all of the first three histological grades were found at every time point in the same pancreas, although maximal infiltration was observed between days 10 and 20, necrosis between day 20 and 30 and fibrosis at day 84. Administration of A77 1726 (5–25 mg/kg per day), concomitantly with MLD-SZ, protected animals from insulitis and destruction of β cells. In comparison with control MLD-SZ-treated mice, in pancreatic islets of leflunomide-treated mice mononuclear cells participating in the insulitis process, e.g. Thy-1.2+, CD4+, CD8+ and α1-AT+ cells, were significantly reduced, as well as IFN-γ+ and iNOS+ cells. Mean histological grade did not exceed 0.5 during the whole investigation period. If A77 1726 was given later (from day 10 to day 19), at a period of apparent MNC infiltration and initial necrosis, protection was less effective, at least when low dose of the drug (5 mg/kg per day) was used. In contrast, A77 1726 given at a higher dose (25 mg/kg per day) preserved numerous islets from further autoimmune attack and necrosis (Fig. 3): at day 20 mean grade was 1.0; at day 30 mean grade was 1.4; and at day 84 mean grade was 1.7, which was significantly lower than in animals treated with MLD-SZ only. In addition, both IFN-γ+ and iNOS+ cells were again reduced in A77 1726-treated mice compared with control MLD-SZ-treated animals.
Fig. 3.
Histology of pancreatic islets in MLD-SZ-induced diabetes in mice. (a) Massive mononuclear cell (MNC) infiltration (arrow) 10 days after disease induction. (H–E staining, mag. × 250.) (b) CD4+ cells (arrows) within MNC infiltrate 20 days after disease induction. (Immunoperoxidase staining, mag. × 250.) (c) α1-antitrypsin (α1-AT)+ cells at the periphery of MNC infiltrate 20 days after disease induction. (Mag. × 312.) (d) Extensive necrosis (arrow) and loss of islet margins 30 days after disease induction. (H–E staining, mag. × 312.) (e) Proliferation of connective tissue 84 days after disease induction. (Maldonado staining, mag. × 125.) Histology of pancreatic islets in A77 1726-treated diabetic mice (dose 25 mg/kg per day, given from day 10–19). (di) Well preserved islets without MNC infiltrate and necrosis 30 days after disease induction. (H–E staining, mag. × 250.) (ei) Absence of islet fibrosis 84 days after disease induction. (Maldonado staining, mag. × 125.)
Effect of A77 1726 on spontaneous MNC aggregation
In order to determine the effect of A77 1726 on the adhesive interactions of MNC, analysis of spontaneous cell aggregation was performed using both PB- and SPL-derived MNC obtained from MLD-SZ-treated and normal mice. Results of these experiments are summarized in Fig. 4. The results revealed that in MLD-SZ-treated mice spontaneous ex vivo MNC clustering was significantly increased, compared with the aggregation of MNC from normal healthy mice. As shown in Fig. 4, higher percentages of cell clusters were detected both in SPL- and PB-derived MNC obtained on day 10, or day 20 after diabetes induction (P < 0.05). However, treatment of mice with A77 1726, started either concomitantly with MLD-SZ or 10 days after the first MLD-SZ challenge, significantly (P < 0.005) suppressed ex vivo aggregation in comparison with MNC clustering of control MLD-SZ-treated mice. The influence of A77 1726 on MNC aggregation was further evaluated in vitro, using the drug during an aggregation assay. MNC were isolated from MLD-SZ-treated animals and incubated for 2 h in medium containing 50 μm of A77 1726, a concentration shown to be the most effective in vitro [18]. The capability of A77 1726 to inhibit cell aggregation in vitro was as effective as in vivo treatment (Fig. 4).
Fig. 4.
Effect of A77 1726 on the spontaneous aggregation of mononuclear cells (MNC). Quantitative aggregation assay was performed by using spleen-derived (A), or peripheral blood (PB)-derived MNC (B). a, Control cells from normal, untreated mice; b, cells from MLD-SZ-treated mice isolated 10 days (□) or 20 days (▪) after the first MLD-SZ challenge; c, cells from mice treated with MLD-SZ and A77 1726 from days 0 to 9 and isolated on day 10 (□), or treated from days 10 to 19 and isolated on day 20 (▪); d, cells from MLD-SZ-treated mice isolated 10 days (□) or 20 days (▪) after the first MLD-SZ challenge incubated in vitro with 50 μm A77 1726. Results represent mean values (bars) ± s.e.m. of individual analyses performed in six different mice from each group. Negative values indicate inhibition of aggregation referring to the cells obtained from MLD-SZ-treated but non-A77 1726-treated control mice. Significantly different from the value of normal untreated mice (b), or of MLD-SZ-treated but non-A77 1726-treated control mice (c,d): *P ≤ 0.05; Student's t-test.
DISCUSSION
In this study, the immunomodulatory potential of a novel compound leflunomide was evaluated in the MLD-SZ-induced autoimmune diabetes in mice. Since this animal model has an autoimmune cell-mediated component [8] and typically shows a chronic and persistent course of disease, it is regarded as useful for studies concerning immunological manipulations of IDDM. To assess the role of the drug in the pathology of a complex disease process, we used leflunomide's primary metabolite A77 1726 and obtained evidence that in vivo administration of the drug during disease induction protects mice from development of hyperglycaemia, massive islet cell invasion and β cell destruction. Furthermore, with late treatment with A77 1726 we presented evidence indicating drug effectiveness in ameliorating IDDM progression. Although the protective and therapeutic potential of leflunomide has been demonstrated in several experimental models of organ-specific autoimmune diseases [4, 5, 7], its mode of action is not completely understood. The drug-induced protection from IDDM may involve several complementary mechanisms. First, by inhibiting tyrosine phosphorylation and subsequent tyrosine kinase-dependent cell activation [6, 19], and/or by inhibiting de novo pyrimidine nucleotide synthesis and cell proliferation [19, 20], leflunomide may interfere with the initial events of activation of autoreactive T cells and their expansion. However, the development of the disease is determined not only by the expansion of β cell-specific autoreactive cells, but also by adhesion cascade-mediated cell–cell interactions and homing of MNC into the target tissue. Sequential mononuclear cell interactions, involving CD28/B7 molecules [21], as well as a number of adhesion receptors and ligands [22], are required for antigen presentation, T cell activation, killer T cell-mediated lysis of target cells, and adhesion and transendothelial migration of leucocytes. Thus, a second mechanism by which leflunomide could exert its effect is by altering the adhesion molecules which mediate cell–cell contacts and provide communication of cells with their environment.
It is worth noting that some adhesion molecules can undergo changes in activity which are independent of their surface expression. Therefore, in order to elucidate the effects of leflunomide upon cell–cell adhesion, in the current study we measured the functional state of adhesion molecules rather than surface expression. In order to test this, we performed studies using an aggregation assay and demonstrated, for the first time, that increased MNC aggregation, which is associated with MLD-SZ treatment of mice [23], was inhibited by A77 1726 both in vivo and in vitro. From this, we can assume that the anti-adhesive property of the drug may interfere with the movement of leucocytes to sites of inflammation and tissue destruction, affecting both their activation and effector function. Beside direct modulation of adhesive cell interactions, leflunomide may also interfere with the production of chemoattractant cytokines, thereby disturbing the process of insulitis. Both of these mechanisms fit well with histological observation, since we provided evidence that leflunomide inhibited accumulation of MNC participating in the insulitis process of the pancreatic islets. In the immunological diabetic process dendritic cells/macrophages are the first cells to infiltrate the islets and therefore initiate the autoreactive process as typical antigen-presenting cells (APC) [12, 14]. In addition, these cells are the first and major source of inflammatory mediators in the islet infiltrates [24]. Since α1-AT+ cells are rare in the islets of A77 1726-treated mice, this indicates that leflunomide may influence the disease by interfering with migration of blood-born APC into the target tissue and/or in situ differentiation of resident APC.
Beside APC, the other cell type which is crucial for the initiation and perpetuation of the disease is islet antigen-reactive T lymphocytes. Reduction of the severity of insulitis, observed in mice treated with A77 1726, thus may result in the impairment of local release of proinflammatory cytokines, as well as the other relevant cytokines by the cells infiltrating the pancreas [13, 14], and reduce subsequent cytokine-inducible generation of the free radical gas NO by β cells [25]. Indeed, immunohistochemical analysis revealed that both IFN-γ+ and iNOS+ intra-islet cells were rare after treatment with A77 1726. Bearing in mind the role for NO in pancreatic β cell damage [26], it is tempting to speculate that leflunomide may reduce NO-mediated destruction of pancreatic β cells.
However, there is a question of the mechanism possibly employed by A77 1726 to suppress iNOS activation in the islets. Previous findings support a role for protein tyrosine kinase (PTK) activity in macrophage iNOS induction [27]. Since A77 1726 has been found to inhibit PTK activity in various cell types [6, 19] it is conceivable that similar down-regulation of PTK might be responsible also for the leflunomide-mediated suppression of NO synthesis in the islets. The precise mechanism of leflunomide's action on NO synthesis at the level of β cells is at present not known, but warrants further study.
Temporal analysis of the effect of A77 1726 on MLD-SZ-induced diabetes has shown that early treatment completely or partially prevented disease induction. Moreover, the drug arrested progression to clinically overt diabetes when administered at a more advanced disease stage, namely when active cell destruction was present. These effects could be related to the A77 1726 prevention of MNC aggregation at the site of autoimmune challenge, as indicated by ex vivo and in vitro experiments (Fig. 4). Effectiveness of A77 1726 in preventing MLD-SZ-induced diabetes, but lack of the protective effect in a ‘toxic’ diabetes induced by a single high dose of SZ, indicate that the drug interferes with immune-mediated events rather than with the direct non-immunological effects of SZ on β cells. It seems that leflunomide protects mice from MLD-SZ-induced IDDM in a permanent fashion, most probably as a result of suppression of a cascade of events associated with progression of the disease from ‘mini autoimmune response’ into chronic immune-mediated damage of endocrine pancreas.
In our study we confirmed the immunosuppressive properties of A77 1726, and showed its dose-dependent effect. Positive effects of leflunomide were achieved in doses ranging between 5 and 25 mg/kg per day. However, caution should be exercised when extrapolating these results to the clinical setting, because leflunomide is metabolized more rapidly in rodents than in humans, and lower doses of the drug are likely to exhibit immunosuppressive properties in humans (clinical trial of the safety and pharmacokinetic profile of leflunomide; Hoechst AG, Wiesbaden, Germany). If maintenance of immunosuppressive effects with concomitant reduction of dose-dependent side-effects may be achieved by combined treatment with ‘low’ doses of leflunomide and other immunosuppressive drugs, this may be a novel safe and feasible form of immunotherapy to be considered for the prophylaxis/therapy of IDDM, and possibly other autoimmune diseases.
Acknowledgments
One of the co-authors of this work, Dr Robert R. Bartlett, deceased this year. We dedicate this paper to his valuable collaboration. The authors wish to thank Dr Miodrag Lukic for critically reading the manuscript. This work was partially supported by grants from Ministry of Science and Technology, Republic of Serbia, Yugoslavia.
REFERENCES
- 1.Mladenovic V, Domljan Z, Rozman B, et al. Safety and effectiveness of leflunomide in the treatment of patients with active rheumatoid arthritis. Results of a randomized, placebo-controlled, phase II study. Arthr Rheum. 1995;38:1595–603. doi: 10.1002/art.1780381111. [DOI] [PubMed] [Google Scholar]
- 2.Thoenes H, Sitter T, Langer KH, et al. Leflunomide (HWA 486) inhibits experimental autoimmune tubulointerstitial nephritis in rats. Int J Immunopharmacol. 1989;11:921–9. doi: 10.1016/0192-0561(89)90114-8. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa T, Inazu M, Gotoh K, Hayashi S. Effects of leflunomide on glomerulonephritis induced by antibasement membrane antibody in rats. Agents Actions. 1990;31:321–8. doi: 10.1007/BF01997627. [DOI] [PubMed] [Google Scholar]
- 4.Smith-Lang L, Glaser B, Weimer LK, et al. Efficacy of novel immunomodulators leflunomide and rapamycin in autoimmune uveitis. FASEB J. 1992;6:A1048. [Google Scholar]
- 5.Schorlemmer HU, Bartlett RR. Therapeutic activity of leflunomide in acute and chronic relapsing experimental allergic encephalomyelitis. Agents Actions. 1994;41:C271–3. [Google Scholar]
- 6.Xu X, Blinder L, Shen J, et al. In vivo mechanism by which leflunomide controls lymphoproliferative and autoimmune disease in MRL/MpJ-lpr/lpr mice. J Immunol. 1997;159:167–74. [PubMed] [Google Scholar]
- 7.Dimitrijevic M, Milenkovic M, Milosavljevic P, et al. Beneficial effects of leflunomide on cardiac myosin-induced experimental autoimmune myocarditis in rats. Int J Immunother. 1998;14:9–21. doi: 10.1016/s0041-1345(98)01374-8. [DOI] [PubMed] [Google Scholar]
- 8.Elliot JI, Dewchand H, Altman DM. Streptozotocin-induced diabetes in mice lacking αβ T cells. Clin Exp Immunol. 1997;109:116–20. doi: 10.1046/j.1365-2249.1997.4241319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartlett RR, Zielinski T, Campion G, Musikic P, Schleyerbach R, Schorlemmer H-U. Leflunomide: a novel immunomodulating drug. In: Lewis AJ, Furst D, editors. Non-steroidal anti-inflammatory drugs II. New York: Marcel Dekker, Inc.; 1994. pp. 349–66. [Google Scholar]
- 10.Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: a new model of diabetes mellitus. Science. 1976;193:415–7. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- 11.Kwon NS, Lee SH, Choi CS, et al. Nitric oxide generation from streptozotocin. FASEB J. 1994;8:529–33. doi: 10.1096/fasebj.8.8.8181671. [DOI] [PubMed] [Google Scholar]
- 12.Kolb-Bachofen V, Epstein S, Kiesel U, Kolb H. Low-dose streptozotocin-induced diabetes in mice. Electron microscopy reveals single-cell insulitis before diabetes onset. Diabetes. 1988;37:21–27. doi: 10.2337/diab.37.1.21. [DOI] [PubMed] [Google Scholar]
- 13.Herold KC, Vezys V, Sun Q, et al. Regulation of cytokine production during development of autoimmune diabetes induced with multiple low doses of streptozotocin. J Immunol. 1996;156:3521–7. [PubMed] [Google Scholar]
- 14.Lukic ML, Stosic-Grujicic S, Shahin A. Effector mechanisms in low dose streptozotocin induced diabetes. Dev Immunol. 1998;6:119–28. doi: 10.1155/1998/92198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothlein R, Springer TA. The requirement for leukocyte function-associated antigen 1 in homotypic leukocyte adhesion stimulated by phorbol ester. J Exp Med. 1986;163:1132–49. doi: 10.1084/jem.163.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukic ML, Stocic-Grujicic S, Ostojic N, et al. Inhibition of nitric oxide generation affects the induction of diabetes by streptozotocin in mice. Biochem Biophys Res Commun. 1991;178:913–20. doi: 10.1016/0006-291x(91)90978-g. [DOI] [PubMed] [Google Scholar]
- 17.Stocic-Grujicic S, Dimitrijevic M, Bartlett RR. A novel immunomodulating agent—leflunomide inhibits experimental autoimmune diabetes in mice. Transplant Proc. 1996;28:3072–3. [PubMed] [Google Scholar]
- 18.Dimitrijevic M, Bartlett RR. Leflunomide, a novel immunomodulating drug, inhibits homotypic adhesion of peripheral blood and synovial fluid mononuclear cells in rheumatoid arthritis. Inflamm Res. 1996;45:550–6. doi: 10.1007/BF02342226. [DOI] [PubMed] [Google Scholar]
- 19.Elder RT, Xu X, Williams JW, et al. The immunosuppressive metabolite of leflunomide, A77 1726, affects murine T cells through two biochemical mechanisms. J Immunol. 1997;159:22–27. [PubMed] [Google Scholar]
- 20.Brazelton TR, Moris RE. Molecular mechanisms of action of new xenobiotic immunosuppressive drugs: tacrolimus (FK506), sirolimus (rapamycin) mycophenolate mofetil and leflunomide. Curr Opin Immunol. 1996;8:710–20. doi: 10.1016/s0952-7915(96)80090-2. [DOI] [PubMed] [Google Scholar]
- 21.Herold KC, Vezys V, Koons A, et al. CD28/B7 costimulation regulates autoimmune diabetes induced with multiple low doses of streptozotocin. J Immunol. 1997;158:984–91. [PubMed] [Google Scholar]
- 22.Hogg N, Berlin C. Structure and function of adhesion receptors in leukocyte trafficking. Immunol Today. 1995;16:327–30. doi: 10.1016/0167-5699(95)80147-2. [DOI] [PubMed] [Google Scholar]
- 23.Feve B, Segain JP, Charbonnel B, Sai P. β-cell adherent splenocytes precede the onset of diabetes in low-dose streptozotocin-treated mice. Diabetologia. 1990;39:9–14. doi: 10.1007/BF00586455. [DOI] [PubMed] [Google Scholar]
- 24.Dahlen E, Dawe K, Ohlsson L, Hedlund G. TNF-α in pancreatic islets in the nonobese diabetic mouse. J Immunol. 1998;160:3585–93. [PubMed] [Google Scholar]
- 25.Corbett JA, McDaniel ML. Intraislet release of interleukin 1 inhibits β cell function by inducing β cell expression of inducible nitric oxide synthase. J Exp Med. 1995;181:559–68. doi: 10.1084/jem.181.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stassi G, De Maria R, Trucco G, et al. Nitric oxide primes pancreatic β cells for Fas-mediated destruction in insulin-dependent diabetes mellitus. J Exp Med. 1997;186:1193–200. doi: 10.1084/jem.186.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong Z, Qi X, Xie K, et al. Protein tyrosine kinase inhibitors decrease induction of nitric oxide synthase activity in lipopolysaccharide-responsive and lipopolysaccharide-nonresponsive murine macrophages. J Immunol. 1993;151:2717–24. [PubMed] [Google Scholar]




