Abstract
The nature of the autoantibody repertoire to the dominant autoantigen in human autoimmune thyroid disease is controversial. There is evidence that autoantibodies to thyroid peroxidase (TPO) interact with overlapping conformational epitopes in an immunodominant region and binding to denatured (DN) protein is decreased. Contrary data demonstrate TPO autoantibody reactivity with DN-TPO or polypeptide fragments. However, none of the TPO-specific, human monoclonal autoantibodies isolated to date preferentially recognize denatured autoantigen. We therefore searched an immunoglobulin gene phage display library for human autoantibodies that bind TPO denatured by reduction and alkylation (DN-TPO). Thyroid-infiltrating B cells from a typical TPO autoantibody-positive patient were the source of mRNA for library construction. Surprisingly, the library enriched after panning on DN-TPO, as well as a panel of individual clones, preferentially bound native (N)-TPO. Of 13 clones selected using DN-TPO or N-TPO, 12 clones recognized the TPO immunodominant region. Moreover, regardless of selection with N-TPO or DN-TPO, their heavy and light chains were encoded by similar VDJ and Vκ combinations. One clone (DN4), isolated using DN-TPO, did not interact with the TPO immunodominant region and its H chain derives from a different VH gene. Although DN4 binds specifically to TPO, its affinity is low, unlike the high affinities of other human TPO autoantibodies. In conclusion, human monoclonal autoantibodies that preferentially recognize denatured TPO could not be isolated from an immunoglobulin gene library despite selection with denatured protein. Our findings demonstrate the bias of the human B cell repertoire towards recognition of an immunodominant region on the conformationally intact form of a major thyroid autoantigen.
Keywords: autoimmunity, autoantibodies, human, monoclonals, thyroid
INTRODUCTION
The nature of the autoantibody repertoire to the dominant autoantigen in human autoimmune thyroid disease is controversial (reviewed in [1]). On the one hand, several studies have shown that autoantibodies to thyroid peroxidase (TPO) interact with epitopes on conformationally intact protein and binding is decreased following protein denaturation [2, 3]. On the other hand, other studies have demonstrated serum autoantibody reactivity with TPO after reduction/denaturation (for example [4, 5]), suggesting that some TPO epitopes may not be highly conformational. Further, several linear epitopes [6, 7], as well as polypeptide fragments [8–11], are recognized by patients' autoantibodies. Indeed, the epitopes of some autoantibodies are reported to be inaccessible in the native molecule [11].
Unlike the debate regarding autoantibody interaction with denatured versus native autoantigen, there is broad agreement that the component of the B cell repertoire directed against native TPO is restricted. First, the epitopes of patients' autoantibodies overlap with only two of a panel of mouse MoAbs that recognize a diverse range of epitopes on TPO [12, 13]. Second, human TPO MoAbs isolated from patients' B cells define a restricted area on TPO that is recognized by TPO autoantibodies in all (> 190) patients' sera so far analysed and by approx. 80% of TPO autoantibodies within each individual serum (reviewed in [14]). The concept of an immunodominant region on native TPO has recently been confirmed [15] in a three-way collaborative comparison of the epitopes of the murine TPO MoAb panel [12] with the original human monoclonal autoantibodies described by our laboratory [16] and another panel of human MoAbs isolated independently by different investigators [17].
To date, none of the human TPO-specific monoclonal autoantibodies isolated by three different laboratories preferentially recognize denatured autoantigen [17–20]. However, all human monoclonal TPO autoantibodies have been isolated using conformationally intact antigen. Given that autoantibodies to both native and denatured TPO are clearly present in autoimmune thyroid disease, we used the phage display approach (reviewed in [21]) to search an immunoglobulin gene library for human autoantibodies that recognize denatured TPO. Remarkably, even when panned on denatured protein, the enriched libraries as well as a panel of individual clones preferentially bound native TPO. These findings indicate the importance of protein conformation in the selection of the autoimmune B cell repertoire.
MATERIALS AND METHODS
Construction of combinatorial immunoglobulin gene libraries
Thyroid tissue was obtained from a patient (GA) with autoimmune thyroid disease. Typical of the majority of such patients, GA had a moderately high serum TPO antibody titre (1:3000 by ELISA, see below). Thyroid tissue (0.5 g) was used to prepare mRNA (Pharmacia, Piscataway, NJ) for reverse-transcription of cDNA (First Strand Synthesis Kit; Stratagene, La Jolla, CA). IgG1 heavy (H) and κ-chain cDNA were amplified by the polymerase chain reaction (PCR) using panels of 5′ VH (or Vκ) oligonucleotide primers capable of cross-priming all VH (or Vκ) gene families [16, 22].
A combinatorial library was constructed in the phage display vector pComb3H [23] (provided by Dr C. Barbas, Scripps Research Institute) by cloning the κ-chain DNA into the Sac1 and Xba1 restriction sites and subsequently introducing the H chain DNA into the Xho1 and Spe1 sites. Electroporation into electrocompetent XL1Blue cells (Gibco BRL, Gaithersburg, MD) was performed using an IBI Gene Zapper (21 μF, 2500 V, 400 W). Infective phagemid were generated by rescue with M13KO7 helper phage (Gibco BRL) and precipitated with polyethylene glycol. The unamplified GA-pComb3H combinatorial library contained approx. 5 × 106 recombinants. Using the same H and κ-chain DNA, a combinatorial library containing approx. 30 × 106 recombinants was generated in the bacteriophage λ vector Immunozap (Stratagene) as described previously [18].
Native and denatured TPO
Human TPO secreted by Chinese hamster ovary (CHO) cells was affinity-purified from culture medium as reported previously [24]. An aliquot (10 μg) of native (N) TPO in PBS pH 7.4 was denatured by reduction and alkylation using 50 mm dithiothreitol (10 min, 20°C) and 40 mm iodoacetamide, 0.2 m Tris, pH 8.3 (10 min, 20°C). This is a standard protocol previously used by Nakajima et al. [2] (but with only 10 mm dithiothreitol) to demonstrate loss of TPO binding activity by patients' autoantibodies. After buffer exchange (to PBS) using Micron 50 columns (Amicon Inc., Beverly, MA), the change in naturation status was assessed by a direct binding assay and reverse immunoblot using TPO autoantibody-positive sera, a human monoclonal TPO-specific autoantibody Fab, WR1.7 [16] and a mouse MoAb (Mab 47) [12] (the latter kindly provided by Dr B. Czarnocka, Medical Centre of Postgraduate Education, Warsaw, Poland). TPO for these assays was labelled using iodogen. Antibody–TPO complexes in the direct binding assay were precipitated for human sera with Protein A (Pansorbin; Calbiochem, La Jolla, CA) [18] and for the murine MoAb with anti-mouse conjugated beads (Sac-Cel; IDS, Boldon, UK) [15]. The reverse immunoblot protocol is outlined below.
Screening the GA-IgG/κ combinatorial libraries with denatured and native TPO
The GA-pComb3H library was screened as described [20] by incubating approx. 1012 phage (1 h, 37°C) in ELISA wells coated with denatured (DN)-TPO or N-TPO (1 μg/well). The wells were washed (four times), bound phagemid eluted with 0.1 m HCl pH 2.2, neutralized with 2 m Tris pH 7.5 and used to infect XL1 Blue cells. Aliquots were withdrawn for titering and culture of the remaining cells was continued overnight at 30°C with M13K07 helper phage. After phagemid precipitation and resuspension, the panning was repeated twice, increasing the stringency of washing before elution to × 5 in round II and × 6 in round III. Twenty individual colonies from the third round of panning on DN-TPO (or N-TPO) were screened in filter lift assays as described [25] for binding to 125I-DN-TPO (or 125I-N-TPO). Aliquots (3 μl) of patients' and control sera, as well as murine MoAb 47 [12], were applied to nitrocellulose filters and incubated with the same preparations of radiolabelled N- and DN-TPO (reverse immunoblot). DNA from TPO-binding colonies was digested with the frequent cutting enzyme BstN1 (Stratagene) to identify clones with different restriction patterns to avoid analysing multiple identical colonies arising from the amplification involved in library panning.
Screening of the GA Immunozap library was as previously described [18] using conventional filter lifts and 125I-N-TPO. Following plaque purification, plasmids were excised from the Immunozap vector using helper phage R408 (Stratagene). Nucleotide sequencing of double-stranded plasmid cDNA for TPO-binding colonies from pComb3H and Immunozap libraries was performed by the dideoxynucleotide termination method or by PCR-based automated sequencing. Sequences were analysed using the V Base Directory [26] to determine the putative germ-line genes encoding the TPO binding H and L chain V regions.
Fab expression from TPO-binding colonies in pComb3H
Fab were expressed as soluble proteins without removal of the cpIII gene from the pComb3H plasmid, as we have shown that this approach provides sufficient Fab for analysis [20]. Plasmid-bearing XL1 Blue cells were incubated (37°C, 6–7 h) and protein synthesis was induced by overnight incubation with isopropyl-thio-galacto-pyranoside (1 mm; Sigma Chemical Co., St Louis, MO). After four freeze–thaw cycles, the cells were centrifuged and the cell pellets were resuspended in PBS containing protease inhibitors.
Fab recognition of N versus DN TPO
A ‘phage’ ELISA was performed by modifying an ELISA previously reported for TPO-specific Fab [18]. In brief, 100-μl aliquots of libraries after one, two or three rounds of panning (diluted 1:102–1:104) were applied to ELISA wells coated with N-TPO or DN-TPO (1 μg/ml). Bound phage were detected with biotinylated sheep antibody to M13 (5 Prime to 3 Prime, Inc., Boulder, CO) and horseradish peroxidase (HRP)-conjugated streptavidin (Pharmingen, San Diego, CA). ELISA for Fab was performed using similarly coated wells and detection, as in previous studies [18], with murine monoclonal anti-human κ (QE11; Recognition Sciences, Birmingham, UK) and affinity-purified anti-mouse IgG conjugated to HRP (Sigma). In both assays, the substrate was o-phenylenediamine + H2O2 and optical densities (OD) were read at 492 nm. Fab specificity was established by comparing OD values for ELISA wells coated with TPO versus the following proteins (1 μg/ml): bovine serum albumin (BSA; Sigma); myeloperoxidase (MPO; Calbiochem), lactoperoxidase (LPO), transferrin, keyhole limpet haemocyanin (KLH) (all from Sigma) and human thyroglobulin (Tg) prepared from Graves' thyroid tissue by gel filtration and ion exchange chromatography.
Recognition of TPO expressed on eukaryotic cells
Flow cytometry of TPO expressed on CHO cells was studied as described [27]. In brief, CHO-TPO cells grown in monolayer were detached by mild trypsinization and incubated with TPO-binding Fab followed by anti-human κ conjugated to PE (Caltag, Burlingame, CA). Fluorescence was analysed using the Becton Dickinson FACScan–CELLQuest system (Mountain View, CA).
Fab binding to 125I-TPO and Fab affinities for TPO
TPO-specific Fab with κ-chains were incubated with 125I-TPO (approx. 20 000 ct/min) and murine monoclonal anti-human κ (QE11) as described [18]. After addition of anti-mouse IgG-coated beads (Sac-cel) to capture the immune complexes, the beads were sedimented and counted to determine the percentage 125I-TPO bound. The Fab affinities for TPO were determined by Scatchard analysis of 125I-TPO binding in the presence of increasing concentrations of unlabelled TPO.
Interaction of TPO-specific Fab with the immunodominant domain on TPO
A modification of the above assay was used to determine the interaction of newly cloned Fab with epitopes in the TPO immunodominant region (reviewed in [14]). As detailed previously [18], test Fab were immobilized with anti-human κ (QE11) and anti-mouse IgG-coupled beads (Sac-cel). Remaining anti-κ binding sites were saturated with normal human serum. Aliquots of 125I-TPO (approx. 20 000 ct/min) preincubated in buffer or with a pool of four Fab that define the immunodominant region (SP1.4, WR1.7, TR1.8 and TR1.9; each at 10−8 m) [16] were added to the immobilized test Fab pellets. After incubation and washing, radioactivity bound to the Sac-cel was counted. Non-specific binding (approx. 3% of total counts) was subtracted to provide specific binding to TPO.
RESULTS
Phage screened on denatured TPO preferentially recognize native TPO
The yield of phage eluted after panning the GA-pComb3H library on DN-TPO increased progressively; approx. 10−4%, approx. 5 × 10−4% and 2 × 10−3% after rounds I, II and III, respectively. Comparable yields were observed for phage from the same library panned on N-TPO (approx. 10−4%, approx. 10−3% and 4 × 10−3%). After three panning rounds, filter lift assays indicated that approx. 50% individual colonies selected using DN-TPO recognized 125I-DN-TPO (Fig. 1a) and approx. 50% colonies selected using N-TPO recognized 125I-N-TPO (Fig. 1b). Surprisingly, in a cross-comparison, the DN-selected colonies recognized N better than DN 125I-TPO (Fig. 1c versus Fig. 1d).
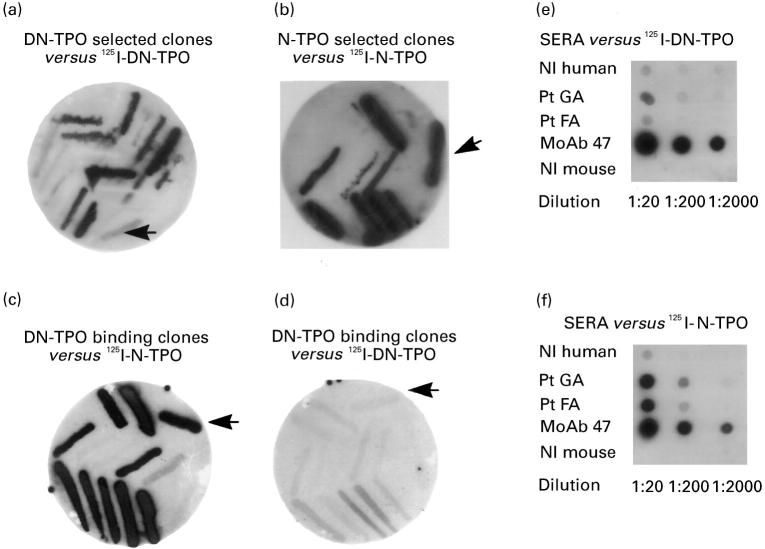
Fig. 1.
Interaction of thyroid-derived clones with native (N) versus denatured (DN) thyroid peroxidase (TPO) after three rounds of panning a pComb3H phage display library constructed from thyroid tissue of a patient with autoimmune thyroid disease. Individual clones from the third round of panning against DN-TPO or N-TPO were tested in filter lifts for binding of radiolabelled antigen. As anticipated, DN-TPO-selected clones bind 125I-DN-TPO (a) and N-TPO-selected clones bind 125I-N-TPO (b). Unexpectedly, DN-TPO binding clones interact better with 125I-N-TPO (c) than with 125I-DN-TPO (d). 125I-DN-TPO used in these studies is recognized very well by mouse MoAb 47, but only weakly or not at all by serum autoantibodies (patients GA and FA) (e). In contrast, 125I-N-TPO is recognized by both serum antibodies and mouse MoAb 47 (f). Controls include normal (Nl) human serum and normal mouse serum (e,f). The arrows indicate binding by the human monoclonal TPO autoantibody Fab WR1.7 (as a bacterial streak) that preferentially recognizes N-TPO [16].
The efficacy of denaturation of DN-TPO used in panning and as tracer in these studies was confirmed in two ways. First, 125I-TPO binding by patients' autoantibodies (sera diluted 1:200) was markedly lower to DN-TPO than to N-TPO, 8.9 + 4.6% (mean + s.e.m.; n = 11 sera) versus 35.4 + 4.5% (t = 14.41, P < 0.001, paired t-test). This magnitude of reduction in TPO autoantibody binding is comparable to values previously obtained by others for Graves' and Hashimoto sera [2]. In contrast, the same DN-TPO was bound to a greater extent than N-TPO (46.5% versus 17.6%, respectively) by murine MoAb 47 (diluted 1:10 000) that recognizes denatured and native TPO [12]. Second, after direct application to filters, sera from patients GA and FA (1:20 and 1:200) bound 125−I-DN-TPO (Fig. 1e) to a much lesser extent than N-TPO (Fig. 1f). Similarly, a streak of TPO-specific Fab WR1.4 bacteria also gave strong signal with N-TPO (Fig. 1b,c) and a much weaker or negative signal with DN-TPO (Fig. 1a,d). Consistent with the direct binding assay, murine MoAb 47 recognized both native and denatured TPO even at a dilution of 1:2000 (Fig. 1e,f).
The autoradiographic observations for TPO selected colonies were confirmed quantitatively by ELISA at different stages of library panning on DN-TPO, as well as for individual clones after the third panning round. Enrichment for TPO-binding phage was evident in the library after round II, at which stage there was preferential recognition of N-TPO versus DN-TPO (Fig. 2a). A similar preference for N-TPO was seen after round III. The greater enrichment at this stage required greater library dilution to overcome saturation of available binding sites on ELISA wells.
Fig. 2.
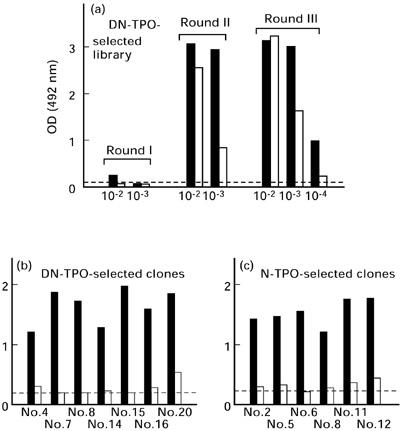
Preferential recognition of native (N) (▪) versus denatured (DN) thyroid peroxidase (TPO) (□) by the phage display library panned on denatured TPO (a) and by Fab expressed by individual clones selected by panning on either DN-TPO (b) or N-TPO (c). Dilutions of phage from the GA-pComb3H library after rounds I, II and III or individual expressed Fab were applied to ELISA wells coated with N- or DN-TPO. Phage were detected with biotinylated anti-M13 phage and horseradish peroxidase (HRP)-conjugated streptavidin and Fab with anti-human k and HRP anti-mouse IgG. The upper limit of binding to bovine serum albumin (BSA)-coated wells is indicated by the broken lines. OD, Optical density.
To reduce the likelihood of characterizing multiple identical clones, we tested 13 DN-TPO-selected and 11 N-TPO-selected clones for differences in BstN1 restriction patterns of their DNA (data not shown). Quantification of binding activity of soluble Fab expressed from individual clones revealed significantly greater recognition of N-TPO versus DN-TPO for 7/7 clones selected with DN-TPO (Fig. 2b, t = 11.369, P < 0.0003; paired t-test), as well as for 6/6 clones selected with N-TPO (Fig. 2c, t = 17.05, P < 0.00001). Therefore, regardless of whether they were selected on DN-TPO or on N-TPO, all clones preferentially recognized N-TPO.
Recognition of the TPO immunodominant region
Despite recognizing conformationally intact TPO, it was possible that clones selected using DN-TPO might differ in other aspects from clones identified using N-TPO. Consequently, we investigated whether the epitopes of the newly isolated TPO-binding clones lay within the TPO immunodominant region recognized by all patients' sera, as previously defined by a pool of four human monoclonal autoantibody Fab [16]; reviewed in [14].
Competition studies with the four Fab pool revealed that, with one exception, the newly isolated TPO-binding Fab interacted with epitopes in the TPO immunodominant region. Thus, these four Fab inhibited 125I-TPO binding by Fab generated from 6/7 clones selected on DN-TPO (Fig. 3a) and from 6/6 clones selected on N-TPO (Fig. 3b). Quantitatively, the mean inhibition for the six DN-TPO-selected and six N-TPO-selected clones was similar: 91 ± 3% and 84 ± 5% inhibition of 125I-TPO binding, respectively. In contrast, in two separate experiments (one shown in Fig. 3), TPO binding by DN-selected clone no. 4 (DN4) was unaffected by the presence of the four Fab pool. Consequently, unlike the epitopes of the other 12 clones from patient GA, the epitope of DN4 lies outside the TPO immunodominant region.
Fig. 3.
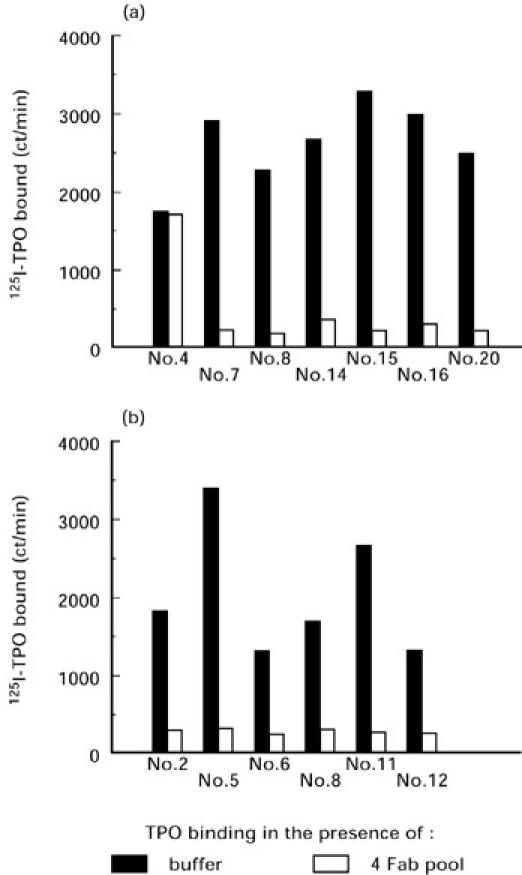
The epitopes of thyroid peroxidase (TPO)-binding clones selected by panning on denatured (DN)-TPO (a) or on native (N)-TPO (b), with one exception (clone DN4), lie within the immunodominant region. Competition assays for radiolabelled TPO binding were performed between newly cloned TPO-binding Fab and four previously isolated Fab that define the immunodominant region [16]. Data are presented as ct/min 125I-TPO bound.
H and L chain variable region genes for clones selected with DN-TPO and N-TPO
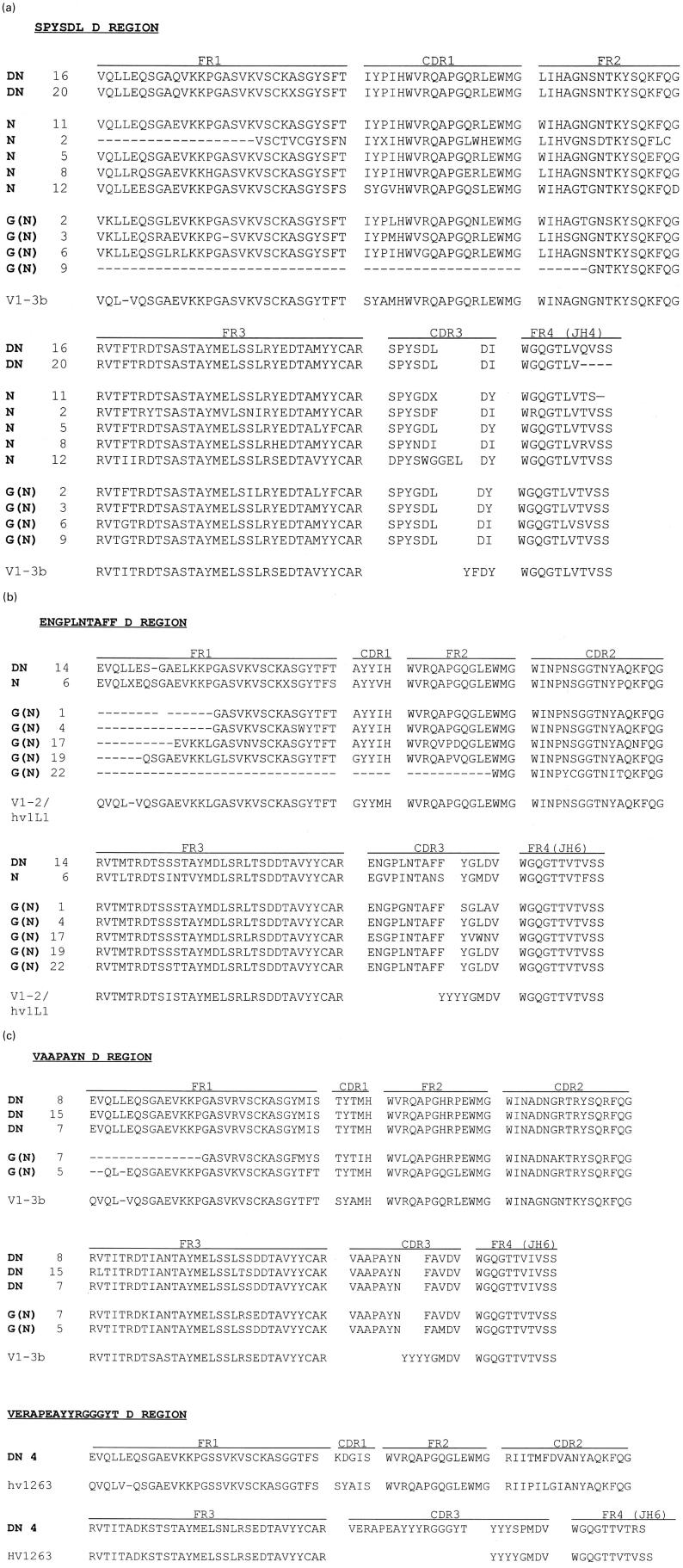
We determined the H and L chain V region nucleotide sequences of the seven DN-TPO-selected clones (‘DN-clones’) and the six N-TPO-selected clones (‘N-clones’) isolated from the GA-pComb3H library. In addition, we characterized 12 clones from a bacteriophage λ (Immunozap) library constructed using H and κ-chain cDNA from the same patient. These clones, termed ‘G(N)’, were identified by filter lift assays using 125I-labelled N-TPO. All TPO binding clones were classified in terms of the derived amino acid sequences of their H chain D regions. Regardless of whether they were selected with DN-TPO or N-TPO (pComb3H vector) or by using 125I-N-TPO (Immunozap vector), the TPO binding clones comprise four groups: (i) the largest group has ‘SPYSDL’ D regions, VH1 family genes derived from V1-3b/DP-25 germ-line genes [26] and JH4 regions (Fig. 4a). This group includes two DN-TPO clones, five N-TPO clones and four G(N) clones obtained by screening with 125I-N-TPO; (ii) the second group has ‘ENGPLNTAFF’ D regions, VH regions related to the germ-line gene V1-2 [26], also known as hv1 l1, and modified JH6 regions (Fig. 4b). This group includes one DN clone, one N clone and five G(N) clones; (iii) the third group has ‘VAAPAYNFAV’ D regions, VH1 genes related to V1-3b/DP-25 [26] and truncated JH6 regions (Fig. 4c). Three three DN clones and three G(N) clones are within this group; (iv) finally, DN4, the unique clone that interacts with an epitope outside the immunodominant region also uses genes distinct from all the other TPO-binding clones. Thus, DN4 has a unique D region, ‘VERAPEAYYGGGYT’, as well as a VH region related to the germ-line gene hv1263 [26] and a complete JH6 region (Fig. 4c). The combination of this D region and a non-truncated JH6 creates a very long CDR3.
Fig. 4.
(See pp 24 and 25) Similar H chain V regions encode thyroid peroxidase (TPO) binding Fab selected using native (N) or denatured (DN)-TPO. Derived amino acid sequences are shown for: (i) clones selected from the GA-pComb3H phage display library by panning on DN-TPO (DN clones); (ii) clones selected from the same library by panning on N-TPO (N clones); (iii) clones isolated using 125I-N-TPO from the GA-bacteriophage λ library (G(N) clones). Clone grouping is based on their D regions: (a) ‘SPYSDL’ group; (b) ‘ENGPLNTAFF’ group; (c) ‘VAAPAYNFAV’ group and the single clone with D region VERAPEAYYYRGGT. Included for comparison are the amino acid sequences of their closest germ-line genes, namely V1–3b, V1–2 and hv1263 [26]. Note, because of their high degree of mutation, the D region germ-line genes could not be assigned and the DJ junctions shown, except for clone DN4, are arbitrary.
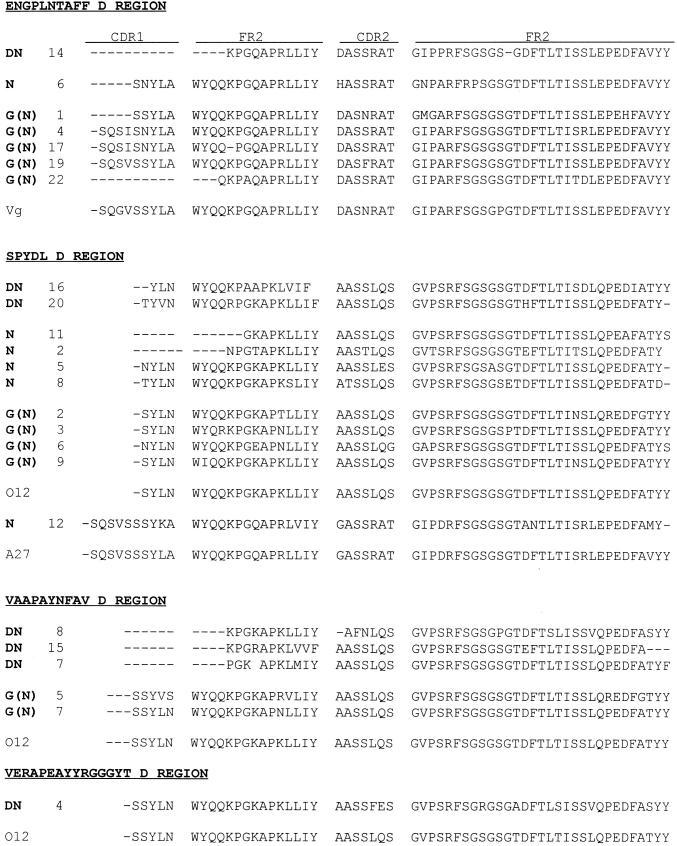
In terms of their κ-chains, partial sequencing of the CDR2 regions (Fig. 5) showed that the majority of the ‘SPYSDL’ D region group, as well as all of the ‘VAAPAYN’ D region group, have O12/O2 [26]-like L chains. In contrast, all clones with ‘ENGPLNTAFF’ D regions have Vκ III chains derived from germ-line gene Vg [26]. One other Fab, N12, with a greatly modified ‘SPYSDL’ D region, has a Vκ III chain derived from A27 [26]. Although the VDJ region of clone DN4 is unique among TPO-binding clones isolated from this patient, it has an O12-like κ-chain (like many other TPO-binding Fab in this panel).
Fig. 5.
Similar L chain variable regions encode thyroid peroxidase (TPO) binding Fab selected using native (N) or denatured (DN)-TPO. Derived amino acid sequences (partial) are shown for clones selected from: (i) the GA-phage display library panned on DN-TPO (DN clones); (ii) the same library panned on N-TPO (N clones); (iii) the GA-bacteriophage λ library using 125I-N-TPO, ‘G(N)’ clones. The clones are grouped according to the derived amino acid sequences of their H chain D regions (see Fig. 6). Included for comparison are the amino acid sequence of their closest germ-line genes, namely Vg; O12/O2 and A27 [26].
Affinity for TPO and specificity of clone DN4 compared with other DN-TPO-selected clones
Because of its unusual VDJ region, and because its epitope lies outside the TPO immunodominant region, we examined other immunological properties of the Fab expressed by clone DN4 Fab: (i) on flow cytometry, Fab DN4, like another representative Fab (DN14), bound native TPO expressed on intact eukaryotic cells (mean fluorescence intensity (MFI) value > 110 units for both Fab compared with 30 units in the presence of second antibody only); (ii) unlike other Fab representative of the three major D region groups (DN8, DN14, and DN16), 125I-TPO binding by DN4 was only marginally inhibited by concentrations of unlabelled TPO as high as 2 × 10−8m unlabelled TPO (Fig. 6a). Fab DN8, DN14 and DN16 had high affinities for TPO, as calculated by Scatchard analysis of the binding data, namely Kd 1.5 × 10−10 m, 2.6 × 10−10m and 1.2 × 10−10 m, respectively. In contrast, the affinity of Fab from DN4 was too low to be measured; (iii) despite its low affinity, Fab DN4 bound specifically to TPO (Fig. 6b). Thus, like Fab DN14, N2 and N6, Fab DN4 gave a strong signal on ELISA wells coated with TPO, but not with the related enzymes LPO and MPO, Tg, and with two non-autoantigens, namely transferrin and KLH.
Fig. 6.
Fab DN4, that does not recognize the thyroid peroxidase (TPO) immunodominant region, has a low affinity for TPO (unlike other TPO-selected Fab), yet it binds specifically to TPO (like other native (N)-TPO and denatured (DN)-TPO selected Fab). (a) 125I-TPO bound (% maximum) in the presence of increasing concentrations of unlabelled TPO by Fab DN4 and three other DN-TPO-selected clones representative of clones with the other D regions. Binding in the absence of unlabelled TPO: DN4, 21.8%; DN8, 27.2%; DN14, 18.3%; DN16, 19.5%. (b) Optical densities (492 nm) for Fab DN4, DN14, N2 and N6 incubated with ELISA wells coated with TPO, lactoperoxidase (LPO), myeloperoxidase (MPO), thyroglobulin (Tg), transferrin (Tsfn) and keyhole limpet haemocyanin (KLH). Fab binding was detected with mouse MoAb to human κ-chains. All samples were analysed simultaneously and data are the means of closely agreeing duplicate wells.
DISCUSSION
The phage display system provides a powerful means of searching an immunoglobulin gene combinatorial library for rare antibody specificities. We therefore used this approach to search for human autoantibodies preferentially reactive with denatured autoantigen in a combinatorial library generated from thyroid-infiltrating plasma cells of a typical patient with autoimmune thyroid disease. Surprisingly, both the library enriched by panning on denatured (reduced and alkylated) TPO, as well as individual clones from this enriched library, interacted preferentially with native TPO. The efficacy of reduction and alkylation in changing the TPO naturation status was confirmed by a direct binding assay and by reverse immunoblot using human sera, a human TPO-specific autoantibody Fab [16] and a mouse MoAb that recognizes N-and DN-TPO [12]. We cannot exclude the possibility that a small proportion of TPO remained in its native form. However, the presence of minimal amounts of N-TPO is unlikely to have influenced the outcome of the study, since previous studies have indicated that antigen-specific phage present at frequencies of less than 1 in a billion can be isolated from a phage display library by repeated rounds of selection [21].
It is possible that extrathyroidal sites of thyroid autoantibody synthesis, such as lymph nodes draining the thyroid gland (reviewed in [28]), may contain B cells specific for denatured TPO. However, TPO-reactive Fab isolated from thyroid and lymph node of the same patient were remarkably similar in terms of their VH and VK gene sequences and recognition of native TPO [17, 29]. Our present observations from panning a thyroid-derived library with N- and DN-TPO, together with information from screening thyroid or lymph node libraries with N-TPO (for example [16–20, 29]), suggest the rarity of human autoantibodies with specificity for denatured TPO. Consequently, conformationally intact TPO, normally expressed on the surface of thyroid cells, is likely to play a critical role in selection of the autoimmune B cell repertoire in autoimmune thyroid disease, the most common organ-specific autoimmune condition in humans.
The second major finding of our study is that, of 12 new human TPO-specific MoAbs isolated using denatured or native autoantigen, all but one recognized the immunodominant region on native TPO (reviewed in [14]). Combining these observations with data on previously characterized MoAbs obtained by two different laboratories [15], 97% (34/35) of TPO-specific human MoAbs identified from seven different patients interact with epitopes in the immunodominant region. Our findings reinforce the concept that the B cell repertoire in human autoimmune thyroid disease is focused on a restricted set of overlapping epitopes on the native 107-kD TPO molecule.
The basis for restricted epitopic recognition on TPO by spontaneously arising human autoantibodies (reviewed in [14]), versus diverse epitopes in experimentally immunized animals [12], is not known. Similar observations have been made for autoantibodies in other autoimmune diseases such as to the acetylcholine receptor in myasthenia gravis [30]. We have suggested [31] that this restricted B cell epitopic recognition may result from the ability of high-affinity antigen-specific B cells to capture and influence the peptides that are ultimately presented to T cells. Several studies have shown that human B cell clones specific for different epitopes on the same antigen (tetanus toxoid) enhance or suppress presentation of different peptides to T cells [32].
The conclusion that clones reactive with native autoantigen dominate the B cell repertoire is supported by our present observation that similar combinations of H and L chain V region genes encode TPO-binding human MoAb isolated from a phage display library using either denatured or native TPO. Similar VDJh and Vκ chain gene combinations were also found in TPO-binding clones isolated from the same thyroid-derived immunoglobulin gene cDNA in a bacteriophage vector using native TPO. Thus, with one exception (see below), all three approaches yielded Fab with three basic VDJh/κ combinations, regardless of whether the libraries were screened on denatured or native TPO (summarized in Table 1). Incidentally, the presence of the same VDJh and VL combinations in TPO-binding Fab isolated from two different libraries (pComb3H and Immunozap), each containing random combinations of the same H and L chain cDNA, supports our earlier findings. That is, in ‘roulette’ studies using a TPO-specific H chain to search the parent L chain library for other L chains compatible with TPO binding, we pulled out the same type of L chains, and vice versa (reviewed in [14]).
Table 1.
Screening of thyroid-derived combinatorial immunoglobulin gene libraries with denatured (DN) or native (N)-thyroid peroxidase (TPO) yields Fab that, with one major exception, are encoded by similar VH and VL chain combinations. Fab grouping is based on the derived amino acid sequences of their D regions and the number of clones represented by each VH/VL combination is indicated in parentheses
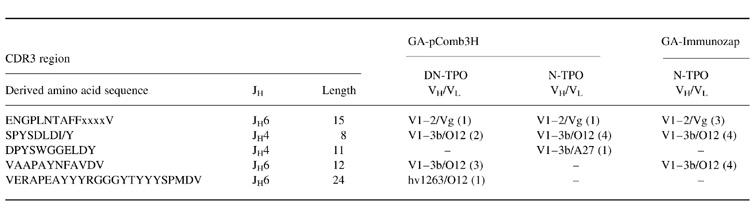
Clone DN4 has a VDhJh/VL combination unique for this patient, namely a VH derived from hv1263, a D region ‘VERAPEAYYYRGGT’ and a JH6 paired with an O12-like Vκ chain (Table 1). Also unique to this autoantibody is that it does not interact with the TPO-immunodominant region. The use of an hv1263-like VH region is not unusual, as H chains derived from this germ-line gene were used by TPO autoantibodies previously isolated from thyroid tissue of a different patient [16, 25]. However, unlike the hv1263/O12 combination in DN4, in previously reported TPO autoantibodies the hv1263-like H chains were paired with L chains related to the Vκ III family gene A3. Most striking about TPO clone DN4 is its very long CDR3 region (24 amino acids), longer than those of (i) other Fab cloned from thyroid cDNA of this patient (8–15 amino acids); (ii) the previously reported TPO autoantibodies with hv1263-like H chains (15 amino acids) [16, 25]; and (iii) other TPO autoantibodies with JH6 regions (12–14 amino acids) [16, 29]. Though antigen-specific, DN4 has a very low affinity for TPO, in contrast to the high affinities of other TPO autoantibodies with hv1263-H chains [16, 25] and the other TPO autoantibodies characterized in the present study (Kd approx. 2 × 10−10m). In the context of DN4's low affinity, it is intriguing that hv1263 is a member of the fetally expressed 51P1 group of genes [33]. However, DN4 uses a mutated, not a germ-line, form of hv1263. Instead, the low affinity of DN4 may be related to its unusually long CDR3 region.
Our observations are relevant to B cell repertoire selection in organ-specific human autoimmune diseases in general. For example, besides TPO, preferential interaction with native rather than denatured autoantigen is observed for IgG class autoantibodies to GAD [34] and the acetylcholine receptor (myasthenia gravis) [30]. Moreover, the association between high serum levels of autoantibodies and reactivity to denatured GAD in Stiff Man syndrome versus much lower titres and lack of binding to denatured autoantigen in insulin-dependent diabetes mellitus (IDDM) type I [34] raises the possibility that B cells specific for the denatured form of the protein only arise late in the autoimmune process.
Autoantibodies can directly mediate disease (as in myasthenia gravis and Graves' hyperthyroidism) and provide invaluable pathological markers (IDDM type I). Equally important, autoantibodies are likely to play a vital role in antigen presentation to T cells (as discussed above). Indeed, very recently it has been shown that B cells are crucial antigen-presenting cells in the non-obese diabetic (NOD) mouse model of IDDM type I [35]. T cell activation requires the processing of antigens to relatively simple linear epitopes. In contrast, because conformationally intact autoantigen is likely to be important during the early stage of B cell repertoire selection in human autoimmunity, understanding this process requires knowledge of the folding, glycosylation and cellular trafficking of full length, native protein.
In conclusion, human monoclonal autoantibodies that preferentially recognize a denatured form of a major thyroid autoantigen (TPO) could not be isolated from an immunoglobulin gene library despite selection with denatured protein. Moreover, unlike the majority of TPO-specific MoAbs isolated in this and previous investigations, only one Fab had its epitope primarily outside the TPO immunodominant region. Our findings demonstrate the bias of the human B cell repertoire towards recognition of an immunodominant region on the conformationally intact form of a major thyroid autoantigen.
Acknowledgments
This work was supported by the National Institutes of Health Grants 1RO1 DK 19289 and DK 36182. We thank Dr Staffan Smeds (University of Linköping, Sweden) for providing us with thyroid tissue from the patient with autoimmune thyroid disease, and Dr Barbara Czanocka (Medical Centre of Postgraduate Education, Warsaw, Poland) for providing us with mouse monoclonal antibody 47.
REFERENCES
- 1.McLachlan SM, Rapoport B. The molecular biology of thyroid peroxidase: cloning, expression and role as autoantigen in autoimmune thyroid disease. Endocr Rev. 1992;13:192–206. doi: 10.1210/edrv-13-2-192. [DOI] [PubMed] [Google Scholar]
- 2.Nakajima Y, Howells RD, Pegg C, Davies Jones E, Rees Smith B. Structure activity analysis of microsomal antigen/thyroid peroxidase. Molec Cell Endocrinol. 1987;53:15–23. doi: 10.1016/0303-7207(87)90187-0. [DOI] [PubMed] [Google Scholar]
- 3.Gardas A, Domek H. The effect of sulphydryl reagents on the human thyroid microsomal antigen. J Endocrinol Invest. 1988;11:385–8. doi: 10.1007/BF03349061. [DOI] [PubMed] [Google Scholar]
- 4.Hamada N, Jaeduck N, Portmann L, Ito K, DeGroot LJ. Antibodies against denatured and reduced thyroid microsomal antigen in autoimmune thyroid disease. J Clin Endocrinol Metab. 1987;64:230–8. doi: 10.1210/jcem-64-2-230. [DOI] [PubMed] [Google Scholar]
- 5.Bermann M, Magee M, Koenig RJ, Kaplan MM, Maastricht J, Johnson J, Baker JR. Differential autoantibody responses to thyroid peroxidase in patients with Graves' disease and Hashimoto's thyroiditis. J Clin Endocrinol Metab. 1993;77:1098–101. doi: 10.1210/jcem.77.4.8408460. [DOI] [PubMed] [Google Scholar]
- 6.Finke R, Seto P, Ruf J, Carayon P, Rapoport B. Determination at the molecular level of a B-cell epitope on thyroid peroxidase likely to be associated with autoimmune thyroid disease. J Clin Endocrinol Metab. 1991;73:919–21. doi: 10.1210/jcem-73-4-919. [DOI] [PubMed] [Google Scholar]
- 7.Libert F, Ludgate M, Dinsart C, Vassart G. Thyroperoxidase, but not the thyrotropin receptor, contains sequential epitopes recognized by autoantibodies in recombinant peptides expressed in the pUEX vector. J Clin Endocrinol Metab. 1991;73:857–60. doi: 10.1210/jcem-73-4-857. [DOI] [PubMed] [Google Scholar]
- 8.Banga JP, Barnett PS, Ewins DL, Page M, McGregor AM. Mapping of autoantigenic epitopes on recombinant thyroid peroxidase fragments using the polymerase chain reaction. Autoimmunity. 1990;6:257–68. doi: 10.3109/08916939008998418. [DOI] [PubMed] [Google Scholar]
- 9.Maastricht J, Koenig RJ, Kaplan MM, Arscott P, Thompson N, Baker JR. Identification of localized autoantibody epitopes in thyroid peroxidase. J Clin Endocrinol Metab. 1992;75:121–6. doi: 10.1210/jcem.75.1.1377703. [DOI] [PubMed] [Google Scholar]
- 10.Zanelli E, Henry M, Malthiery Y. Use of recombinant epitopes to study the heterogeneous nature of the autoantibodies against thyroid peroxidase in autoimmune thyroid disease. Clin Exp Immunol. 1992;87:80–86. doi: 10.1111/j.1365-2249.1992.tb06417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arscott PL, Koenig RJ, Kaplan MM, Glick GD, Baker JR. Unique autoantibody epitopes in an immunodominant region of thyroid peroxidase. J Biol Chem. 1996;271:4966–73. doi: 10.1074/jbc.271.9.4966. [DOI] [PubMed] [Google Scholar]
- 12.Ruf J, Toubert M, Czarnocka B, Durand-Gorde J, Ferrand M, Carayon P. Relationship between immunological structure and biochemical properties of human thyroid peroxidase. Endocrinol. 1989;125:1211–8. doi: 10.1210/endo-125-3-1211. [DOI] [PubMed] [Google Scholar]
- 13.Czarnocka B, Pastuszko D, Carayon P, Ruf J, Gardas A. Majority of thyroid peroxidase in patients with autoimmune thyroid disease are directed to a single TPO domain. Autoimmunity. 1996;23:145–54. doi: 10.3109/08916939608995338. [DOI] [PubMed] [Google Scholar]
- 14.McLachlan SM, Rapoport B. Genetic and epitopic analysis of thyroid peroxidase (TPO) autoantibodies: markers of the human thyroid autoimmune response. Clin Exp Immunol. 1995;101:200–6. doi: 10.1111/j.1365-2249.1995.tb08339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo J, McIntosh RS, Czarnocka B, Weetman A, Rapoport B, McLachlan SM. Relationship between autoantibody epitopic recognition and immunoglobulin gene usage. Clin Exp Immunol. 1998;111:408–14. doi: 10.1046/j.1365-2249.1998.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chazenbalk GD, Portolano S, Russo D, Hutchison JS, Rapoport B, McLachlan SM. Human organ-specific autoimmune disease: molecular cloning and expression of an autoantibody gene repertoire for a major autoantigen reveals an antigenic dominant region and restricted immunoglobulin gene usage in the target organ. J Clin Invest. 1993;92:62–74. doi: 10.1172/JCI116600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czarnocka B, Janota-Bzowski M, McIntosh RS, Asghar MS, Watson PF, Kemp EH, Carayon P, Weetman AP. Immunoglobulin G kappa anti-thyroid peroxidase antibodies in Hashimoto's thyroiditis: epitope mapping analysis. J Clin Endocrinol Metab. 1997;82:2639–44. doi: 10.1210/jcem.82.8.4124. [DOI] [PubMed] [Google Scholar]
- 18.Portolano S, Chazenbalk GD, Seto P, Hutchison JS, Rapoport B, McLachlan SM. Recognition by recombinant autoimmune thyroid disease-derived Fab fragments of a dominant conformational epitope on human thyroid peroxidase. J Clin Invest. 1992;90:720–6. doi: 10.1172/JCI115943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hexham JM, Partridge LJ, Furmaniak J, Petersen VB, Colls JCJ, Pegg CR, Rees Smith B, Burton DR. Cloning and characterisation of TPO autoantibodies using combinatorial phage display libraries. Autoimmunity. 1994;17:167–79. doi: 10.3109/08916939409010651. [DOI] [PubMed] [Google Scholar]
- 20.Portolano S, Prummel MF, Rapoport B, McLachlan SM. Molecular cloning and characterization of human thyroid peroxidase autoantibodies of lambda light chain type. Molec Immunol. 1995;32:1157–69. doi: 10.1016/0161-5890(95)00060-7. [DOI] [PubMed] [Google Scholar]
- 21.Marks C, Marks JD. Phage libraries—a new route to clinically useful antibodies. N Engl J Med. 1996;335:730–3. doi: 10.1056/NEJM199609053351008. [DOI] [PubMed] [Google Scholar]
- 22.Jaume JC, Portolano S, Rapoport B, McLachlan SM. Influence of the light chain repertoire on immunoglobulin genes encoding thyroid autoantibody Fab from combinatorial libraries. Autoimmunity. 1996;24:11–23. doi: 10.3109/08916939608995353. [DOI] [PubMed] [Google Scholar]
- 23.Barbas CF, III, Wagner J. Synthetic human antibodies: selecting and evolving functional proteins. Meth: Comp Meth Enzymol. 1995;8:94–103. [Google Scholar]
- 24.Guo J, McLachlan SM, Hutchison JS, Rapoport B. The greater glycan content of recombinant human thyroid peroxidase of mammalian than on insect cell origin facilitates purification to homogeneity of enzymatically active protein remaining soluble at high concentration. Endocrinol. 1998;139:999–1005. doi: 10.1210/endo.139.3.5782. [DOI] [PubMed] [Google Scholar]
- 25.Portolano S, McLachlan SM, Rapoport B. High affinity, thyroid-specific human autoantibodies displayed on the surface of filamentous phage use V genes similar to other autoantibodies. J Immunol. 1993;151:2839–51. [PubMed] [Google Scholar]
- 26.Tomlinson IMS, Williams SC, Corbett SJ, Cox JBL, Winter G. V BASE Sequence Directory. Medical Research Council Center for Protein Engineering. 1998 [Google Scholar]
- 27.Nishikawa T, Rapoport B, McLachlan SM. Exclusion of two major areas on thyroid peroxidase from the immunodominant region containing the conformational epitopes recognized by human autoantibodies. J Clin Endocrinol Metab. 1994;79:1648–54. doi: 10.1210/jcem.79.6.7527407. [DOI] [PubMed] [Google Scholar]
- 28.Rees Smith B, McLachlan SM, Furmaniak J. Autoantibodies to the thyrotropin receptor. Endocr Rev. 1988;9:106–21. doi: 10.1210/edrv-9-1-106. [DOI] [PubMed] [Google Scholar]
- 29.McIntosh RS, Asghar MS, Kemp EH, Watson PF, Gardas A, Banga JP, Weetman AP. Analysis of IgG kappa anti-thyroid peroxidase antibodies from different tissues in Hashimoto's thyroiditis. J Clin Endocrinol Metab. 1997;82:3818–25. doi: 10.1210/jcem.82.11.4348. [DOI] [PubMed] [Google Scholar]
- 30.Tzartos SJ, Seybold ME, Lindstrom JM. Specificities of antibodies to acetylcholine receptors in sera from myasthenia gravis patients measured by monoclonal antibodies. Proc Natl Acad Sci USA. 1982;79:188–92. doi: 10.1073/pnas.79.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo J, Quaratino S, Jaume JC, Costante G, Londei M, McLachlan SM, Rapoport B. Autoantibody-mediated capture and presentation of autoantigen to T cells via the Fc epsilon receptor by a recombinant human autoantibody Fab converted to IgE. J Immunol Methods. 1996;195:81–92. doi: 10.1016/0022-1759(96)00091-9. [DOI] [PubMed] [Google Scholar]
- 32.Simitsek PD, Campbell DG, Lanzavecchia A, Fairweather N, Watts C. Modulation of antigen processing by bound antibodies can boost or suppress class II major histocompatibility complex presentation of different T cell determinants. J Exp Med. 1995;181:1957–63. doi: 10.1084/jem.181.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasso EH, van Dijk KW, Bull AP, Milner ECB. A fetally expressed immunoglobulin VH1 gene belongs to a complex set of alleles. J Clin Invest. 1993;91:2358–67. doi: 10.1172/JCI116468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J, Namchuk M, Bugawan T, et al. Higher autoantibody levels and recognition of a linear NH2-terminal epitope in the autoantigen GAD65, distinguish stiff-man syndrome from insulin-dependent diabetes mellitus. J Exp Med. 1994;180:595–606. doi: 10.1084/jem.180.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falcone M, Lee J, Patstone G, Yeung B, Sarvetnick N. B lymphocytes are crucial antigen-presenting cells in the pathogenic autoimmune response to GAD65 antigen in nonobese diabetic mice. J Immunol. 1998;161:1163–8. [PubMed] [Google Scholar]