Abstract
Langerhans cell histiocytosis (LCH) is related to the proliferation of cells, which are similar to Langerhans cells (LC) but possess many abnormal characteristics. Lesions are widespread and this fact suggests that LCH cells or their precursors are present in the blood of patients. In five adult patients, we have isolated and cultured CD34+ blood progenitors of dendritic cells. We studied their phenotype by flow cytometry and their functional properties in mixed culture with heterologous lymphocytes and with autologous lymphocytes in the presence of tri-nitro-phenyl antigen (TNP). The amount of CD34+ precursors was dramatically higher than controls but a high mortality occurred during the in vitro differentiation. The phenotype of surviving cells was similar to LC phenotype (CD1a+, CD83+, Lag+) but some of them expressed CD2. These cells were able to induce T cell proliferation in mixed culture. They could not initiate primary response to TNP, except in a patient treated with thalidomide. In our hands, these CD34+ cells may be precursors of LCH cells.
Keywords: dendritic cells, Langerhans cells, Langerhans cell histiocytosis, precursors, CD34
INTRODUCTION
Langerhans cells (LC) are dendritic cells. Their main function is antigen presentation to lymphocytes. They originate in bone marrow and migrate to the skin and other epithelia, such as lung, mouth or genital mucosa.
Histiocytoses are a group of disorders involving cells coming from monocytes/macrophages or dendritic cells [1]. In 1953, Lichtenstein [2] coined the generic term ‘histiocytosis X’ to encompass eosinophilic granuloma, Hand–Schüller– Christian disease and Letterer–Siwe disease. Histiocytosis X is now known to involve proliferation of LC or their precursors and it has been proposed that this generic term be changed to Langerhans cell histiocytosis (LCH) [1, 3]. The pathophysiogeny of LCH remains unknown. According to the Working Group of Histiocyte Society, it seems to be convenient to divide LCH into three categories: single system disease, multi-system disease and multi-system disease with evidence of organ failure. LCH represents a rare condition in adults [4] and may be associated with monocytic leukaemias [5]. Spreading of lesions (multi-system but also multiple cutaneous lesions) strongly suggests that LCH cells are not only present in involved organs but are probably circulating with the blood flow.
The presence of LCH cells in the blood has never been demonstrated. In a recent paper, Hosmalin et al. [6] did not find an increased amount of CD1a+ cells in peripheral blood from LCH patients. We suggest that LCH cell precursors could be found in peripheral blood. It is difficult to differentiate normal LC and their blood precursors from, respectively, LCH cells and their precursors. Indeed, LCH cells share many characteristics with normal LC, which establish the relationship of this disease with a proliferation of abnormal LC [7]. Nonetheless, a recent review [8] makes an inventory of the numerous possible phenotypic and functional differences between normal LC and LCH cells. We have cultured blood CD34+ cells from adult LCH patients and studied their phenotype, their differentiation in culture and their antigen-presenting capacity. We have chosen adults because it is not possible to obtain enough cells from children.
PATIENTS AND METHODS
Patients and controls
Patient 1 was a 40 year-old man, suffering for 3 months from cutaneous lesions of LCH (Letterer–Siwe's disease), without visceral involvement. The patient was treated only with local applications of nitrogen mustard.
Patient 2 was a 35-year-old man. He had been suffering from LCH for 12 years, with involvement of skin, nails, lung and pituitary gland. The patient was treated by oral administration of thalidomide for 6 months.
Patient 3 was a 65-year-old man, suffering from pulmonary LCH for 4 years. He was treated with vepeside.
Patient 4 was a 28-year-old man, suffering from pulmonary LCH for 4 months. He was treated with vepeside.
Patient 5 was a 44-year-old man, suffering from pulmonary LCH for 12 years and treated with interferon-alpha (IFN-α).
In all patients, blood and bone marrow cells showed no abnormality in their morphology and distribution. Especially, there was no detectable monocytic or histiocytic proliferation. Blood samples from the four patients were studied. Cord blood (twenty samples) and blood from four healthy adult volunteers were used as controls.
Isolation and culture of CD34+ haematopoietic progenitor cells
Mononuclear cells were isolated by flotation on Ficoll–Paque (Pharmacia Biotech, Uppsala, Sweden) and adherent cells depleted by overnight culture in plastic flasks with 5 × 106 cells/cm2 in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (FCS) and antibiotics. CD34+ cells were purified by immunomagnetic selection with mini-MACS (Myltenyi-Biotec, GmbH, Bergisch Gladbach, Germany). This procedure gave regularly a suspension with > 95% of CD34+ cells. Isolated progenitors were cultured according to the method described by Caux et al. [9]. They were cultured in RPMI 1640 containing glutamax-1 (Gibco-BRL, Grand Island, NY), 25 mm HEPES, 10% heat-inactivated FCS (Gibco-BRL), 5 × 10−5 m 2-mercaptoethanol (2-ME; Sigma Chemical Co., St Louis, MO), antibiotics (PSF; Sigma) and 200 U/ml of human recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF; specific activity 2 × 106 U/mg, kindly provided by Schering-Plough Labs, Dardilly, France) and 50 U/ml of recombinant human tumour necrosis factor-alpha (TNF-α; specific activity 2 × 107 U/mg; Genzyme Corp., Boston, MA). Mature dendritic cells were harvested at days 12–14 and the phenotype controlled with a panel of MoAbs.
Purification of T cells
Mononuclear cells were isolated from peripheral blood or cord blood by flotation on Ficoll–Paque, then depleted of adherent cells by overnight culture on a plastic surface. T cells were purified by rosetting with aminoethylisothiouronium bromide (AET)-treated sheep erythrocytes followed by Ficoll–Paque flotation. Control by cytometry analysis showed that CD2 or CD3 expression was > 95% and B or NK markers were absent. T cells were frozen at 10 × 106 cells per vial in RPMI 1640 with 30% FCS and 10% dimethylsulfoxide (DMSO).
Chemical tests
We used 2,4,6-trinitrobenzene sulfonic acid (TNBS, P-2297; Sigma) known as a strong contact allergen. To circumvent problems related to the toxicity of TNBS, antigen-presenting cells (APC) were haptenated before they were cultured with T cells. Modification of APC with the tri-nitro-phenyl antigen (TNP) and subsequent culture were performed according to the method already described [10]. Cell pellets were resuspended in Hanks' balanced salt solution (HBSS) containing 5 mm TNBS for 10 min at 37°C and then washed out extensively. An irritant, SDS (L-4509; Sigma) was used at 10 μg/ml for control and added directly to cell cultures.
Phenotypic analysis
Cells were washed with isotonic NaCI/Pi buffer containing 1% bovine serum albumin and 0.2% sodium azide (PBS–BSA–azide). Cells (2.5 × 105) were incubated with 10 μl of labelled MoAb for 30 min at 4°C. The MoAbs used recognize numerous antigens: CD1a (B-B5, IgG1) from Innotest (Besançon, France), CD2 (IOT11, IgG1), CD3 (UCHT 1, IgG1), CD4 (IOT4, IgG2a), CD8 (B9-11, IgG1), CD83 (HB15, IgG2k) and HLA-DR (1OT2a, IgG2b) from Immunotech (Marseille, France), CD14 (TÜK4, IgG2κ), CD19 (HD37, IgG2b), CD21 (1F8, IgG1κ) and CD25 (ACT-1, IgG1κ) from Dakopatts (Aarhus, Denmark). Anti-Lag MoAb (Langerhans associated granule) was kindly provided by Kashihara-Sawami (Kyoto, Japan). After two washes in PBS–BSA–azide, cells were fixed with 1% formaldehyde in PBS–BSA–azide. Analysis was performed on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA). Cell size was estimate by light diffraction at small angles (FSC). With unlabelled antibodies we added a second step with a GAMIg–FITC or a GAMIg–PE, from Zymed Labs (South San Francisco, CA).
Cytochemical and immunophenotypic staining
Samples of 0.5 × 104 cells were resuspended in 100 μl of PBS and deposited by 20-μl volumes onto glass slides previously treated by 1% alcyan blue solution. After 30 min at ambient temperature, slides were air-dried and acetone-fixed or fixed with 1% glutaraldehyde. Cells were incubated with various MoAbs for 30 min at 4°C and stained with alkaline phosphatase-conjugated second antibody and fuchsin, from Dako LSAB 2 kit (Aarhus, Denmark).
T cell proliferative assays, mixed lymphocyte reaction
Stimulator cells were co-cultured with 105 allogeneic or autologous responder T cells in round-bottomed 96-well microtitre plates in RPMI with 10% FCS, humidified atmosphere, 5% CO2 at 37°C. Triplicate cultures for each allergen concentration were maintained for 3–6 days. Proliferation was assessed by addition of 1 μCi 3H-methyl-thymidine per well (2 Ci/mmol; Amersham, Orsay, France) 18 h before harvesting. Incorporated thymidine was quantified in a β-counter (Matrix 96; Packard Instrument Co., Downers Grove, IL). Results are expressed as the mean dpm ± s.d. of triplicate cultures.
RESULTS
Differentiation of CD34+ cells
Isolation of CD34+ cells from blood samples allowed recovery of a high number of cells in comparison with that recovered from healthy subjects' blood or cord blood. Two blood samples were taken from patient 1 at 3-month intervals and the percentages of CD34+ cells recovered were 0.28% and 0.22% of the mononuclear cells. For patients 2, 3, 4 and 5, the CD34+ cells represented respectively 0.21%, 0.14%, 0.26% and 0.11%. For healthy subjects, the numbers were 0.03%, 0.08%, 0.09% and 0.06%. In cord blood, the mean percentage of CD34+ cells was 0.53% (range 0.2–1.25%).
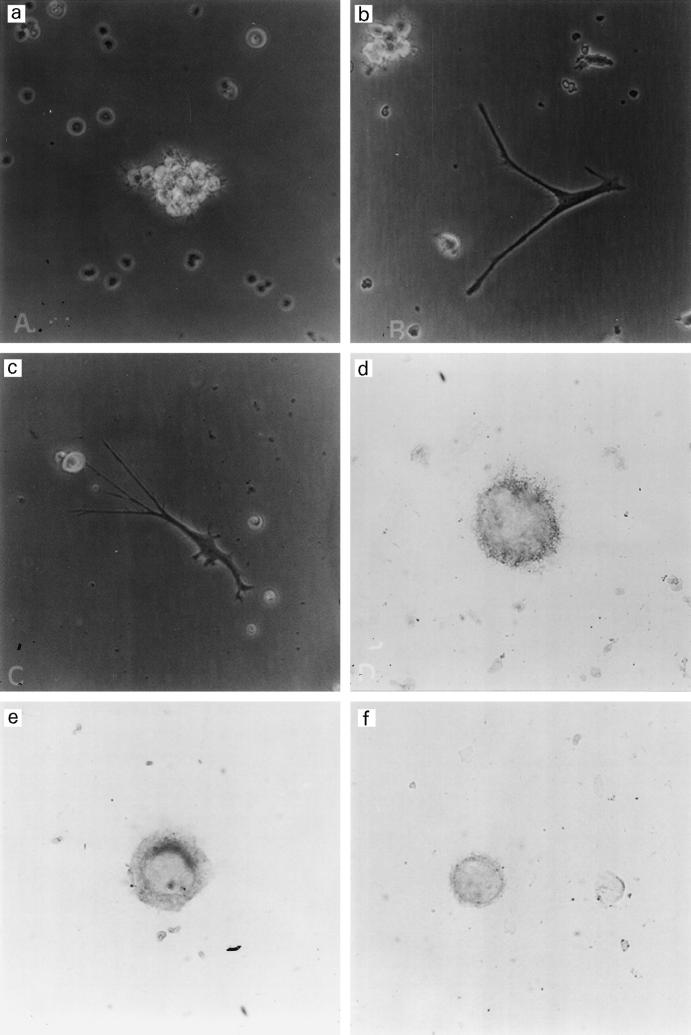
Culture of CD34+ cells with GM-CSF and TNF-α allowed the generation of dendritic cells (DC) with characteristics of LC at day 12 or 14, in particular with the expression of CD1a, CD83 and Lag antigen. CD34+ precursors are small round cells. Within 72 h, part of the cells formed small aggregates. At days 4–6 aggregates increased in size (Fig. 1a), various amounts of non-aggregated cells were observed according to the preparations; they were either small round cells or adherent cells with irregular shape; furthermore, characteristic DC appeared at day 5 around aggregates (Fig. 1b).
Fig. 1.
(a,b,c) Observation at day 6 by phase contrast microscopy with a magnification coefficient of 200. Aggregate of dendritic cells (DC) (a); and isolated adherent DC (b and c). (d,e,f) Immunoenzymatic labellings with fuchsin red of day 12 DC. (d,e) Staining with anti-Lag, mag. × 600. (f) Staining with anti-CD2, mag. × 400.
From day 6 to day 12, the size of aggregates increased again; however, unlike the CD34 cord blood cell differentiation, a high amount of cells died during this period of time (Fig. 2). By days 12–14, the amount of adherent DC was maximum; the dendritic adherent cells did not proliferate and lost their structure within 3–5 days. With all patients, the number of viable DC recovered at day 12 was near the number of isolated CD34 progenitors observed with cells isolated from the blood adult controls, whereas the ratio was over 50 with cord blood progenitors.
Fig. 2.
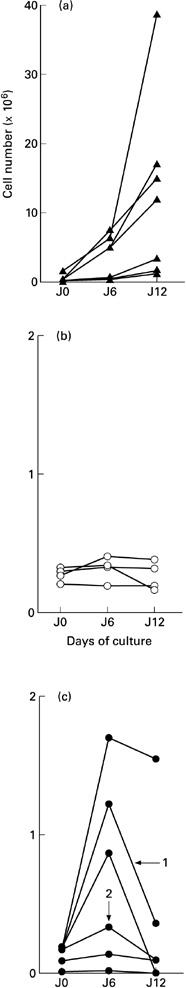
Total number of cells during the differentiation of CD34+ progenitors in dendritic cells. (a) CD34+ cells were isolated from 40–70-ml samples of cord blood. (b) CD34+ cells were isolated from 450-ml samples of peripheral blood of healthy volunteers. (c) CD34+ cells were isolated from 40–70-ml samples of peripheral blood of Langerhans cell histiocytosis (LCH) patients (numbers with arrow identify the two blood samples from patient 1).
Phenotype of LCH cells
The low amount of cells recovered at day 12 did not allow the determination of a complete phenotype; however, some characteristic markers of DC were tested either by FACS or by slide staining. With both patients, DC with markers of mature LC such as CD83 and Lag were identified (Table 1 and Fig. 1c,d). About 15% (10–20%) of these DC presented a significant labelling with anti-CD2 antibodies (Fig. 1e), but CD3 was not expressed on DC generated from peripheral blood. The same markers were present on cord blood-derived cells, except CD2, which was absent.
Table 1.
Phenotype of day 12 dendritic cells
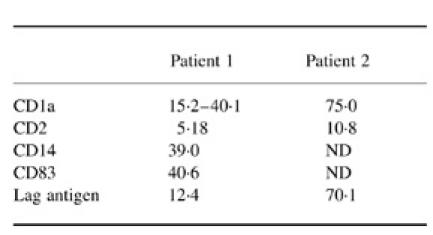
Results are expressed as % of labelled cells analysed by FACs or by cell count on stained slides.
ND, Not done.
Functional potency of LCH cells
Isolated T lymphocytes from patients were normal with respect to their phenotype with CD2 and CD3 markers > 95%, CD4 and CD8 between 40% and 50%. Other markers such as CD1a, CD14, CD19 and DR were < 10% and CD25 labelled 10–18% of the cells according to the preparation. They proliferated in mixed lymphocyte reaction (MLR) when they were cultured in the presence of allogeneic DC differentiated from cord blood or healthy subjects' blood progenitors (Fig. 3, left panels). When DC from LCH patients were cultured with allogeneic T cells, a significant response was observed; however, with patient 1, DC presented a lower potency (Fig. 2, left panels). Treatment of patients' DC with TNP led to an absence of T cell activation with patients 1, 3, 4 and 5, whereas a normal response was observed with patient 2 (Fig. 3, right panel).
Fig. 3.
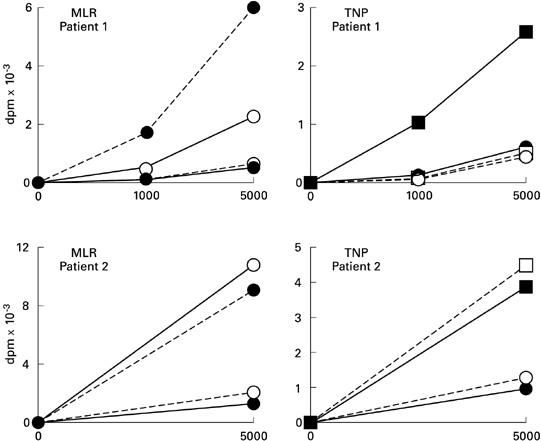
Proliferation of T lymphocytes induced by allogeneic dendritic cells (DC) or DC treated with tri-nitro-phenyl antigen (TNP). Different combinations of cells were used: untreated patients' DC (○) or treated with TNP (□); cord blood-derived DC untreated (•) or treated with TNP (▪); and T lymphocytes from patients (–––––) or from cord blood (—–). The same results were observed with DC generated from peripheral blood or controls (data not shown).
DISCUSSION
In all patients, the percentage of CD34+ peripheral blood cells was around three times higher than the percentage observed in normal human peripheral blood. Their proliferation potency was similar to that of cord blood CD34+ cells, but the mortality was abnormally high during the culture and the amount of differentiated cells was similar to that of peripheral blood controls. At day 12, part of these cells had the morphology and the phenotype of LC. Allogeneic T cell response in MLR indicated the presence of functional DR and accessory molecules, but DC were unable to activate autologous T cells in primary response (except for one patient).
Taken together, our findings suggest that LCH cell progenitors were present in the blood of patients suffering from LCH. The higher amount of CD34+ cells in our patients may indicate the presence of two populations of CD34+ cells: normal LC progenitors and LCH cell progenitors. LCH cell progenitors had a higher proliferation potency, as indicated by the cell number at day 6, but most of these cells died in vitro. In vivo, LCH cells may be rescued by the microenvironment in skin, lung or other organs. As epidermal LC [8] or in vitro generated LC [9], they were CD1a+, CD14−, CD83+ and expressed Lag antigen; however, part of these cells possessed CD2, as also LCH cells isolated from the skin. This marker is absent on normal epidermal LC and expressed by LCH cells isolated from skin biopsies [11]. CD2 could induce adhesion of LCH cells in numerous tissues [11]. At day 12, cells derived from CD34+ cells of LCH patients possessed a functional MHC class II and adequate accessory molecules, as shown by their potency in MLR. The allogeneic capacity of our generated DC to activate normal T cells contrasts with another study using LCH cells isolated from patients' skin but also spleen and lymph nodes [12].
LC are now known to originate from a subpopulation of bone marrow cells which are recovered in the blood as CD34+ precursors [13]. They belong to the family of ‘myeloid’ cells. They share common precursors with macrophages in bone marrow and probably blood, but the precise point at which LC, as other DC, differentiate from the myelomonocytic lineage remains unclear [14]. Nonetheless, differentiation of DC from monocytes is possible from cord blood [15, 16] or peripheral blood of adults [17]. Thus, normal epidermal LC arise probably from a very restricted precursor population and represent a clonal population of cells. LCH seems to represent a clonal proliferation of cells derived from LC-like cells [18, 19]. When LCH cells differ from normal LC is not resolved, but our study affords new arguments. The presence of abnormal cells in the blood of patients suffering from LCH strongly suggests that the differentiation takes place before maturation of LC in the skin. We suggest that LCH cells originate from blood CD34+ precursors, which are able to migrate to different tissues. After 6 days of culture with GM-CSF and TNf-α, cells derived from CD34+ cells are similar to ‘indeterminate cells’, normally present in the dermis [20]. In vivo, we suggest that the equivalent of these cells migrate in the dermis or other tissues in LCH patients. Then, they could induce lesions. They are probably able to multiply or die but not to differentiate in normal LC. LCH cells or their precursors could stimulate normal LC production through the secretion of growth factors, but normal LC are not more numerous in LCH patients than in healthy subjects and we do not think that the high number of CD34+ cells in LCH patients could be related to this hypothetical phenomenon.
Only one patient (no. 2) was able to activate T cells after TNP treatment, whereas the other did not. This could be related to the lower percentage of CD1a+ cells generated, but we cannot exclude a defect at the progenitor level that led to an abnormal internalization and/or processing of the mature DC. Unfortunately, the low amount of cells available did not allow us to test this hypothesis. The abnormal hapten-presenting capacity might be also related to an abnormal production of cytokines, as demonstrated with LCH cells [8]. Patient 2 activated autologous T cells in a normal manner after TNP incubation, suggesting a differentiation of the DC from a normal progenitor subpopulation. This patient was treated with thalidomide, which acts directly on mature LC, inducing a decrease of TNF-α synthesis [21]; however, it does affect in vitro mixed LC–lymphocyte reaction [22]. Yu et al. have shown that LCH cells produce higher amounts of TNF-α than normal LC [23]. We suggest that thalidomide might restore the ability of LC to initiate T cell proliferation through the inhibition of TNF-α production. Indeed, TNF-α is known to be able to inhibit antigen presentation [24].
LCH patients are often treated by dermatologists with topical treatments [1]. Our study shows that LCH cell progenitors are probably present in relatively high amounts in the blood of these patients, even at early stages of the disease, when LCH is supposed to be located only in the skin. The presence of LCH cell progenitors in the blood might explain the spreading of cutaneous lesions. It suggests that new lesions may appear in the skin or in other organs, the dissemination might be rather the consequence of cell defects such as anomaly of homing than the consequence of local dedifferentiation followed by cell dissemination. Thus, topical treatments are effective but a systemic treatment might be more preventive against relapses of the disease. This conclusion has to be moderated by the ability of some LCH patients to undergo a spontaneous healing.
Acknowledgments
This work was supported by Institut National de la Santé et de la Recherche Médicale and Hospices Civils de Lyon. We thank Marie-Josèphe Gariazzo for her skilful technical assistance, Valérie Sagot for her medical assistance and Géraldine Guironnet for her bibliographical assistance.
REFERENCES
- 1.Cline MJ. Histiocytes and histiocytosis. Blood. 1995;84:2840–53. [PubMed] [Google Scholar]
- 2.Lichtenstein L. Integration of eosinophilic granuloma of bone, Letterer–Siwe disease and Schüller–Christian disease as related manifestations of a single nosologic entity. Arch Pathol. 1953;56:84–102. [PubMed] [Google Scholar]
- 3.Misery L, Lyonnet S, Cambazard F, Faure M. Encyclopidie médico-chirurgicale-dermatologie. 12-798-A-10. Paris: Editions Techniques; 1993. Histiocytose X (histiocytose langerhansienne) p. 5. [Google Scholar]
- 4.Malpas JS, Norton AJ. Langerhans cell histiocytosis in the adult. Med Ped Oncol. 1996;27:540–6. doi: 10.1002/(SICI)1096-911X(199612)27:6<540::AID-MPO6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 5.Cambazard F, Misery L, Kanitakis J, Archimbaud E, Hemier C. Acute monoblastic leukemia and histiocytosis X: a case report and review of the literature. Eur J Dermatol. 1991;1:11–17. [Google Scholar]
- 6.Hosmalin A, MacIlroy D, Autran B, Ragot JP, Debré P, Herson S, Karmochkine M. Imbalanced ‘memory’ T lymphocyte subsets and analysis of dendritic cell precursors in the peripheral blood of adult patients with Langerhans cell histiocytosis. Clin Exp Rheumatol. 1997;15:649–54. [PubMed] [Google Scholar]
- 7.Basset F, Turiat J. Identification par la microscopie électronique de particules de nature probablement virale dans les lésions granulomateuses d'une histiocytose X pulmonaire. CR Acad Sci. 1965;261:3701–3. [PubMed] [Google Scholar]
- 8.Chu T, Jaffe R. The normal Langerhans cell and the LCH cell. Br J Cancer. 1994;70:S4–S10. [PMC free article] [PubMed] [Google Scholar]
- 9.Caux C, Vanbervliet B, Massacrier C, et al. CD34+ haematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF + TNFalpha. J Exp Med. 1996;184:695–701. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rougier N, Redziniak G, Schmitt D, Vincent C. Evaluation of the capacity of dendritic cells derived from cord blood CD34 precursors to present haptens to unsensitized autologous T cells in vitro. J Invest Dermatol. 1998;110:348–52. doi: 10.1046/j.1523-1747.1998.00150.x. [DOI] [PubMed] [Google Scholar]
- 11.De Graaf J, Tamminga R, Kamps W, Timens W. Expression of cellular adhesion molecules in Langerhans cell histiocytosis and in normal Langerhans cells. Am J Pathol. 1994;147:1161–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Yu RCH, Morris JF, Pritchard L, Chu TC. Defective alloantigen- presenting capacity of Langerhans cell histiocytosis cells. Arch Dis Child. 1992;67:1370–2. doi: 10.1136/adc.67.11.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caux C, Massacrier C, Dezutter-Dambuyant C, Vanbervliet B, Jacquet C, Schmitt D, Banchereau J. Human dendritic Langerhans cells generated in vitro from CD34+ progenitors can prime naive CD4+ T cells and process soluble antigen. J Immunol. 1995;155:5427–32. [PubMed] [Google Scholar]
- 14.Peters JH, Gieseler R, Thiele B, Steinbach F. Dendritic cells: from ontogenetic orphans to myelomonocytic descendants. Immunol Today. 1996;17:273–8. doi: 10.1016/0167-5699(96)80544-5. [DOI] [PubMed] [Google Scholar]
- 15.Caux C, Dezutter-Darnbuyant C, Schmitt D, Banchereau J. GM-CSF and TNFα cooperate for the generation of dendritic/Langerhans cells from human hematopoietic progenitors. Nature. 1992;360:258–63. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 16.Rougier N, Schmitt D, Vincent C. IL-4 addition during differentiation of CD34+ progenitors delays maturation of dendritic cells while promoting their survival. Eur J Cell Biol. 1998;75:287–93. doi: 10.1016/S0171-9335(98)80124-6. [DOI] [PubMed] [Google Scholar]
- 17.Strunk D, Rappersberger K, Egger C, Strobl H, Kromer E, Elbe A, Maurer D, Stingl G. Generation of human dendritic/Langerhans cells from circulating CD34+ hematopoietic progenitor cells. Blood. 1996;87:1292–8. [PubMed] [Google Scholar]
- 18.Willman CL, Busque L, Griffith BG, Favara BE, MacClain KL, Duncan MH, Gilliland DG. Langerhans cell histiocytosis (Histiocytosis X)—A clonal proliferative disease. N Engl J Med. 1994;331:154–60. doi: 10.1056/NEJM199407213310303. [DOI] [PubMed] [Google Scholar]
- 19.Yu R, Chu C, Buluwela L, Chu AC. Clonal proliferation of Langerhans cells in Langerhans cell histiocytosis. Lancet. 1994;1:767–8. doi: 10.1016/s0140-6736(94)91842-2. [DOI] [PubMed] [Google Scholar]
- 20.Caux C. Pathways of development of human dendritic cells. Eur J Dermatol. 1998;8:375–84. [PubMed] [Google Scholar]
- 21.Charue D, Chauvin E, Duguet C, Revuz L, Bagot M. Thalidomide decreases the production of GM-CSF and TNF-α in the mixed epidermal cell–lymphocyte reaction. Eur J Dermatol. 1996;6:373–6. [Google Scholar]
- 22.Misery L, Péguet-Navarro J, Thivolet J, Faure M, Schmitt D, Claudy AL. Thalidomide does not affect mixed skin cell–lymphocyte reactions. Clin Exp Dermatol. 1995;20:85–86. doi: 10.1111/j.1365-2230.1995.tb01296.x. [DOI] [PubMed] [Google Scholar]
- 23.Yu RCH, Buluwela L, Alaibac M, Chu A. Cytokine expression by human epidermal Langerhans cells and Langerhans cell histiocytosis cells. Br J Dermatol. 1993;129:29. [Google Scholar]
- 24.Kämpgen E, Romani N, Koch F, Eggert A, Schuler G. Cytokine receptors on epidermal Langerhans cells. In: Moll H, editor. The immune functions of epidermal Langerhans cells. Berlin: Springer-Verlag; 1995. pp. 37–52. [Google Scholar]